Abstract
The goal of this in vitro study was to evaluate the relative biocompatibility of four endodontic sealers on the cell culture of the human fibroblast through cytotoxicity.
Materials and Methods:
In this study four endodontics sealers was used GuttaFlow (Roeko)silicone based sealer, AH plus (De Tray-DENTSPLY) epoxy resin based, Apexit (Vivadent) calcium hydroxide based and Endorez (Ultradent) methacrylate based sealer. Sealers were tested on primary cell lines of human gingival fibroblasts. Experiments were preformed in laboratories of Hacettepe University of Ankara, Turkey and Faculty of Dentistry, University of Sarajevo, Bosnia and Herzegovina Cytotoxicity was determinate using WST-1 assay.
Results:
Results were analyzed by SPSS 19 program. Kolgomorov-Smirnov test, Shapiro-Wilk and descriptive statistics also were used, as well as Kriskall-Wallis, ANOVA test and T- test. According to our results all four sealers showed different cytotoxicity effects on human gingival fibroblast cell culture, but all of them are slightly cytotoxic.
Conclusions:
According to results of this study it can be concluded: all four sealers showed different cytotoxicity effects on primary cell lines of human gingival fibroblasts, but all of them are slightly cytotoxicity.
Keywords: four root canal sealers, cytotoxicity, human gingival fibroblasts
1. INTRODUCTION
Endodontic treatment aims to eliminate infection of the root canal follow by three-dimensional obturation of the endodontic space in order to prevent apical and coronal penetration of liquids and microorganisms (1). The most used obturation technique is combination gutta-percha points with an endodontic sealer. Main functions of the sealer is to fill and seal the gaps between the gutta-percha points and the walls of the root canal and to fills the voids between individual gutta-percha points.
Elutable substances and degradation products from root canal sealers may gain access to periodontal tissues in a number of ways. Most products exert some toxic effect, when they are fresh and the effect is reduced over time as the concentration of leachable components decreases (2,3).
The biocompability of different root canal sealers varies considerably, but it is of primary importance for successful endodontic treatment (4,5).
The aim of this study was to investigate the cytotoxic effects of eluates of methacrylate resin (EndoREZ), epoxy resin(AH Plus), calcium hydroxide (Apexit) and silicone (GuttaFlow) based root canal sealers on primary cell line of human gingival fibroblasts in different setting times.
2. MATERIAL AND METHODS
2.1. Sealers
Four endodontic sealers were evaluated : methacrylate resin (EndoREZ, Ultradent), epoxy resin(AH Plus, De Tray-Dentsply), calcium hydroxide (Apexit, Vivadent) and silicone (GuttaFlow, Roeko) based.
2.2. Cell Culture
Tissue samples were taken from human gingiva after the routine extraction of wisdom teeth. These samples were then sliced into 1mm3 pieces and their primer culture was done by seeding them in flasks of DMEM which include %10 FBS, 0.25mg/ml amphotericin B, 100 units/ml penicillin and 100 mg/ml streptomycin. One week later the primer focus were checked. Once the cells began to drop from the focus to flasks, they were fed with growth media every other day while their growth was obtained in incubator at 37ºC, %5 CO2. When the cells covered their flasks, the passaging was done with %0.25 Tripsin-EDTA. After passaging, some of the cells were frozen and stored. Other cells were seeded in new flasks (1×106 cell/80 cm2) to grow.
Figure 1.
Gingival fibroblast
Figure 2.
Fibroblasts cell growth
2.3. Preparation of Sealer Specimens
Root canal sealers were prepared according to the manufacturer’s recommendation. The sealers were then placed into sterile, cylindrical teflon molds which had 4 mm diameter and 2 mm height. First group four samples from each sealer immediately after setting were immersed in medium. The second, third and fourth group of specimens were stored in humid environment at 37 ºC for 24 hours, 48 hours and 7 days and then taken to the cell culture medium for testing.
2.4. Preparation of Extraction Medium
Extraction medium was prepared in cell culture medium as 1.25cm²/ml. It was the proportion of the surfaces of the specimens and the volume of medium. The petri dishes in which the extracts stored, were incubated for 24 hours at 37°C.
The specimens were removed and the extracts were sterile filtered using Millex-GS sterile filter (Milipore S.A.S., Molsheim, Cedex, France). Undiluted extracts were used for the testing.
2.5. Cytotoxicity Test
The cytotoxicity of the specimens were analyzed by WST-1 method (6). This method was based on the diminish of the WST’s yellow-orange color, mitochondrial dehydrogenation of the respiratory chain reaction and the formazon products from degradation. Thereby viability of cells were measured with optical density of the formazan products. WST-1 method was applied according to the manufacturer’s recommendation.
Human gingival fibroblasts were seeded in plates with 96 wells as to be 1×104 cell/well.
100 µlt culture medium was added to each of wells. Medium was taken away when the human gingival fibroblasts became sub-confluent.
The cell layer was washed with phosphate buffered saline and treated with 100 µlt culture medium, 10%FBS (control) and the extracts for 24 hours.
At the end of the process, 10 µlt WST-1 was added and incubated for 4 hours at 37 ºC. Absorption of cells was read with micro ELISA at 420-600nm. For the viability of cells the formula below was used:
% Viability of cells = [(mean absorbancy of treated cells) – (mean absorbancy of blank cells)/(mean absorbancy of control cells) – (mean absorbancy of blank cells)]×100
2.6. Statistical analysis
The mean absorbencies of the six wells containing the same extract and their standard deviation were calculated. Optical density values of test cultures were expressed as percentage of optical density obtained for the control medium. The absorption value obtained with the control was considered as indicating 100% viability. Cytotoxicity was also rated based on cell viability relative to controls as not cytotoxic –>90% cell viability, slightly cytotoxic – 60 90% cell viability, moderately cytotoxic – 30–59% cell viability and strongly cytotoxic – <30% cell viability.
3. RESULTS
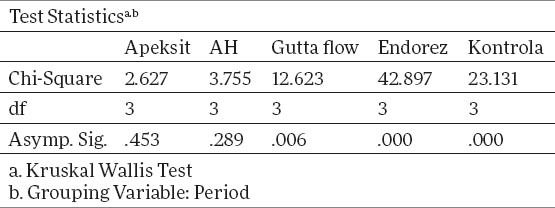
Tables 1 and 2 show descriptive statistics of total human gingival fibroblasts. The middle value for Apexit is 3.00±0.24, AH 2.89±0.53, Gutta flow 2.96±0.53, Endorez 2.51±0.37, Control 3.15±0.33. X2 test proves that there is a statistically significant difference between groups for p<0.05, for Gutta flow, Endorez and Control, while for Apexit and AH there is no statistically significant difference. The value of X2 test for Apexit 2.67, for AH 3.75, for Gutta Flow 12.623, for Endorez 42.8 and for control 23.13.
Table 1.
Total descriptive statistics for humang gingival fibroblasts with different sealers
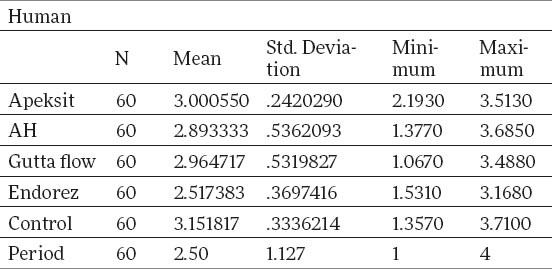
Table 2.
Testing ranks by X2 test
Tables 3 and 4 show a descriptive statistics of total human fibroblasts expressed in percents. The percents of surviving cells are calculated by the formula of group control/control x 100. The middle value for Apexit is 96.7%±17.8, AH 93.6%±24,1, Gutta flow 95.5%±24.1, Endorez 80.8%±16.2. X2 test proves that there is a statistically significant difference between groups for p<0.05, for Gutta flow, Endorez and Apexit, while for AH there is no statistically significant difference. The value of X2 test for Apexit is 13.6, for AH 4.43, for Gutta Flow 9.698, and for Endorez 28.2. The aforementioned results for surviving cells are given on graph 2, while the true cytotoxicity of tested sealers in given on graph 3.
Table 3.
Descriptive statistics for the total value of human fibroblasts expressed in %
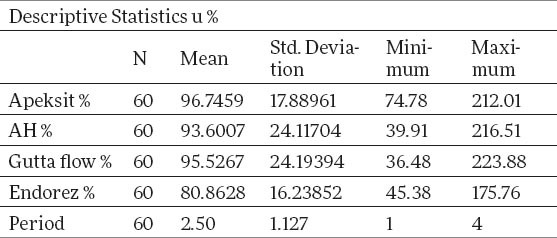
Table 4.
Testing ranks of human fibroblasts expressed in percents by X2 test
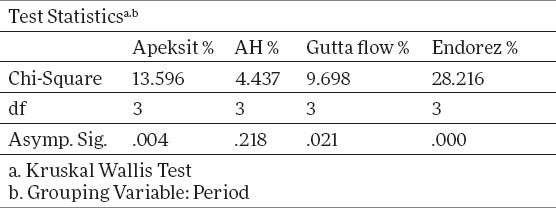
Graph 2.
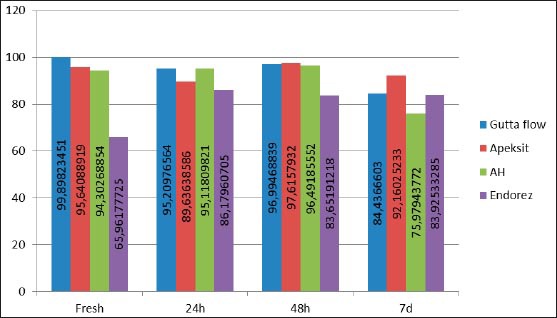
Values of surviving cells in human fibroblasts through time expressed in percents
Graph 3.
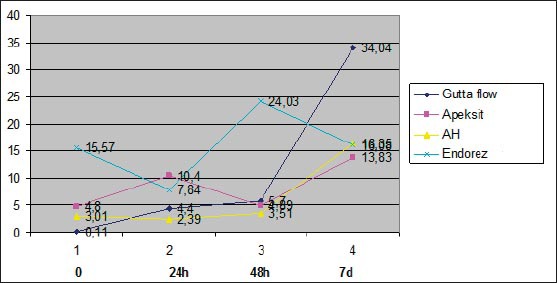
Cytotoxicity of sealers expressed in precents through time
Graph 1.
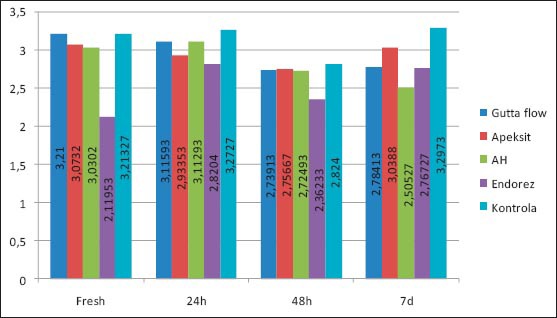
Values of surviving cells in human gingival fibroblasts through time
4. DISCUSSION
In this study, calcium hydroxide based sealer Apexit Plus showed a low cytotoxicity through the observed time periods. Freshly mixed Apexit Plus had a 95.6% cell visibility; after 24 hours 89.6% and after 48 hours 97.6%, with cellular survival of 92.1% after 7 days, which points out that sealer is slightly cytotoxic. Results of this study for calcium hydroxide based sealer Apexit Plus, are in compliance with findings of Beltes et all 1995, Vajrabhaya and Sithisarn 1997, Geurtsen et all 2001, Miletić et all 2000, Schwarze et all 2002. (7,8,9,10,11)
Silicone based sealer GuttaFlow, showed non-cytotoxicity in a freshly mixed stated, where live cell visibility on the culture of human gingival fibroblast was 99%. 24 hours later, there was still no cytotoxic effect and live cell visibility was 95.2% on the culture of human gingival fibroblast, and after 48 hours with live cell visibility of 96.9%.
Such results correlate with the findings of Bouillaguet et all.,2006 and Willershausen et all 2011, where they conclude that the silicone based sealer GuttaFlow is not cytotoxic and is considered to be biocompatible (12,13). It is an interesting fact that, in this study, GuttaFlow, on the culture of human gingival fibroblast after 7 days shows a slightly increased cytotoxicity for the percentage of live cells after 7 days is 84.4%. We find that cytotoxicity of GuttaFlow sealers was increased, though quite mildly. This data corresponds to the results of Bouillaguet et all. 2006, where it is mentioned that a toxic response to is increased in time. It is believed, that the reason to it is release of silver particles that are added to this sealer as a preservative (12). Furthermore, research shows that the existence of small voids in the core of GuttaFlow, and that allows possible releasing of unreacted components in such porosities(14,15).
In this research, AHA Plus Jet epoxy resin based sealer, on the culture of human gingival fibroblast, showed no cytotoxic effect, neither in the initial time, nor in other observed periods. This finding is consistent to the findings of Leyhausen et all 1999 and Willershausen et all 2011, where AHA Plus Jet sealer is considered biocompatible (13).
In this study, freshly mixed AHA Plus had 94.3% of live cells; after 24 hours it is 95.1%; after 48 hours it is 96.4%, and after 7 days it is 75.9%. These results show that cytotoxicity of AHA Plus after 7 days is somewhat higher in relation to previously tested periods of time. This decrease of visible live cells is still within the limits of insignificant cytotoxicity, but we can say that these results correspond to Bouillaguet et all.,2006, in which it is stated that cytotoxicity of this sealer increased in time (12).
In this research, methacrylate based sealer Endorez, in the culture of human gingival fibroblast, as a freshly mixed sample has 65.9% visible live cells. A sample of this sealer, after 24 hours gives a cellular visibility of 86.6%; after 48 hours it is 83.6% and after 7 days, the visibility of live cells is 83.9%. These results show that freshly mixed Endorez had the highest rate of cytotoxicity, which decreased in time, which corresponds the results of Lodiene et all 2008, Zmener 2004, Miletić et all. 2005 (16, 17, 18).
With human gingival fibroblast, using Kruskal Wallis rank sum test by comparing middle values of live cells in time, it was shown that there was a statistically proven difference (p<0,05) for Gutta flow, Endorez and Apexit. Therefore, their cytotoxicity changes in time, while there was no statistically proven difference for AHA Plus sealer, that is, its cytotoxicity does not change significantly in time. These results, for AHA Plus sealer, we can take with reserve, since we experienced significant deviations from the normal distribution with this sealer after 7 days measuring.
5. CONCLUSIONS
According to results of this study it can be concluded: all four sealers showed different cytotoxicity effects on human gingival fibroblast cell culture, but all of them are slightly cytotoxic.
Endorez and Apexit their cytotoxicity changes in time, while there was no statistically proven difference for AHA Plus and GuttaFlow sealer, its cytotoxicity does not change significantly in time.
Acknowledgement
The research process of this study was accomplished in Hacettepe University Ankara/Turkey and supported by Hacettepe University Scientific Research Coordination Unit (Project number: 011 D01 201 003). Also the paper ‘In Vitro Comparison of Cytotoxicity of Four Root Canal Sealers on Human Gingival Fibroblasts’ (doi: 10.5455/medarh.2015.69.24-27) was a part of the same grant. Author wish to affirm gratitude to all colleagues on Department of Endodontics, Faculty of Dentistry, Hacettepe University and Department of Histology, Faculty of Medicine, Hacettepe Universityof Ankara, Turkey for all help and support in this research..
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of new resin calcium hydroxide - and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. International Endodontic Journal. 2007;40:329–337. doi: 10.1111/j.1365-2591.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 2.Araki K, Suda H, Spangberg LS. Indirect longitudinal cytotoxicity of root canal sealers on L929 cells and human periodontal ligament fıbroblasts. Journal of Endodontics. 1994;20:67–70. doi: 10.1016/S0099-2399(06)81183-0. [DOI] [PubMed] [Google Scholar]
- 3.Thom DC, Davies JE, Santerre JP, Friedman S. The hemolytic and cytotoxic properties of a zeolite-containing root filling material in vitro. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics. 2003;95:101–108. doi: 10.1067/moe.2003.90. [DOI] [PubMed] [Google Scholar]
- 4.Geurtsen W. Biocompatibility of resin-modified filling materials. Critical Reviews in Oral Biology and Medicine. 2000;11:333–355. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 5.Geurtsen W. Biocompatibility of root canal filling materials. Australian Endodontic Journal. 2001;27:12–21. doi: 10.1111/j.1747-4477.2001.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Francoeur AM, Assalian A. Microcat: a novel cell proliferation and cytotoxicity assay based on WST-1. Biochemica. 1996;3:19–25. [Google Scholar]
- 7.Beltes P, Koulaouzidou E, Kotoula V, Kortsaris AH. In vitro evaluation of the cytotoxicity of calcium hydroxidebased root canal sealers. Endodontics and Dental Traumatology. 1995;11:245–249. doi: 10.1111/j.1600-9657.1995.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwarze T, Fiedler I, Leyhausen G, Geurtsen W. The cellular Compatibility of Five Endodontic Sealers during the Setting Period. Journal of Endodontics. 2002;28:784–778. doi: 10.1097/00004770-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Vajrabhaya L, Sithisarn P. Multilayer and monolayer cell cultures in a cytotoxicity assay of root canal sealers. International Endodontic Journal. 1997;30:141–144. doi: 10.1046/j.1365-2591.1997.00056.x. [DOI] [PubMed] [Google Scholar]
- 10.Miletic I, Anic I, Karlovic Z, Maršan T, Pezelj-Ribaric S, Osmak M. Cytotoxic effect of four root filling materials. Endodontics and Dental Traumatology. 2000;16:287–290. doi: 10.1034/j.1600-9657.2000.016006287.x. [DOI] [PubMed] [Google Scholar]
- 11.Geurtsen W. Biocompatibility of root canal filling materials. Australian Endodontic Journal. 2001;27:12–21. doi: 10.1111/j.1747-4477.2001.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 12.Bouillaguet, et al. Initial In Vitro Biological Response to Contemporary Endodontic Sealers. JOE. 2006;32:989–992. doi: 10.1016/j.joen.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Willershausen, et al. In Vitro Analysis of the Cytotoxicity and the Antimicrobial Effect of Four Endodontic Sealers. Head and Face Medicine. 2011;7:15. doi: 10.1186/1746-160X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjo¨gren U, Sundqvist G, Nair PNR. Tissue reaction to gutta-percha particles of various sizes when implanted subcutaneously in guinea pigs. European Journal of Science. 1995;103:313–321. doi: 10.1111/j.1600-0722.1995.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang FQ, She WJ, Fu YF. Comparison of the cytotoxicity in vitro among six types of nano-silver base inorganic antibacterial agents. Zhonghua Kou Giang Yi Xue Za Zhi. 2005;40:504–507. [PubMed] [Google Scholar]
- 16.Lodiene G, Morisbak E, Bruzell E, Ørstavik D. Toxicity Evaluation of Root Canal Sealers in Vitro. International Endodontic Journal. 2008;41:72–77. doi: 10.1111/j.1365-2591.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 17.Zmener O. Tissue response to a new methacrylatebased root canal sealer: preliminary observations in the subcutaneous connective tissue of rats. Journal of Endodontics. 2004;30:348–351. doi: 10.1097/00004770-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Miletić I, Devčić N, Anić I, Borčić J, Karlović Z, Osmac M. The cytotoxicity of RoekoSeal and AH Plus compared during different setting periods. Journal of Endodontics. 2005;32:307–309. doi: 10.1097/01.don.0000140570.95688.ee. [DOI] [PubMed] [Google Scholar]


