Abstract
Cancer immunoediting, the process by which the immune system controls tumor growth and shapes tumor immunogenicity, consists of three stages, elimination, equilibrium and escape. The molecular mechanisms that underlie the equilibrium phase, during which the immune system maintains tumor dormancy, remain incompletely defined. Here, we investigated the length of the equilibrium phase during immune control of methycholanthrene (MCA)-induced or p53 mutant cancers and demonstrated the critical and opposing roles of IL-23 and IL-12 in maintaining cancer cells in a state of immune-mediated dormancy. Inhibition of IL-23p19 was shown to reduce the malignant potential of lesions established by MCA inoculation, while inhibition of IL-12/23p40 enhanced tumor outgrowth. Furthermore, agonistic anti-CD40 antibody treatment mimicked the effects of anti-IL-23p19 mAb treatment. Other cytokines such as IL-4, IL-17, TNF, and IFNαβ, which are known to play important roles either in MCA tumorigenesis or in the elimination phase of cancer immunoediting, did not play critical roles in maintaining the equilibrium phase. Taken together, our findings demonstrate opposing roles for IL-23 and IL-12 in determining the outgrowth versus dormancy of occult neoplasia and suggest a potential long-term danger in using IL-12/23p40 antibodies for treating human autoimmune inflammatory disorders.
Keywords: IL-23, IL-12, T cell, cancer, surveillance
Introduction
Cancer immunoediting, the process whereby the immune system controls tumor outgrowth and shapes tumor immunogenicity, is comprised of three phases: elimination, equilibrium and escape (reviewed in (1, 2)). The elimination phase involves the immune detection and destruction of tumor cells that have developed as a result of a failure of intrinsic tumor suppressor mechanisms. Elimination is a modernized view of the older concept of cancer immunosurveillance that now takes into account that efficient tumor destruction requires the coordinated effects of both innate and adaptive immunity. Sometimes not all tumor cells are eliminated and the residual tumor cells enter the equilibrium phase where the immune system and developing tumor enter into a temporary state of balance that controls tumor outgrowth. During equilibrium, tumor cells undergo editing as a consequence of genetic and epigenetic changes (such as DNA mutations or changes in gene expression) in the tumor cell population and a selective pressure exerted by adaptive immunity. While this pressure is sufficient to control net tumor progression, eventually, if the immune response fails to completely eliminate the tumor, the process results in the selection of tumor cell variants that are able to resist, avoid, or suppress the anti-tumor immune response, leading to the escape phase and the outgrowth of clinically apparent cancers (3).
Although many immune components that participate in the equilibrium phase are known, the underlying mechanisms remain poorly defined. The presence of an equilibrium phase was suspected from earlier studies with experimental tumors (4–6), but only recently was experimentally demonstrated using de novo carcinogen-induced tumors in mice (7) In that report we demonstrated the presence of equilibrium lesions following a low-dose regimen of the chemical carcinogen, methylcholanthrene (MCA), and showed that dormant cancer cells were present in these lesions together with actively proliferating immune infiltrates. Using depleting or blocking monoclonal antibodies, we further demonstrated that equilibrium was maintained by components of adaptive immunity, namely CD4+ and CD8+ T cells, IFN-γ and IL-12p40 (7). In contrast, depletion of NK cells, blockade of NKG2D, or inhibition of TRAIL effector function failed to cause the emergence of progressively growing tumors (7). Since this initial demonstration of the existence of an equilibrium phase in 2007, two additional studies in mice demonstrated that immunity controlled neoplastic disease for extended periods of time (8, 9). However the questions remain whether additional immune components play a critical role in maintaining the equilibrium phase and whether there is a distinct function for IL-12 and IL-23, since these cytokines share the IL-12p40 subunit that was blocked in the previous report.
To address these questions we have since sought to 1) determine the length of time that immunity can manifest the equilibrium phase, 2) determine the relative roles of two cytokines that contain the IL-12p40 subunit i.e., IL-23 and IL-12 in maintaining equilibrium and 3) assess the role of other cytokines and cell types known to regulate T cell polarity in immune responses. The heterodimeric cytokine IL-23, that consists of the p40 and p19 subunits (as opposed to p40 and p35 forming IL-12) has emerged as a new player in promoting tumor growth and development (10, 11). Importantly, anti-IL-12/23p40 and anti-IL-23p19 antibodies are in early phase clinical trials in patients with psoriasis and inflammatory bowel syndromes (IBD)(12, 13). The potential oncogenicity of each of these therapeutic approaches is of significant clinical importance given the chronicity of disease and elevated risk of malignancies in these patient groups (14, 15). Here we show that the equilibrium phase can occur over extended periods of time and that both IL-23 and IL-12 maintain malignant cells in equilibrium with host immunity, but that IL-23 promotes cancer persistence while IL-12 prevents cancer outgrowth.
Materials and Methods
Mice
Inbred C57BL/6 wild type (WT) mice were bred and maintained at the Peter MacCallum Cancer Centre (Peter Mac) as described previously (16). Mice 6–14 weeks old at the time of study initiation were used in all experiments. All experiments were performed in accordance with guidelines set out by the Peter Mac Animal Experimental Ethics Committee.
Tumor Models
Male WT mice were injected subcutaneously with low doses of MCA (Sigma Fine Chemicals) as described (7) and monitored for tumor development. At various time points after inoculation, MCA-treated mice bearing progressively growing primary fibrosarcomas were removed from the experiment (~10–20% of group)(1st stage). The remaining mice in each group were then treated weekly with either cIg or mAbs that deplete or block specific immune components for 3–8 weeks (2nd stage). In most experiments (Fig. 2–6, Table 1), after a 1–3 week break after the last immune intervention treatment, mice then received either cIg or anti-IFN-γ or a cocktail of anti-CD4/CD8/IFN-γ for a further 6 weeks (3rd stage). The timing and dose for each experiment are indicated in the figure legends. Mice were monitored for tumor development throughout for up to 600 days. Tumor size (cm2) for each individual mouse was recorded as described previously (7).
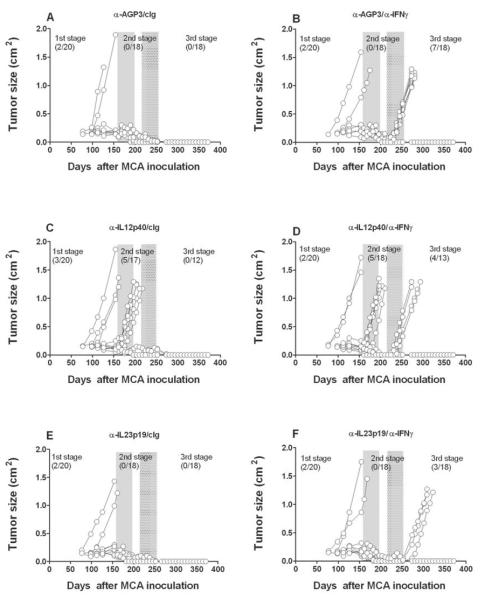
Fig. 2. Opposing roles of IL-23 and IL-12 maintain tumors in equilibrium.
Groups of 20 male C57BL/6 WT mice were inoculated subcutaneously with 10 μg MCA. At day 161 the tumor-free mice were injected weekly for 6 weeks (until day 196) with (A, B) cIg (anti-AGP3), (C, D) anti-p40, or (E, F) anti-IL-23p19 (500 μg i.p.). Mice then received cIg (A, C, E) or anti-IFN-γ weekly (B, D, F) for 6 weeks (250 μg i.p.) from day 217 to day 252. Mice were monitored for the appearance of late-forming sarcomas until day 400 after MCA inoculation. The proportion of tumors arising prior and post each mAb treatment are indicated. Tumor size (cm2) for each individual mouse is plotted.
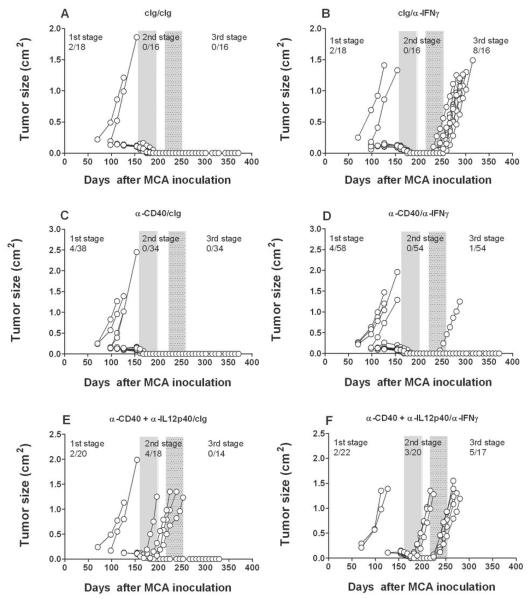
Fig. 6. Anti-CD40 mAb treatment resolves equilibrium lesions.
Groups of 18–38 male C57BL/6 WT mice were inoculated subcutaneously with 10 μg MCA. At day 161 the tumor-free mice were injected weekly for 6 weeks (until day 196) with (A, B) cIg (PIP2, 100 μg i.p.), (C, D) anti-CD40 (100 μg i.p.), or (E, F) anti-CD40 (100 μg i.p.) plus anti-IL-12p40 (500 μg i.p.). Mice then received cIg (A, C, E) or anti-IFN-γ weekly (B, D, F) (250 μg i.p.) for 6 weeks from day 217 to day 252. Mice were monitored for the appearance of late-forming sarcomas until day 400 after MCA inoculation. The proportion of tumors arising prior and post each mAb treatment are indicated. Tumor size (cm2) for each individual mouse is plotted.
Table 1.
IL-23 maintains malignant potential of tumors in equilibrium.
| α-AGP3/α-IFNγ | α-IL23p19/α-IFNγ | ||
| Outgrowth | 16 | 5* | |
| Tumor free | 18 | 32 | |
| α-AGP3/cIg | α-IL23p19/cIg | ||
| Outgrowth | 0 | 0 | |
| Tumor free | 34 | 36 | |
| α-AGP3/α-CD4/-CD8/-IFNγ | α-IL23p19/α-CD4/-CD8/-IFNγ | ||
| Outgrowth | 22 | 11** | |
| Tumor free | 20 | 34 | |
| α-AGP3/cIg | α-IL23p19/cIg | ||
| Outgrowth | 2 | 3 | |
| Tumor free | 41 | 39 | |
| α-AGP3/α-IFNγ | α-IL10R/α-IFNγ | α-IL23p19/-IL-10R/α-IFNγ | |
| Outgrowth | 8 | 10*** | 0**** |
| Tumor free | 8 | 26 | 18 |
| α-AGP3/cIg | α-IL10R/cIg | α-IL23p19/-IL-10R/cIg | |
| Outgrowth | 1 | 0 | 0 |
| Tumor free | 15 | 35 | 18 |
Composite of data from Fig. 2, Fig. 3, and Supplementary Fig. S4, demonstrating the number of mice with tumor outgrowth versus those remaining tumor free following intervention with cIg, anti-IFN-γ or anti-CD4/CD8/IFN-γ cocktail (stage 3). Statistical analysis was performed by Fishers Exact test, compared against equivalent α-AGP3 group,
p = 0.0036
p = 0.0087
p = 0.2056
p = 0.0007
MAbs
The majority of the mAbs used for neutralization or depletion have been previously described (7). These include control Igs (PIP, a mAb specific for bacterial glutathione S-transferase), anti-CD8α (YTS169.4), anti-CD4 (GK1.5), and anti-IFN-γ (H22). These were produced from hybridoma supernatants and purified in endotoxin-free form by Protein G affinity chromatography (Leinco Technologies, St. Louis, MO). Anti-AGP3 (control Ig (cIg), 4D2), anti-IL-23p19 (16E5), and anti-IL-12/23p40 (C17.8) were provided by AMGEN and have been described previously (11). Anti-IL-10R (1B1.3) was produced in house and used as indicated. Anti-IL-17RA (M751) was also grafted on the same murine IgG1 background and kindly provided by AMGEN Inc. Anti-IL-10R (1B1.3), anti-IFNAR1 (MAR1-5A3), anti-IL-4 (11B11), and anti-TNF (TN3 19.12) were produced in house and used as indicated.
Statistical Analysis
Significant differences in proportions of mice with tumor at each stage were determined by the Fishers Exact test. Values of p < 0.05 were considered significant.
Results
The equilibrium phase is a protracted process
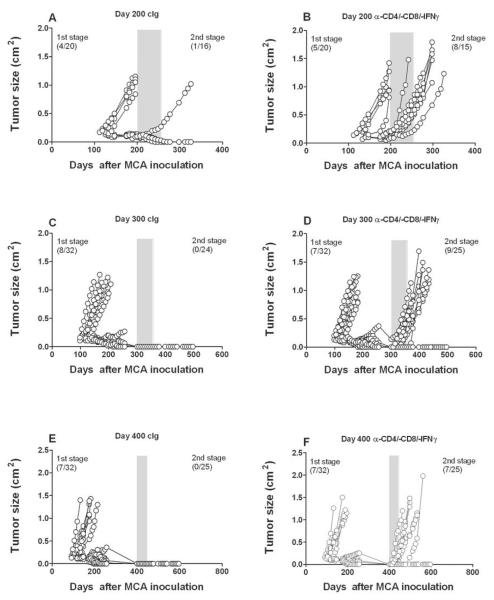
We previously described the presence of occult neoplastic lesions created by subcutaneous inoculation of low doses of the carcinogen, MCA (5–25 μg)(7). Primary fibrosarcomas were shown to typically arise between 10 and 20 weeks post carcinogen inoculation, while the occult equilibrium lesions were dormant for up to 200 days before T cell depletion and IFN-γ neutralization allowed escape from immune control (7). In the current study, we similarly found a low proportion of primary fibrosarcomas (4/20) and either tumor free mice or those with stable masses (<0.5 cm2) that developed and disappeared over time (Fig. 1A). In addition, approximately 56% (8/15) of mice treated with a cocktail of anti-CD4/anti-CD8 and anti-IFN-γ for 8 weeks from day 200 after MCA inoculation developed fibrosarcomas, compared with only 1/16 treated with cIg over the same period (Fig. 1A and B). Similar results were found in female mice (Supplementary Fig. S1), confirming that equilibrium displays host sex independence. To investigate the latency period of equilibrium, we delayed conditional immune depletion for 300 (Fig. 1C and D) and 400 (Fig. 1E and F) days. Notably, immune depletion caused the outgrowth of fibrosarcomas (9/25 and 7/25, respectively), suggesting that significant numbers of mice still harbored occult malignant fibroblasts up to 400 days after MCA inoculation and long after stable lesions had macroscopically disappeared. These data revealed that immune-mediated dormancy of primary MCA sarcomas is a protracted process and that few equilibrium lesions spontaneously resolved (ie. elimination) or escaped immune control over time.
Fig. 1. Occult tumors persist in equilibrium.
Groups of 20–32 male C57BL/6 WT mice were inoculated subcutaneously with 25 μg MCA. At (A, B) 200 days, (C, D) 300 days, or (E, F) 400 days the tumor-free mice were injected weekly for 8 weeks with (A, C, E) cIg (PIP2, 300 μg i.p.) or (B, D, F) a cocktail of anti-CD4/-CD8/-IFN-γ (100 μg i.p. each). Mice were monitored for the appearance of late-forming sarcomas for 200 days after the commencement of antibody treatment. The proportion of tumors arising prior and post mAb treatment are indicated. Tumor size (cm2) for each individual mouse is plotted.
To extend our findings to a spontaneous tumor model, we aged large cohorts of male and female C57BL/6 p53+/− mice for 750 days and monitored tumor development. We have previously shown that 45–60% of p53+/− mice develop tumors (predominantly sarcomas and lymphomas) by day 750(17) and we observed similar findings in the current study (47.5–56.3% cohort, males mean age of death 616 ± 16 days, females 534 ± 17 days) (Supplementary Fig. S2A)(18). Mice that were macroscopically tumor free at 750 days were then injected weekly for 8 weeks (days 750–799) with cIg or a cocktail of anti-CD4/-CD8/-IFN-γ antibodies. Over the next 200 days, a similar number of mice died in each group (Supplementary Fig. S2B), but the cohort of mice receiving a cocktail of anti-CD4/-CD8/-IFN-γ had a significantly higher proportion (6/25) of tumors develop (mostly low grade and large cell lymphoma) compared to the cohort of mice receiving cIg (1/26) (p = 0.0496) (Supplementary Fig. S2C). Although it is more difficult to define the duration of tumor dormancy in this tumor model, judging from earlier studies where the impact on host immunity (eg. perforin, TRAIL, IFN, NKT cells) has been observed on tumor incidence over the first 300–500 days of life of these mice (17–20), these data support the concept that host adaptive immunity controls outgrowth of tumors, even late in life, in tumor prone p53+/− mice.
Anti-IL-23p19 reduces malignant potential of MCA-induced stable masses
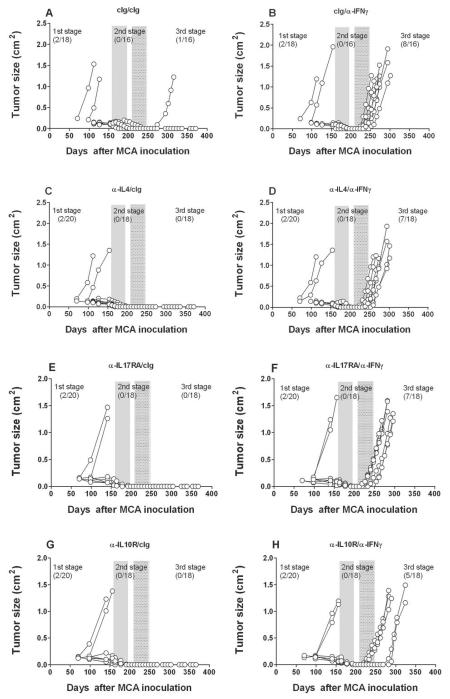
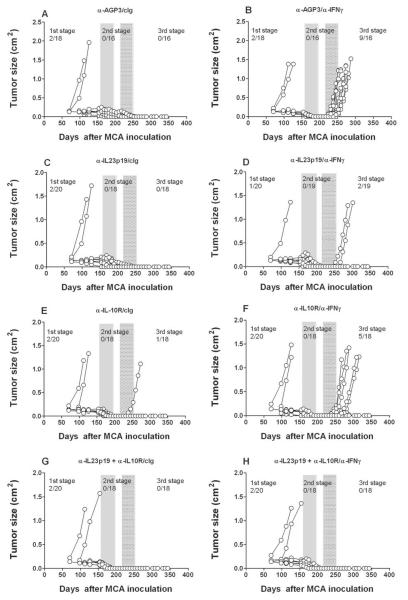
Given that IL-12p40 mAb blockade results in the outgrowth of stable masses in MCA-treated C57BL/6 wild-type mice (7), it was possible that either IL-23 or IL-12 might be responsible for controlling the outgrowth of equilibrium lesions. In the absence of a method to conditionally and specifically delete IL-12p70 heterodimer, we used an IL-23-specific anti-IL-23p19 mAb to assess the comparative effect of neutralizing IL-23 versus IL-12/23p40 in mice harboring stable masses that had been induced by MCA. As in earlier experiments, mice that developed a progressively growing primary tumor up to 200 days were excluded. We then treated mice lacking clinically apparent tumors for 6 weeks with either anti-IL-23p19, anti-IL-12/23p40 mAbs or control Ig (anti-AGP3), ceased treatment for 3 weeks and then treated the cohorts with either cIg or anti-IFN-γ for an additional 6 weeks (Fig. 2). Specifically, fewer tumors developed and tumor appearance displayed delayed kinetics in mice pre-treated with anti-IL-23p19 (3/18) (Fig. 2F) than with cIg (7/18) (Fig. 2B) after IFN-γ neutralization, suggesting that IL-23 promotes cancer persistence and tumor maintenance during the equilibrium phase and that blockade of IL-23 with anti-IL-23p19 is host-protective. In contrast to cIg (0/18) or anti-IL-23p19 (0/18) pre-treatment, mice receiving anti-IL-12/23p40 developed tumors prior to secondary cIg (5/17) or anti-IFN-γ (5/18) treatment, confirming the role of IL-12 in preventing the outgrowth of occult primary cancer cells. Additional tumors grew out in mice initially treated with anti-IL-12p40 and subsequently treated with anti-IFN-γ (4/13). These data were supported by two other similarly constructed experiments (results of all three pooled and summarized in Table 1), where we observed that treatment with anti-IL-23p19 prevented the outgrowth of MCA-tumors from mice subsequently treated with anti-IFN-γ. Overall, regardless of whether anti-IFN-γ (p = 0.0036) or anti-CD4/CD8/IFN-γ (p = 0.0087) treatment was used to permit tumor growth and escape from the equilibrium phase, IL-23 blockade had a statistically profound effect in protecting the host compared with cIg (Table 1). Furthermore across several experiments where tumors arose post immune depletion, anti-IL-23p19 delayed the outgrowth compared to cIg (Supplementary Fig. S3)(p < 0.05). Thus, IL-23 promotes cancer persistence during the equilibrium phase, a result that enhances our knowledge of the previously demonstrated role of IL-23 as a potent cancer-promoter, enabling the initiation of MCA-induced sarcomas (11).
Other cytokines that activate and polarize T cells do not appear to regulate equilibrium
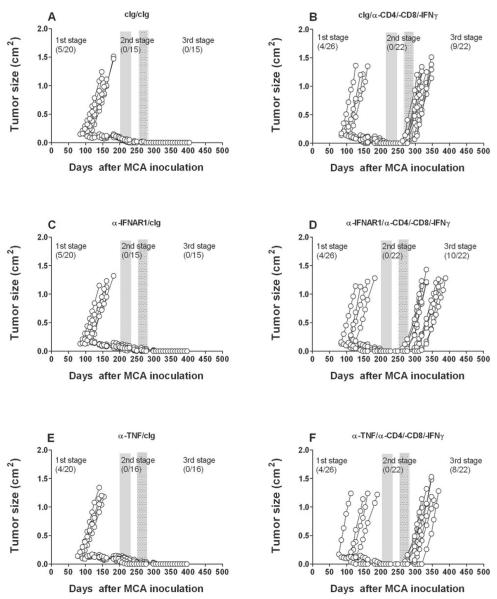
To further explore the selectivity of IL-12/IL-23 and IFNγ as critical components of the equilibrium phase, we used a similar loss-of-function approach to assess potential roles for other immunomodulatory/pro-inflammatory cytokines in the process. Mice deficient in tumor necrosis factor alpha (TNF) and IFNAR1 have been demonstrated to have an elevated incidence of MCA-induced sarcomas (21) (22), indicating that these two cytokines are critical players in cancer immunosurveillance. However, the role of TNF in tumor dormancy remains controversial due to contradictory findings in studies of tumor dormancy (23, 24). Here, equilibrium was not altered in different cohorts of MCA treated mice given neutralizing/blocking mAbs to either TNFα or the type I IFN receptor (Fig. 3). Similarly, other key cytokines that define T cell polarity, IL-4 and IL-17RA, also did not critically regulate the equilibrium phase (Fig. 4). Interestingly however, anti-IL-10R treatment produced slightly fewer tumors and delayed tumor emergence from equilibrium when compared to mice treated with cIg (Fig. 4 and Supplementary Fig. S3). This effect was more evident when anti-IL-10R and anti-IL-23p19 were used in combination (0/18 tumors emerged from equilibrium after IFN-γ neutralization, versus 5/18 anti-IL-10R and 2/19 anti-IL-23p19) and suggests that combinatorial anti-IL-10R/IL-23p19 therapy eliminates most, if not all, persistent cancer cells present in occult tumor masses (Fig. 5) (Table 1). Nevertheless, these studies point to the very special roles of IFNγ, IL-12 and IL-23 in induction/maintenance of the equilibrium phase.
Fig. 3. TNF and IFNAR1 do not regulate tumors in equilibrium.
Groups of 20–26 male C57BL/6 WT mice were inoculated subcutaneously with 5 μg MCA. At day 200 the tumor-free mice were injected weekly for 6 weeks (until day 235) with (A, B) cIg, (C, D) anti-IFNAR1, or (E, F) anti-TNF (300 μg i.p.). Mice then received cIg (A, C, E) (300 μg i.p.) or anti-CD4/-CD8/-IFN-γ weekly (B, D, F)(100 μg i.p. each) for 5 weeks from day 256 to day 284. Mice were monitored for the appearance of late-forming sarcomas until day 400 after MCA inoculation. The proportion of tumors arising prior and post each mAb treatment are indicated. Tumor size (cm2) for each individual mouse is plotted.
Fig. 4. IL-10, but not IL-4 or IL-17, regulates tumors in equilibrium.
Groups of 18–20 male C57BL/6 WT mice were inoculated subcutaneously with 10 μg MCA. At day 161–163 the tumor-free mice were injected weekly for 6 weeks (until day 196–198) with (A, B) cIg, (C, D) anti-IL-4, (E, F) anti-IL-17RA, or (G, H) anti-IL-10R (300 μg i.p.). Mice then received cIg (A, C, E, G) (250 μg i.p.) or anti-IFN-γ weekly (B, D, F, H)(250 μg i.p.) for 6 weeks from day 212–217 to day 247–252. Mice were monitored for the appearance of late-forming sarcomas until day 400 after MCA inoculation. The proportion of tumors arising prior and post each mAb treatment are indicated. Tumor size (cm2) for each individual mouse is plotted.
Fig. 5. IL-23 and IL-10R blockade collectively resolve equilibrium lesions.
Groups of 18–20 male C57BL/6 WT mice were inoculated subcutaneously with 10 μg MCA. At day 161 the tumor-free mice were injected weekly for 6 weeks (until day 196) with (A, B) cIg (anti-AGP3), (C, D) anti-IL-23p19, (E, F) anti-IL-10R or (G, H) anti-p19 and anti-IL-10R (500 μg i.p. each). Mice then received cIg (A, C, E, G) or anti-IFN-γ (B, D, F, H) weekly (250 μg i.p.) for 6 weeks from day 217 to day 252. Mice were monitored for the appearance of late-forming sarcomas until day 400 after MCA inoculation. The proportion of tumors arising prior and post each mAb treatment are indicated. Tumor size (cm2) for each individual mouse is plotted.
Triggering CD40 mimics IL-23 neutralization
Since IL-12 is reported to maintain MCA sarcomas in equilibrium to a similar degree as T cells (Fig. 2)(7), we next sought to determine whether agonist anti-CD40, a powerful stimulator of IL-12p70 production from antigen presenting cells could eliminate persistent cancer cells in equilibrium lesions. Mice harboring equilibrium tumors that were treated with anti-CD40 for 6 weeks developed fewer (1/54) tumors after anti-IFN-γ treatment than the equivalent group treated with cIg (8/16) (Fig. 6), demonstrating a protective effect for anti-CD40 mAb. The reduction in tumor outgrowth was similar or better than that obtained using anti-IL-23p19 (Fig. 2, 5 and Table 1). These data suggest that either blockade of IL-23p19 or a significant induction of IL-12p70 from CD40 stimulation could resolve dormant fibrosarcomas in the equilibrium phase. The role of IL-12p40, and most logically IL-12p70 in the protective effect of anti-CD40, was supported by the impact of additionally neutralizing IL-12p40 plus anti-CD40 in the second stage where now 3/20 tumors arose, and then a further 5/17 tumors in the third stage following anti-IFN-γ treatment.
Discussion
This study significantly expands our rudimentary understanding of the mechanisms controlling the immune-mediated dormancy of the equilibrium phase. First, we have demonstrated that the equilibrium phase is a protracted process resulting in long latency from carcinogen inoculation to tumor outgrowth/escape that may extend for the entire lifetime of the host (up to 850 days in mice). Second, we have determined, for the first time, that IL-23 plays a critical role in opposing the anti-tumor effects of IL-12 and thus, allows cancer cells to persist in a state of immune-mediated dormancy and prevent cancer cell elimination. Thirdly, we extend and corroborate our previous findings that adaptive immunity maintains occult cancer in an equilibrium state and that the elimination phase and the equilibrium phase are temporally and mechanistically distinct events. Specifically, cytokines known to regulate tumor immunity such as IL-4, IL-17, TNF and IFNαβ do not appear to play a critical role in controlling the equilibrium phase. This is despite the fact that many of these cytokines are critical for the initiation or elimination of MCA-induced sarcomas. Collectively, these data support a model of tumor development and immune system interactions occurring in three distinct phases as we have previously described (1, 2), but suggest that specific cytokines such as IL-12 and IL-23 play roles during the earlier phase of tumor initiation and elimination as well determining the dormancy or outgrowth of occult neoplasia during the equilibrium phase.
We (11) and others (10, 25) have recently demonstrated the effect of IL-23 in suppressing both innate and adaptive anti-tumor effector responses, and we speculate that local tumor infiltrating myeloid cell production of IL-23 and IL-10 quite possibly directly or indirectly suppresses these anti-tumor activities. It will be important to determine which leukocytes potentially produce these cytokines and demonstrably regulate the process. In our preliminary studies, since CD11b macrophages produce IL-23 and IL-10 (25) (26), we administered either vehicle control liposomes or the macrophage depleting agent clodrolip (27) prior to treating the mice with cIg or anti-IFN-γ (Supplementary Fig. S4). Depletion of macrophages abrogated most fibrosarcoma outgrowth (4/36) when compared with cIg pre-treatment (7/16 tumors), suggesting that macrophages were promoting the survival of dormant fibrosarcomas during the equilibrium phase. In contrast, depletion of CD11chi cells (including most DC) using diptheria toxin and CD11c DOG transgenic mice, or Ly-6G+ cells (neutrophils) in wild-type mice did not alter equilibrium tumor outgrowth after anti-IFN-γ treatment (Supplementary Fig. S5). However, while it is true that these depleting antibodies, liposomes and transgenic mice are the best available tools to eliminate these leukocyte subsets, we realize that these approaches have limited specificity and that deleting these cells may have immune modulating effects that interfere with the establishment of the equilibrium phase. Future studies that specifically delete DCs and other myeloid and granulocyte subsets will be required to elucidate the precise roles these subsets have in generating and maintaining occult tumor cells in equilibrium with anti-tumor immunity.
Anti-IL-12/23p40 therapies are highly effective in treating psoriasis (28, 29). However, from our studies it is clear there may be a potential risk of compromising cancer surveillance mechanisms through blocking IL-12. More research is needed into the long-term effects of anti-IL-12/23p40 agents in mice, and soon data in humans will be available. Ustekinumab (anti-human IL-12/23p40 antibody, Stelara) is approved in Canada, Europe and the United States to treat moderate to severe plaque psoriasis. Thus far it has proven both effective and safe. Briakinumab (anti-human IL-12/23p40, ABT-874) has also been tested in patients with psoriasis, including higher dosing, but despite the report of highly promising efficacy(13), it has been withdrawn from FDA consideration likely due to safety concerns including an imbalance between briakinumab and placebo in serious infections, major adverse cardiac events and skin cancers (30). IL-12/23p40 antibodies have also shown promise in early clinical trials in Crohn's disease (31) and ustekinumab is currently in clinical trials for Crohn's disease (Clinical trials identifiers: NCT01369342, NCT01369329, NCT01369355). Inflammatory bowel disease (IBD) is associated with increased expression of IL-23 (32) and polymorphisms within the IL-23R gene locus are linked to susceptibility to Crohn's disease (33). People who suffer from ulcerative colitis or Crohn's disease are at an increased risk of developing colon cancer. It is not yet clear whether inhibition of IL-12/23p40 may increase the risk of cancer in this patient population. Anti-IL-23 mAbs are currently in clinical trials for the treatment of psoriasis (Clinical trials identifier - NCT01225731). We should soon be able to evaluate the therapeutic potential of neutralizing IL-23 in patients with IBD, and it will be interesting to monitor these patients long term for malignancies. Given our data herein, and others concerning the role of IL-23 in tumor initiation, anti-IL-23p19 mAb therapy may be seriously considered for use in a tumor preventative setting. Indeed, the ability of anti-CD40 to prevent tumors emerging from equilibrium suggests there may be some merit in preventing tumor outgrowth by using a combination of anti-CD40 and anti-IL-23p19.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Qerime Mundrea and Shellee Brown for maintenance of the mice at the Peter MacCallum Cancer Centre and Bianca von Scheidt for technical assistance. We thank Alison Budelsky (AMGEN) for provision of the anti-IL-17RA mAb.
Grant Support This work was supported in part by a commercial research agreement with AMGEN Incorporated, the National Health and Medical Research Council of Australia (NH&MRC) Program Grant (454569), and The Association for International Cancer Research. MWLT was supported by a NH&MRC CDF1 award. MJS received support from a NH&MRC Australia Fellowship.
Abbreviations
- ASGM1
asialoGM1
- IFN-γ
interferon-gamma
- mAb
monoclonal antibody
- MCA
3′-methylcholanthrene
- WT
wild-type
REFERENCES
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–4. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinhold KJ, Miller DA, Wheelock EF. The tumor dormant state. Comparison of L5178Y cells used to establish dormancy with those that emerge after its termination. J Exp Med. 1979;149:745–57. doi: 10.1084/jem.149.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinhold KJ, Goldstein LT, Wheelock EF. The tumor dormant state. Quantitation of L5178Y cells and host immune responses during the establishment and course of dormancy in syngeneic DBA/2 mice. J Exp Med. 1979;149:732–44. doi: 10.1084/jem.149.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzawa A, Takeda Y, Narita M, Ozawa H. Survival of leukemic cells in a dormant state following cyclophosphamide-induced cure of strongly immunogenic mouse leukemia (DL811) Int J Cancer. 1991;49:303–9. doi: 10.1002/ijc.2910490227. [DOI] [PubMed] [Google Scholar]
- 7.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 8.Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, et al. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med. 2007;204:879–91. doi: 10.1084/jem.20061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030–9. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 11.Teng MW, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Moller A, et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci U S A. 2010;107:8328–33. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon KB, Papp KA, Langley RG, Ho V, Kimball AB, Guzzo C, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): Results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2011 doi: 10.1016/j.jaad.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Reich K, Langley RG, Papp KA, Ortonne JP, Unnebrink K, Kaul M, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. NEngl J Med. 2011;365:1586–96. doi: 10.1056/NEJMoa1010858. [DOI] [PubMed] [Google Scholar]
- 14.Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. photochemotherapy Follow-up Study. Cancer. 1994;73:2759–64. doi: 10.1002/1097-0142(19940601)73:11<2759::aid-cncr2820731118>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Haynes NM, Hawkins ED, Li M, McLaughlin NM, Hammerling GJ, Schwendener R, et al. CD11c+ dendritic cells and B cells contribute to the tumoricidal activity of anti-DR5 antibody therapy in established tumors. J Immunol. 2010;185:532–41. doi: 10.4049/jimmunol.0903624. [DOI] [PubMed] [Google Scholar]
- 17.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113:6382–5. doi: 10.1182/blood-2009-01-198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161–9. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 22.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–18. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Houghton J, Li H, Fan X, Liu Y, Liu JH, Rao VP, et al. Mutations in bone marrow-derived stromal stem cells unmask latent malignancy. Stem Cells Dev. 2010;19:1153–66. doi: 10.1089/scd.2009.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–23. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 27.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody,in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–74. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 29.Croxtall JD. Ustekinumab: a review of its use in the management of moderate to severe plaque psoriasis. Drugs. 2011;71:1733–53. doi: 10.2165/11207530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A Phase III, Randomized, Controlled Trial of the Fully Human IL-12/23 mAb Briakinumab in Moderate-to-Severe Psoriasis. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.304. [DOI] [PubMed] [Google Scholar]
- 31.Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135:1130–41. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–88. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.