Abstract
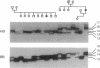
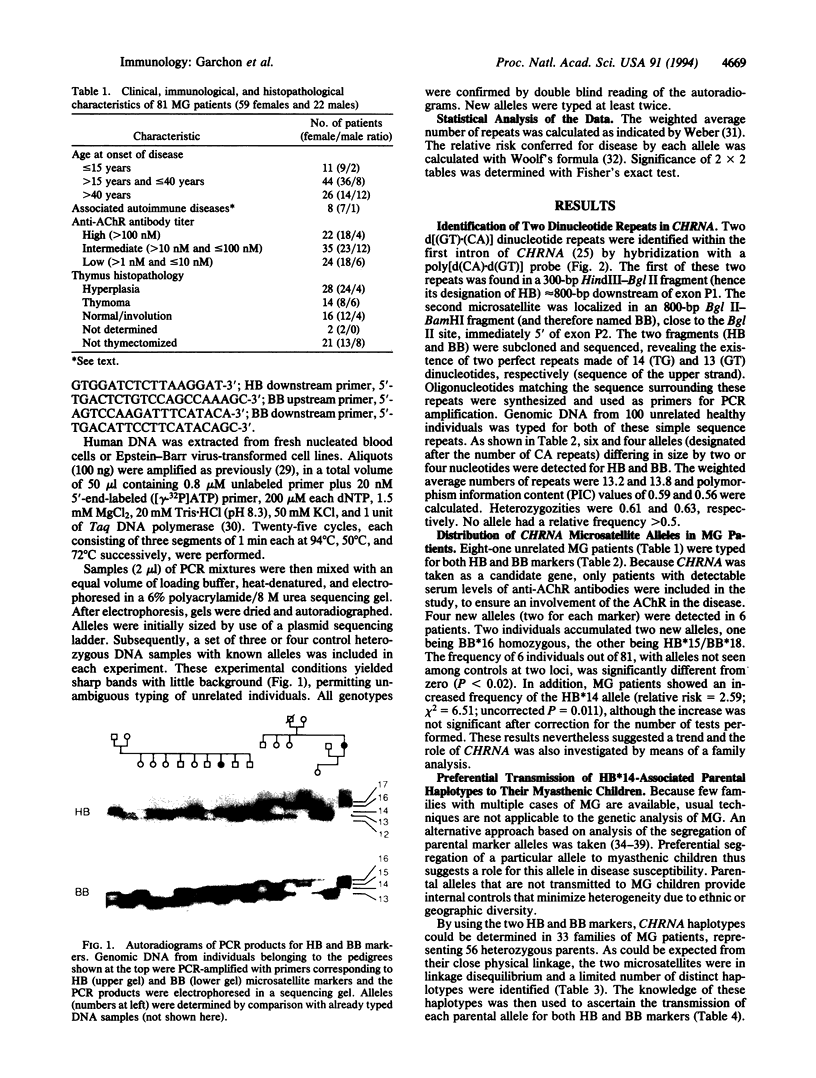
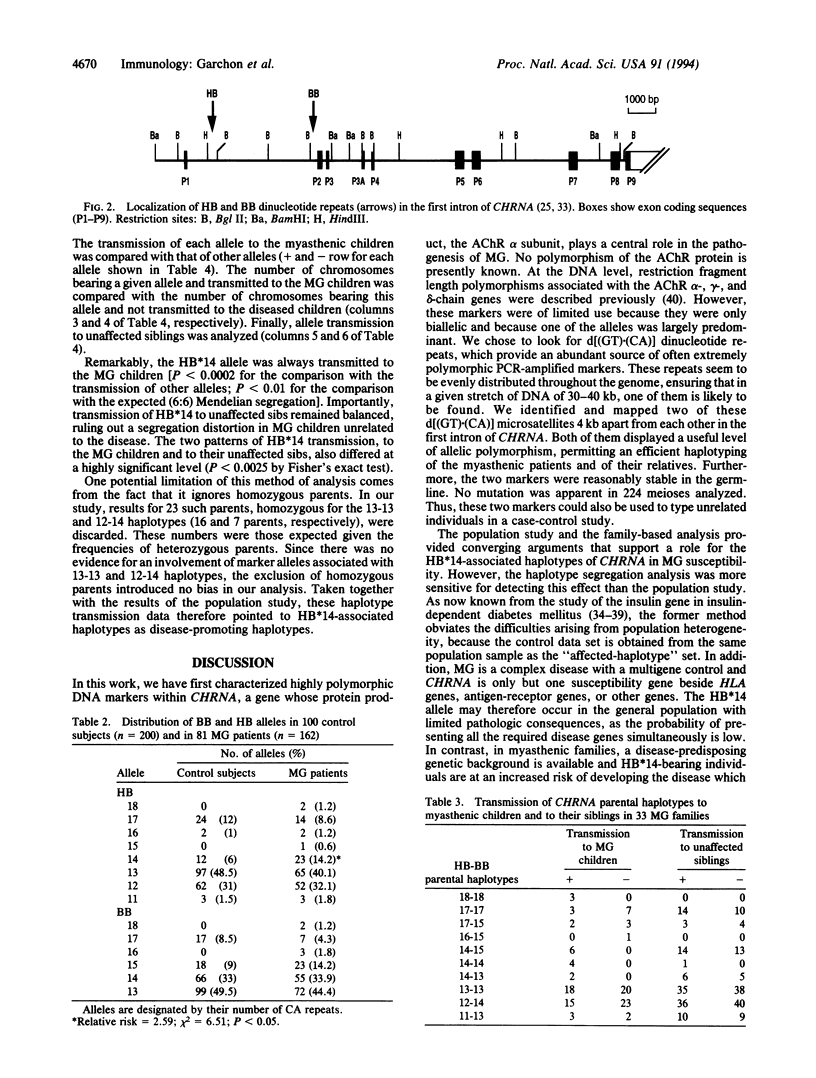
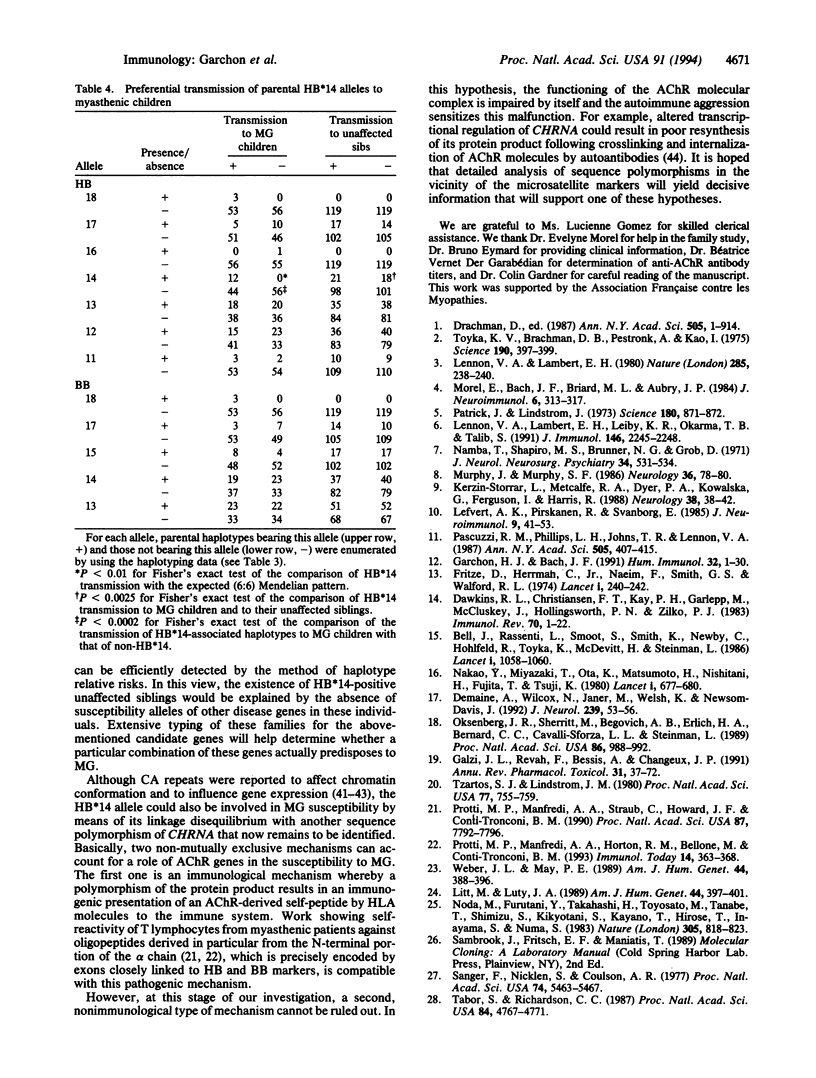
The muscle acetylcholine receptor is the major target of the autoimmune response in generalized myasthenia gravis. To investigate the role of the gene encoding the alpha subunit of the receptor (CHRNA), two stable polymorphic d[(GT).(CA)]dinucleotide repeats, designated HB and BB, were characterized within the first intron of CHRNA. The HB*14 allele conferred a relative risk for myasthenia gravis of 2.5 in 81 unrelated patients compared with 100 control subjects. Very significantly, family analysis based on haplotype segregation data indicated that parental haplotypes associated with HB*14 always segregated to the child with myasthenia gravis (P < 0.0002 for the comparison with the transmission of haplotypes not bearing HB*14), whereas their transmission to unaffected siblings was equilibrated. Myasthenia gravis patients also showed a high frequency of microsatellite variants unseen in controls. These findings implicate the CHRNA in susceptibility to myasthenia gravis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeson D., Morris A., Vincent A., Newsom-Davis J. The human muscle nicotinic acetylcholine receptor alpha-subunit exist as two isoforms: a novel exon. EMBO J. 1990 Jul;9(7):2101–2106. doi: 10.1002/j.1460-2075.1990.tb07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J., Rassenti L., Smoot S., Smith K., Newby C., Hohlfeld R., Toyka K., McDevitt H., Steinman L. HLA-DQ beta-chain polymorphism linked to myasthenia gravis. Lancet. 1986 May 10;1(8489):1058–1060. doi: 10.1016/s0140-6736(86)91330-9. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L., Christiansen F. T., Kay P. H., Garlepp M., McCluskey J., Hollingsworth P. N., Zilko P. J. Disease associations with complotypes, supratypes and haplotypes. Immunol Rev. 1983;70:5–22. doi: 10.1111/j.1600-065x.1983.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Demaine A., Willcox N., Janer M., Welsh K., Newsom-Davis J. Immunoglobulin heavy chain gene associations in myasthenia gravis: new evidence for disease heterogeneity. J Neurol. 1992 Jan;239(1):53–56. doi: 10.1007/BF00839214. [DOI] [PubMed] [Google Scholar]
- Falk C. T., Rubinstein P. Haplotype relative risks: an easy reliable way to construct a proper control sample for risk calculations. Ann Hum Genet. 1987 Jul;51(Pt 3):227–233. doi: 10.1111/j.1469-1809.1987.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Fritze D., Herrman C., Jr, Naeim F., Smith G. S., Walford R. L. HL-A antigens in myasthenia gravis. Lancet. 1974 Feb 16;1(7851):240–242. doi: 10.1016/s0140-6736(74)92548-3. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bessis A., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Garchon H. J., Bach J. F. The contribution of non-MHC genes to susceptibility to autoimmune diseases. Hum Immunol. 1991 Sep;32(1):1–30. doi: 10.1016/0198-8859(91)90113-n. [DOI] [PubMed] [Google Scholar]
- Garchon H. J., Bedossa P., Eloy L., Bach J. F. Identification and mapping to chromosome 1 of a susceptibility locus for periinsulitis in non-obese diabetic mice. Nature. 1991 Sep 19;353(6341):260–262. doi: 10.1038/353260a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzin-Storrar L., Metcalfe R. A., Dyer P. A., Kowalska G., Ferguson I., Harris R. Genetic factors in myasthenia gravis: a family study. Neurology. 1988 Jan;38(1):38–42. doi: 10.1212/wnl.38.1.38. [DOI] [PubMed] [Google Scholar]
- Knapp M., Seuchter S. A., Baur M. P. The haplotype-relative-risk (HRR) method for analysis of association in nuclear families. Am J Hum Genet. 1993 Jun;52(6):1085–1093. [PMC free article] [PubMed] [Google Scholar]
- Lefvert A. K., Pirskanen R., Svanborg E. Anti-idiotypic antibodies, acetylcholine receptor antibodies and disturbed neuromuscular function in healthy relatives to patients with myasthenia gravis. J Neuroimmunol. 1985 Jul;9(1-2):41–53. doi: 10.1016/s0165-5728(85)80005-9. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H., Leiby K. R., Okarma T. B., Talib S. Recombinant human acetylcholine receptor alpha-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol. 1991 Apr 1;146(7):2245–2248. [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H. Myasthenia gravis induced by monoclonal antibodies to acetylcholine receptors. Nature. 1980 May 22;285(5762):238–240. doi: 10.1038/285238a0. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Lobos E. A., Rudnick C. H., Watson M. S., Isenberg K. E. Linkage disequilibrium study of RFLPs detected at the human muscle nicotinic acetylcholine receptor subunit genes. Am J Hum Genet. 1989 Apr;44(4):522–533. [PMC free article] [PubMed] [Google Scholar]
- Loutrari H., Kokla A., Tzartos S. J. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur J Immunol. 1992 Sep;22(9):2449–2452. doi: 10.1002/eji.1830220939. [DOI] [PubMed] [Google Scholar]
- Morel E., Bach J. F., Briard M. L., Aubry J. P. Neonatal myasthenia gravis. Anti-acetylcholine receptor antibodies in the amniotic fluid. J Neuroimmunol. 1984 Aug;6(5):313–317. doi: 10.1016/0165-5728(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Murphy J., Murphy S. F. Myasthenia gravis in identical twins. Neurology. 1986 Jan;36(1):78–80. doi: 10.1212/wnl.36.1.78. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Nishitani H., Ota K., Fujita T., Tsuji K. Gm allotypes in myasthenia gravis. Lancet. 1980 Mar 29;1(8170):677–680. [PubMed] [Google Scholar]
- Namba T., Shapiro M. S., Brunner N. G., Grob D. Myasthenia gravis occurring in twins. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):531–534. doi: 10.1136/jnnp.34.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor L. H., Clark E. M. d(TG)n.d(CA)n sequences upstream of the rat prolactin gene form Z-DNA and inhibit gene transcription. Nucleic Acids Res. 1990 Mar 25;18(6):1595–1601. doi: 10.1093/nar/18.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Sherritt M., Begovich A. B., Erlich H. A., Bernard C. C., Cavalli-Sforza L. L., Steinman L. T-cell receptor V alpha and C alpha alleles associated with multiple and myasthenia gravis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):988–992. doi: 10.1073/pnas.86.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. Statistical properties of the haplotype relative risk. Genet Epidemiol. 1989;6(1):127–130. doi: 10.1002/gepi.1370060124. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Gunn S., Gabbay K. H. Multigenic basis for type I diabetes. Association of HRAS1 polymorphism with HLA-DR3, DQw2/DR4, DQw8. Diabetes. 1990 Dec;39(12):1504–1509. doi: 10.2337/diab.39.12.1504. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Lowenhaupt K., Rich A., Nordheim A. (dC-dA)n.(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J. 1987 Jun;6(6):1781–1789. doi: 10.1002/j.1460-2075.1987.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascuzzi R. M., Phillips L. H., 2nd, Johns T. R., Lennon V. A. The prevalence of electrophysiological and immunological abnormalities in asymptomatic relatives of patients with myasthenia gravis. Ann N Y Acad Sci. 1987;505:407–415. doi: 10.1111/j.1749-6632.1987.tb51311.x. [DOI] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Horton R. M., Bellone M., Conti-Tronconi B. M. Myasthenia gravis: recognition of a human autoantigen at the molecular level. Immunol Today. 1993 Jul;14(7):363–368. doi: 10.1016/0167-5699(93)90237-F. [DOI] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Howard J. F., Jr, Conti-Tronconi B. M. Immunodominant regions for T helper-cell sensitization on the human nicotinic receptor alpha subunit in myasthenia gravis. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7792–7796. doi: 10.1073/pnas.87.19.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman R. S., McGinnis R. E., Ewens W. J. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet. 1993 Mar;52(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G., Robinson W. P., Kuhner M. K., Joe S., Klitz W. HLA and insulin gene associations with IDDM. Genet Epidemiol. 1989;6(1):155–160. doi: 10.1002/gepi.1370060129. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Brachman D. B., Pestronk A., Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975 Oct 24;190(4212):397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955 Jun;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Weber J. L. Informativeness of human (dC-dA)n.(dG-dT)n polymorphisms. Genomics. 1990 Aug;7(4):524–530. doi: 10.1016/0888-7543(90)90195-z. [DOI] [PubMed] [Google Scholar]