Abstract
Background:
The efficacy of isotretinoin at 0.5 to 1.0 mg/kg per day in the treatment of acne is well established and considered safe, although it is sometimes not easily tolerated because of its cutaneous side effects.
Objective:
The purpose of this study was to determine the efficacy of low-dose isotretinoin in the treatment of acne.
Methods:
In this prospective, non comparative, open-label study, 50 patients, both male and female, with moderate acne were enrolled and treated with isotretinoin at 20 mg/d (approximately 0.3-0.4 mg/kg per day) for 3 months. The patients were divided into two age groups: 12 to 20 and 21 to 35 years old. Patients were evaluated at 2-month intervals by means of clinical and laboratory examinations. A 4-year follow-up was also carried out.
Results:
At the end of the treatment phase, good results were observed in 90.8% of the patients aged 12 to 20 years, and in 89.6% of the patients aged 21 to 35 years. Failure of the treatment occurred in 5.2% and 7.4% of the two groups, respectively. Three patients dropped out of the study because of lack of compliance, and another patient discontinued participation because of a laboratory side effect. During the 2-year follow-up period, relapses of the acne occurred in 3.9% of the patients aged 12 to 20 years and in 5.9% of the patients aged 21 to 35 years. Elevated serum lipid levels (up to 20% higher than the upper limit of normal value) were found in 4.2% of the patients and abnormal (<twice the upper limit of normal values) liver tests were observed in 4.8%.
Limitations:
This was a non comparative, open-label study.
Conclusion:
Three months of treatment with low-dose isotretinoin (20 mg/d) was found to be effective in the treatment of moderate acne, with a low incidence of severe side effects and at a lower cost than higher doses.
Keywords: acne vulgaris, low-dose vitamin “A” tablets
1. INTRODUCTION
Acne is chronic inflammatory disease of the pilosebaceous units. The driving forces for the development of acne are an increased sebum production, ductal cornification, propionibacterium acnes.(P. acnes) bacterial colonization of the pilosebaceous duct and inflammation (1). Inflammatory lesions may be superficial or deep, and may arise from non-inflamed lesions (comedones).
The superficial lesions are usually papules and pustules and the deep lesions are deep pustules and nodules (2). The ratio of P. acnes counts in patients with acne, and without acne, is very high. Cutaneus P. acnes are slow-growing bacteria and may be unable to multiply in follicles in which the sebum excretion rate is high, as end products of bacterial metabolism escape with the out-flowing sebum. When a follicle becomes blocked, it behaves as a closed culture system from which bacteria and their end products cannot escape (4). Acne is dermatosis, very frequent at puberty and which can affect 80 to 100% of teenagers according to estimates. In a great majority of cases, acne disappears without leaving any sequels. However, in about 15% of cases, it can take a severe form requiring medical management. Isotretionoin is nowadays recognized in the treatment of severe forms (nodulare and nodulocystic, Conglobata and antibiotic-resistant acne) (5). Daily medical practice is affected when it comes to making a choice. What medical strategy, what drug, among all those available which have an equivalent efficacy and are interested in the same indications, should be adopted against a given condition and for a given type of patients. Through this work, our wish was to describe the impact of a modification of the cost of consumed medical resources on the overall cost of management, for health insurance, of the condition that affects a young population, most of the time still at school. In the first chapter, it sounded relevant to give a reminder of the bases of economic evaluation in order to familiarize the reader which the terminology inherent to this discipline.
Isotretinoin
Oral isotretinoin is a synthetic vitamin A analog that should be considered for all patients with moderate-or-severe recalcitrant acne, provided there are no contraindications. It is the only treatment that has an effect on all four major pathogenic factors involved in acne and is therefore, unsurprisingly, the most clinically effective anti-acne therapy available (6, 7). Since its first approval for severe treatment-resistant acne in 1982, it has revolutionized the management of acne, producing long-term remission or significant improvements in many patients (8).
Isotretinoin decreases the size and secretion of the sebaceous gland, normalizes follicular keratinization and prevents comedogenesis, inhibits the growth of surface and ductal P. acnes via changes of the follicular milieu, and has anti-inflammatory effects (6-9). During treatment, isotretinoin reduces sebum production by 90% or greater (within 6 weeks) and P. acnes populations decrease substantially (9). However, both sebum and P. acnes levels increase upon cessation of treatment, albeit to a lesser extent (10).
This profound effect on sebaceous gland activity can be achieved in most patients with a dose of isotretinoin 0.5–1.0 mg/kg/day. A recent European Directive on isotretinoin prescribing has recommended a starting dose for all patients of 0.5 mg/kg/day, with the dose titrated to obtain a maximal early response offset against the development and tolerance of side effects (12). Being lipophilic, it should be administered with food, which has been shown by pharmacokinetic studies to double absorption (13). A heavy alcohol intake should be avoided while on treatment as isotretinoin is metabolized by cytochrome P450 enzymes, which are induced by ethanol, resulting in reduced efficacy (14).
Retinoic (vitamin A) acid is available in the form of tretinoin gel or cream (0.01–0.025%) and its isomer, isotretinoin gel (0.05%). A third-generation retinoid-like drug, adapalene (gel or cream; 0.1%) is also licensed for mild-to-moderate acne, has a significant and rapid anti-inflammatory action and is better tolerated than its predecessors (15, 16). Vitamin A and other retinoids reduce abnormal growth and development of keratinocytes within the pilosebaceous unit. Reversal of the hypercornification within the follicular canal, as well as the induction of accelerated proliferation of the follicular epithelium helps to ‘unplug’ the follicle (17). This in turn inhibits development of the microcomedo and noninflammatory lesions, resulting in fewer anaerobic conditions, a reduction in P. acnes growth and a microenvironment less favorable for the development of inflammation. In addition, the newer retinoids reduce the rupture of comedones into the surrounding skin, also resulting in less inflammation (18, 19).
In terms of preference, both 0.1% adapalene and 0.025% tretinoin are shown to be equally effective against total lesion count, although adapalene has a faster onset of action with greater activity observed after 1 week of continuous daily use. By 12 weeks, both agents reduce noninflammatory lesions and inflammatory lesions by 83 and 70%, respectively (15, 21, 24).
All topical retinoids can produce irritant dermatitis, but this is less problematic with second- and third-generation agents and with cream formulations rather than gels (21). Patients should be warned that they may experience an initial flare of inflammatory lesions at the start of treatment (22). These adverse effects can be minimized by starting at lower concentrations for a short period with incremental increases in contact time and preparation strength. Owing to potential photo sensitivity, topical retinoids are best applied at night and patients should not expose themselves to excessive UV light. The link between retinoids and teratogenicity is well established but significant systemic absorption of topical retinoids has not been demonstrated (24). Recommendations are, however, that female patients should be advised to avoid pregnancy and discontinue use immediately should they conceive while on treatment.
The efficacy of isotretinoin at 0.5 to 1.0 mg/kg per day in the treatment of acne is well established and considered safe, although it is sometimes not easily tolerated because of its cutaneous side effects.
2. OBJECTIVE
The purpose of this study was to determine the efficacy of low-dose isotretinoin in the treatment of acne.
3. METHODS
We Studies, 50 patients were taken in two genera (25–25) of which half was treated with isotretionin orally and the other half with the local isotretionin. The patients were divided into two age groups: 12 to 20 and 21 to 35 years old. Patients were evaluated at 2-month intervals by means of clinical and laboratory examinations. For the purpose of our example, the reference elements we have decided to retain are the medical recommendations inherent to isotretinoin prescription. A blood test including triglyceride, total cholesterol and transaminase noising is also necessary. Regular monitoring of these results is only necessary in the case of noticed abnormalities in patients with risk factors (diabetes, obesity, hepatitis B or C).
4. RESULTS
At the end of the treatment phase, good results were observed in 90.8% of the patients aged 12 to 20 years, and in 89.6% of the patients aged 21 to 35 years. Failure of the treatment occurred in 5.2% and 7.4% of the two groups, respectively. Three patients dropped out of the study because of lack of compliance, and another patient discontinued participation because of a laboratory side effect. During the 2-year follow-up period, relapses of the acne occurred in 3.9% of the patients aged 12 to 20 years and in 5.9% of the patients aged 21 to 35 years. Elevated serum lipid levels (up to 20% higher than the upper limit of normal value) were found in 4.2% of the patients and abnormal (<twice the upper limit of normal values) liver tests were observed in 4.8%. Results of treatment of two our patients with acne vulgaris by isotretionin low dose are presented on Figures 1 (a, b, c and d) and 2 (a, b, c and d). Side effects; mostywere; dry skin, dry mouth, slight depression.
Figure 1a, 1b, 1c and 1d.
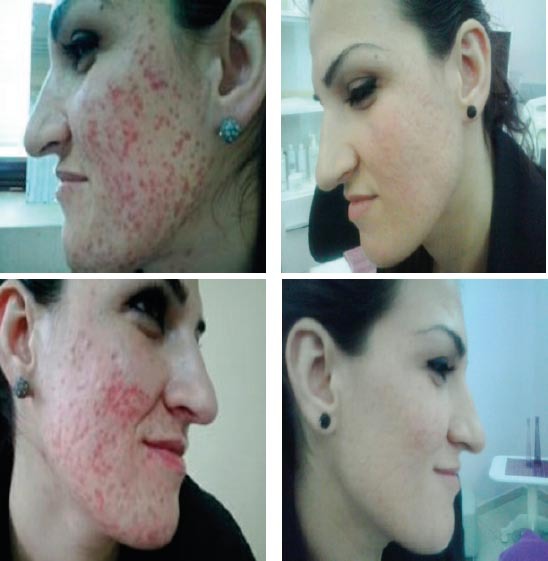
Result of patient treatment with low dose of isotretinoin
Figure 2a, 2b, 2c and 2d.
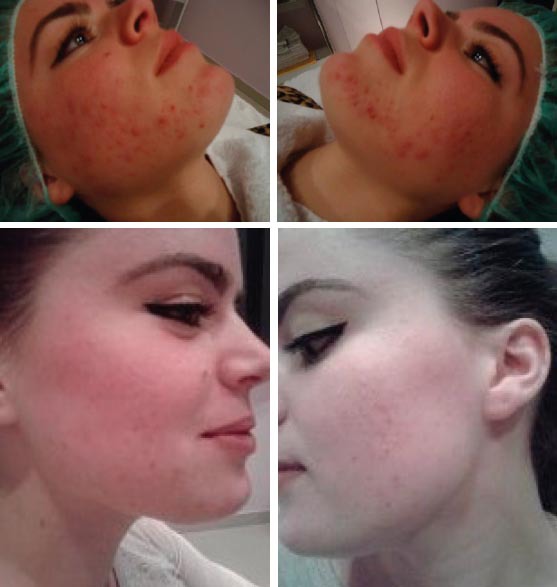
Result of patient treatment with low dose of isotretinoin
Figure 3.
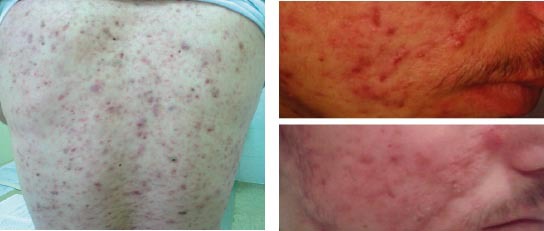
Acne vulgaris before and after treatment with isotretinoin
5. CONCLUSION
Three months of treatment with low-dose isotretinoin (20 mg/day) was found to be effective in the treatment of moderate to severe acne vulgaris. The benefits accrued to the society from using isotretinoin outweigh the risks, and thus low-dose isotretinoin can be used in the treatment of moderate to severe acne vulgaris as an effective modality of treatment, with a low incidence of severe side effects and at a lesser cost. We recommend judicious use of low-dose isotretinoin in patients with moderate to severe acne, because acne not only scars the face, but also the mind and the heart. Three months of treatment with low-dose isotretinoin (20 mg/d) was found to be effective in the treatment of moderate acne, with a low incidence of severe side effects and at a lower cost than higher doses.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Cunliffe WJ. The sebaceous gland and acne-40 years on. Dermatology. 1998;196:9–15. doi: 10.1159/000017859. [DOI] [PubMed] [Google Scholar]
- 2.Orentreich N, Durr NP. The natural evolution of comedones into inflammatory papules and pustules. J Invest Dermatol. 1974;62:316–320. [Google Scholar]
- 3.Leyden JJ. Absorption of minocycline hydrochloride: effect of milk, food, and iron. J Am Acad Dermatol. 1985;12:308. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 4.Simpson NB. Functional blockage of open comedones. Br J Dermatol. 1987;17:43–47. doi: 10.1111/j.1365-2133.1987.tb04089.x. [DOI] [PubMed] [Google Scholar]
- 5.Cunliffe WJ, Gould DJ. Prevalence of facial acne vulgaris in late adolescents and adults. 1979 Apr;28:1–10. doi: 10.1136/bmj.1.6171.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King K, Jones DH, Daltry DC, et al. A double-blind study of the effects of 13-cis-retinoic acid on acne, sebum excretion rate and microbial population. Br J Dermatol. 1982;107:583–590. doi: 10.1111/j.1365-2133.1982.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada M, Schroder M, Roos TC, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerisation to all-trans-retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115:321–327. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Layton AM, Knaggs HE, Taylor J, et al. Isotretinoin for acne vulgaris - 10 years later. A safe and successful treatment. Br J Dermatol. 1993;129:292–296. doi: 10.1111/j.1365-2133.1993.tb11849.x. [DOI] [PubMed] [Google Scholar]
- 9.Ward A, Brogden RN, Heel RC, et al. Isotretinoin: a review of its pharmacological properties and therapeutic efficacy in acne and other skin disorders. Drugs. 1984;28:6–37. doi: 10.2165/00003495-198428010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Leyden JJ, Mc Ginley KJ, Foglia AN. Qualitative and quantitative changes in cutaneous bacteria associated with systemic isotretinoin therapy for acne conglobata. J Invest Dermatol. 1986;86:390–393. doi: 10.1111/1523-1747.ep12285658. [DOI] [PubMed] [Google Scholar]
- 11.Strauss JS, Stranieri AM. Changes in long-term sebum production from isotretinoin therapy. J Am Acad. Dermatol. 1982;6:751–755. doi: 10.1016/s0190-9622(82)80055-8. [DOI] [PubMed] [Google Scholar]
- 12.Layton AM, Dreno B, Gollnick HPM, et al. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20:773–776. doi: 10.1111/j.1468-3083.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 13.Colburn WA, Gibson DM, Wiens RE, et al. Food increases the bioavailability of isotretinoin. J Clin Pharmacol. 1983;23:534–538. doi: 10.1002/j.1552-4604.1983.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 14.Soria C, Allegue F, Galiana J, et al. Decreased isotretinoin efficacy during acute alcohol intake. Dermatologica. 1991;182:203. doi: 10.1159/000247785. [DOI] [PubMed] [Google Scholar]
- 15.Caron D, Sorba V, Kerrouche N, et al. A split-face comparison of adapalene 0.1% gel and tretinoin gel 0.025% gel in acne patients. J Am Acad Dermatol. 1997;36:S110–S112. doi: 10.1016/s0190-9622(97)70052-5. [DOI] [PubMed] [Google Scholar]
- 16.Ioannides D, Rigopoulos D, Katsambas A. Topical adapalene gel 0.1% vsisotretinoin gel 0.05% in the treatment of acne vulgaris: a randomised open-label clinical trial. Br J Dermatol. 2002;147:523–527. doi: 10.1046/j.1365-2133.2002.04873.x. [DOI] [PubMed] [Google Scholar]
- 17.Christophers E, Wolfe HH. Effect of vitamin A acid on skin: in vivo and in vitro studies. Acta Derm Venereol. 1975;74:42–53. [PubMed] [Google Scholar]
- 18.Leyden JJ. Rational therapy for acne vulgaris: an update on topical treatment. J Am Acad Dermatol. 1986;15:907–915. doi: 10.1016/s0190-9622(86)70250-8. [DOI] [PubMed] [Google Scholar]
- 19.Bouclier M, Chatelus A, Ferracin J, et al. Quantification of epidermal histological changes induced by topical retinoids and CD271 in the rhino mouse model using a standardized image analysis technique. Skin Pharmacol. 1991;4:65–73. doi: 10.1159/000210926. [DOI] [PubMed] [Google Scholar]
- 20.Cunliffe WJ, Poncet M, Loesche C, et al. A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris. A meta-analysis of five randomised trials. Br J Dermatol. 1998;139(Suppl. 52):48–56. doi: 10.1046/j.1365-2133.1998.1390s2048.x. [DOI] [PubMed] [Google Scholar]
- 21.Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicentre trial. J Am Acad Dermatol. 1996;34:482–485. doi: 10.1016/s0190-9622(96)90443-0. [DOI] [PubMed] [Google Scholar]
- 22.Layton AM. Disorders of the sebaceous glands. In: Burns DA, Breathnach SM, Cox NH, et al., editors. Rook's Textbook of Dermatology. 8th Edition. London, UK: Blackwell Publishing; 2010. [Google Scholar]
- 23.Schefer H. Penetration and percutaneous absorption of topical retinoids. A review. Skin Pharmacol. 1993;6:17–23. doi: 10.1159/000211160. [DOI] [PubMed] [Google Scholar]


