Abstract
Background
Previous reports describe false positive urine immunoassay screens for phencyclidine (PCP) associated with use of tramadol, dextromethorphan, or diphenhydramine. The likelihood of these false positives is unknown.
Objective
We sought to find the relative frequency of false-positive PCP screens associated with these medications and to look for any other medications with similar associations.
Methods
In an IRB-approved study, we retrospectively reviewed charts of all ED encounters with positive urine screens for PCP in our hospital from 2007 through 2011, inclusive. Our laboratory routinely confirmed all positive screens using GC-MS with results classified as either “confirmed” (true positive) or “failed to confirm” (false positive). We recorded all medications mentioned in the chart as current medications or medications given before the urine sample. We compared frequencies of tramadol, dextromethorphan, diphenhydramine, and other medications between the two groups.
Results
Tramadol, dextromethorphan, alprazolam, clonazepam, and carvedilol all were significantly more frequent among the false positive group. Diphenhydramine was more frequently recorded among the false positive group, but this was not statistically significant.
Conclusion
False positive urine screens for PCP are common with tramadol, dextromethorphan, alprazolam, clonazepam, and carvedilol and may also occur with diphenhydramine.
Introduction
In 2010, the Drug Abuse Warning Network (DAWN) recorded over 4.9 million Emergency Department (ED) visits in the ten US metropolitan areas covered in the network.1 Of these, 53,716 ED visits were classified as involving phencyclidine (PCP) with positive toxicology testing in 28,938 of these visits.
Urine drug screening in the ED usually involves immunoassay testing for major classes of drugs of abuse. Most hospitals do not routinely perform secondary testing to confirm positive screen results. Therefore, the true rate of false positive urine drug screens is unknown. False positive drug screens for illicit drugs can lead to an incorrect treatment, prejudice from nursing and physician staff, increased healthcare expenditures, and adverse legal or employment action.
Published reports describe false positive urine screen for PCP associated with certain commonly used medications, such as tramadol (Ultram ®),2, 3, 4 dextromethorphan (cough suppressants with ‘DM’ in the name),5, 6, 7 and diphenhydramine (Benadryl ®). 8, 9 This is likely due to structural similarities including two six-member rings separated by one atom of carbon or nitrogen (Figure 1). However, the frequency of such false positive results is unknown. The objective of this study was to determine the frequency of false positive urine screens for PCP in association with reported use of medications.
Figure 1.
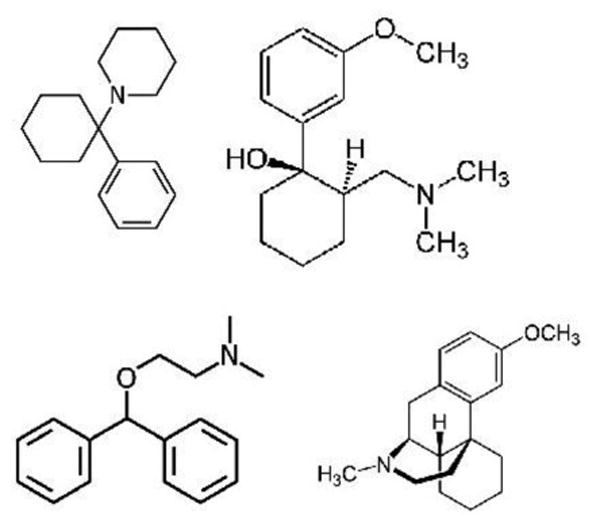
Structures of PCP, tramadol, diphenhydramine, and dextromethorphan. Adapted from Wikipedia (http://en.wikipedia.org/wiki/Tramadol, http://en.wikipedia.org/wiki/Diphenhydramine, http://en.wikipedia.org/wiki/Dextromethorphan)
Methods
The approach for this IRB-approved, retrospective study was very similar to a previous study of false positive urine drug screens for amphetamines associated with the use of bupropion by Casey et al.10 We reviewed ED and hospital charts for patients presenting to the Barnes-Jewish Hospital ED from 2007-2011 with a positive urine screen for PCP. We then divided these patients into two groups: those that were “confirmed” by GC-MS, and those that were “non-confirmed”, either because of an interfering substance or because the concentration of PCP was below the reportable threshold.
We recorded documented use of tramadol, diphenhydramine, and dextromethorphan, as well as any other medications that frequently appeared. We defined “documented use” of a particular medication if one of the following was applicable: 1) a medication the patient was already taking at the time of the visit on which the UDS was performed, 2) a medication that was administered in the ED on the visit the UDS was performed but before the results of the UDS were given, and 3) a medication that was prescribed on the preceding ED visit. We then compared the proportions of each medication within the “confirmed” and “non-confirmed” groups. The null hypothesis of each comparison was that the frequency of a given medication would be the same in both groups.
Before starting the data extraction phase of the project, researchers met to clearly define variables and standardize data collection. Weekly meetings were scheduled to discuss progress and to ensure uniform handling of data that were conflicting, missing, or ambiguous. The rater was not blinded to the purpose of the study.
All protected health information (PHI) was de-identified and recorded into a password-protected Microsoft Excel® (Microsoft, Redmond WA) file for tabulation and statistical comparisons.
Results
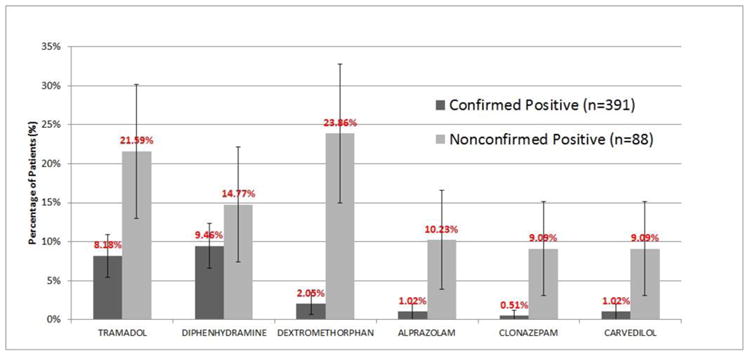
Between January 1st 2007 and December 31st 2011, 33,972 urine drug screens were performed by the laboratory at Barnes-Jewish Hospital on patients presenting to the ED. Out of 33,972 urine screens, 491 (1.4%) screens were positive for PCP. Of those 491, 391 (79.6%) were confirmed positive for PCP by GC-MS, 88 (17.9%) failed to confirm, and 12 (2.4%) were excluded because of inadequate urine volume.
Among the 391 confirmed positives, patient charts reflected documented use of tramadol in 32 patients (8.2%, 95% CI: 5.5%-10.9%), diphenhydramine in 37 patients (9.5 %, 95% CI: 6.6%-12.4%), and dextromethorphan in 8 patients (2.0%, 95% CI: 0.6%-3.4%). Among the 88 who failed to confirm, records reflected documented use of tramadol in 19 patients (21.6%, 95% CI: 13.0%-30.2%), diphenhydramine in 13 patients (14.8%, 95% CI: 7.4%-22.2%), and dextromethorphan in 21 patients (23.9%, 95% CI: 15.0%-32.8%).
Alprazolam, clonazepam, and carvedilol unexpectedly were also significantly overrepresented among patients with PCP screens which failed confirmation. Of the 391 confirmed positives, four patients had documented use of alprazolam (1.0%, 95% CI: 0.0%-2.0%), two patients with clonazepam use (0.5%, 95% CI: -0.2%-1.2%), and four patients on carvedilol (1.0%, 95% CI: 0.0%-2.0%). The 88 patients with PCP screens that failed to confirm included nine patients with recorded use of alprazolam (10.2%, 95% CI: 3.9%-16.6%), eight patients with clonazepam (9.1%, 95% CI: 3.1%-15.1%), and eight patients with carvedilol (9.1%, 95% CI: 3.1%-15.1%).
Discussion
All three of the medications suggested by previous case reports had higher proportions in the non-confirmed group than among the confirmed positive group. However, these differences were significantly different for tramadol and dextromethorphan but not for diphenhydramine, which showed a non-significant trend for positive PCP screens which failed confirmation by GC-MS.
Unexpected drugs with significant disproportion of false positive PCP screens included alprazolam, clonazepam, and carvedilol. This finding may be due to the fact that these medications share structural similarity with PCP, each with two six-member rings that are one atom apart (Figure 4).
Figure 4.
Structures of PCP, alprazolam, clonazepam, and carvedilol. Adapted from Wikipedia (http://en.wikipedia.org/wiki/Alprazolam, http://en.wikipedia.org/wiki/Clonazepam, http://en.wikipedia.org/wiki/Carvedilol)
As with most retrospective studies, a limitation of our study is the assumption that the information recorded in patient's chart was correct, complete, and accurate. Also, this was a single-center study, of which the results might not be generalizable to other patient populations.
The investigator who abstracted data from the charts was not blinded to the purpose of the study. However, the data elements were recorded by ED nurses, physicians, and/or hospital laboratory technicians at the time of the ED encounters, when the study was not yet conceived. Identifying the presence or absence of the data elements in a given chart was objective, so we believe that unblinded data abstraction did not appreciably bias the results.
Another potential limitation is that we did not stratify our sample based upon renal or hepatic function. We did not record serum concentrations of creatinine or hepatic enzymes because most patients were missing one or both. We believe that any hepatic or renal abnormalities would be equally distributed among both of our study groups.
Conclusion
Tramadol, dextromethorphan, alprazolam, clonazepam, and carvedilol were significantly associated with false positive urine screens for phencyclidine. Diphenhydramine showed a non-significant trend and was among the most frequently identified medications among patients with false positive screens for phencyclidine.
Figure 2. Sample sizes of study population.
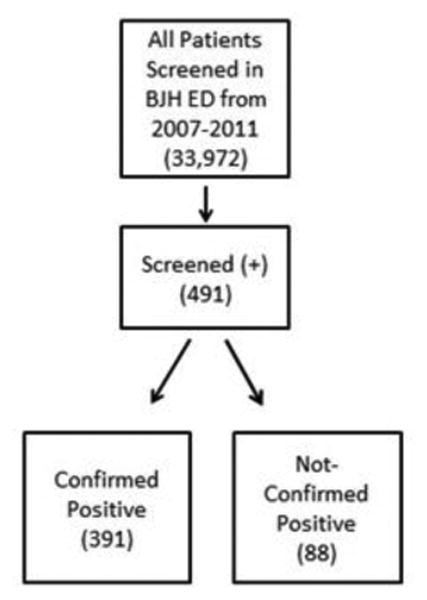
Figure 3. Distribution of commonly documented medications.
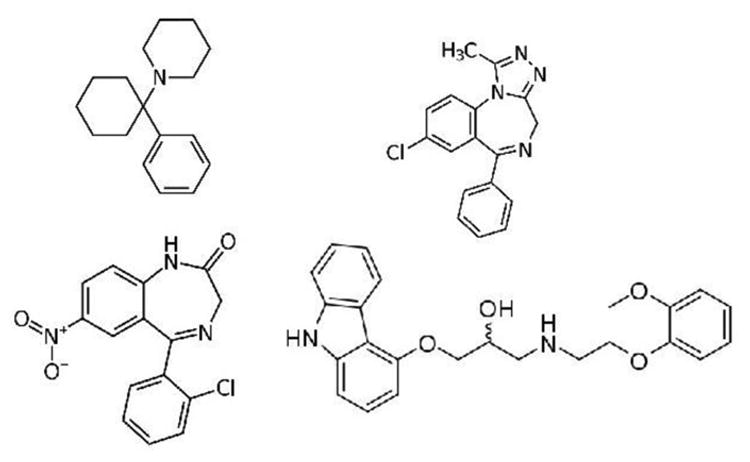
Table 1. Demographics of patients with confirmed and non-confirmed positive PCP screens.
| Confirmed Positive(n=391) | Non-confirmed Positive(n=88) | |
|---|---|---|
| Gender | ||
| Male | 82.9% (324) | 70.5% (62) |
| Female | 17.1% (67) | 29.5% (26) |
| Age (SD) | 38.0 (9.5) | 36.6 (13.7) |
Acknowledgments
This work was supported by NIH Clinical and Translational Science Award program through the National Center for Advancing Translational Sciences under award numbers UL1 TR000448 and TL1 TR000449.
Footnotes
This work has previously been presented at the SAEM Great Plains Regional Research Forum on September 29, 2012 and the Graduate Research Symposium at Washington University in St. Louis on February 16, 2013.
The authors have no conflicts to disclose.
References
- 1.SAMHSA. Rockville, MD: Center for Behavioral Health Statistics and Quality; 2011. [last accessed date 2/19/2013]. Drug Abuse Warning Network, 2010: Selected Tables of National Estimates of Drug-Related Emergency Department Visits. Available at: http://www.samhsa.gov/data/DAWN.aspx. [Google Scholar]
- 2.Hull MJ, Griggs D, Knoepp SM, Smogorzewska A, Nixon A, Flood JG. Postmortem urine immunoassay showing false-positive phencyclidine reactivity in a case of fatal tramadol overdose. Am J Forensic Med Pathol. 2006;27:359–362. doi: 10.1097/01.paf.0000233534.59330.c2. [DOI] [PubMed] [Google Scholar]
- 3.Ly BT, Thornton SL, Buono C, Stone JA, Wu AHB. False-positive urine phencyclidine immunoassay screen result caused by interference by tramadol and its metabolites. Ann Emerg Med. 2012;59:545–547. doi: 10.1016/j.annemergmed.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 4.LeLievre B, Bretaudeau M, Urvois A, Boels D, Turcant A. Immunochromatographic tests: false-positive results for methadone and phencyclidine after acute poisoning with tramadol and dextropropoxyphene. Acta Clinica Belgica. 2010;65:12–17. [Google Scholar]
- 5.Schier J, Diaz JE. Avoid unfavorable consequences: dextromethorpan can bring about a false-positive phencyclidine urine drug screen. J Emerg Med. 2000;18:379–381. doi: 10.1016/s0736-4679(99)00234-6. [DOI] [PubMed] [Google Scholar]
- 6.Budai B, Iskandar H. Dextromethorphan can produce false positive phencyclidine testing with HPLC. Am J Emerg Med. 2002;20:61–62. doi: 10.1053/ajem.2002.29556. [DOI] [PubMed] [Google Scholar]
- 7.Marchei E, Pellegrini M, Pichini S, Martín I, García-Algar O, Vall O. Are false-positive phencyclidine immunoassay instant-view multi-test results caused by overdose concentrations of ibuprofen, metamizol, and dextromethorphan? Ther Drug Monitor. 2007;29:671–673. doi: 10.1097/FTD.0b013e318156e983. [DOI] [PubMed] [Google Scholar]
- 8.Levine BS, Smith ML. Effects of diphenhydramine on immunoassay of phencyclidine in the urine. Clin Chem. 1990;36:1258. [PubMed] [Google Scholar]
- 9.Brahm NC, Yeager LL, Fox MD, Farmer KC, Palmer TA. Commonly prescribed medications and potential false-positives urine drug screens. Am J Hosp Pharm. 2010;67:1344–1350. doi: 10.2146/ajhp090477. [DOI] [PubMed] [Google Scholar]
- 10.Casey ER, Scott MG, Tang S, Mullins ME. Incidence of false positive amphetamine results due to bupropion in the Syva Emit II urine drug screens. J Med Toxicol. 2011 doi: 10.1007/s13181-010-0131-5. doi:10.1007/s13181-010- 0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


