Abstract
Background
Dopaminergic drugs remain the mainstay of Parkinson’s disease therapy but often fail to improve cognitive problems such as impulsivity. This may be due to the loss of other neurotransmitters, including noradrenaline, which is linked to impulsivity and response inhibition. We therefore examined the effect of the selective noradrenaline reuptake inhibitor atomoxetine on response inhibition in a stop-signal paradigm.
Methods
This pharmacological functional magnetic resonance imaging study used a double-blinded randomized crossover design with low-frequency inhibition trials distributed among frequent Go trials. Twenty-one patients received 40 mg atomoxetine or placebo. Control subjects were tested on no-drug. The effects of disease and drug on behavioral performance, regional brain activity, and functional connectivity were analyzed using general linear models. Anatomical connectivity was examined using diffusion-weighted imaging.
Results
Patients with Parkinson’s disease had longer stop-signal reaction times, less stop-related activation in the right inferior frontal gyrus (RIFG), and weaker functional connectivity between the RIFG and striatum compared with control subjects. Atomoxetine enhanced stop-related RIFG activation in proportion to disease severity. Although there was no overall behavioral benefit from atomoxetine, analyses of individual differences revealed that enhanced response inhibition by atomoxetine was associated with increased RIFG activation and functional frontostriatal connectivity. Improved performance was more likely in patients with higher structural frontostriatal connectivity.
Conclusions
This study suggests that enhanced prefrontal cortical activation and frontostriatal connectivity by atomoxetine may improve response inhibition in Parkinson’s disease. These results point the way to new stratified clinical trials of atomoxetine to treat impulsivity in selected patients with Parkinson’s disease.
Keywords: Atomoxetine, Impulsivity, Noradrenaline, Parkinson’s disease, Response inhibition, SSRT
Dopaminergic drugs remain the mainstay of Parkinson’s disease (PD) therapy but often fail to improve cognitive and behavioral problems such as impulsivity and poor executive control (1, 2, 3). Their limited effect may be because they do not address the loss of other monoaminergic projections to the forebrain, including noradrenaline (4, 5, 6), or changes in the frontostriatal connections that support inhibition and other executive functions (7). Noradrenergic agents have been suggested as a treatment for impulsivity and executive dysfunction (8, 9, 10, 11). This study focuses on one important facet of the multidimensional construct of impulsivity, the impairment in response inhibition.
We proposed that noradrenaline plays a crucial role in regulating the neurocognitive systems for response inhibition in the context of PD, based on preclinical evidence from animal and human studies. We distinguished the prevention of prepotent action (restraint) from stopping an initiated action (cancellation). These two forms of response inhibition are typically studied with the NoGo paradigm and stop-signal reaction time (SSRT) task, respectively. In rats and humans, the selective noradrenaline reuptake inhibitor atomoxetine improves stop-signal performance (12, 13, 14, 15, 16), reduces premature decisions (17, 18), and increases right inferior frontal gyrus (RIFG) activation (19). In contrast, a similar dose of atomoxetine had no effect (or even negative impact) on NoGo inhibition in healthy adults (20, 21), which supports preclinical evidence of a neuropharmacologic distinction between restraint and cancellation type inhibition (22, 23). Evidence for a noradrenergic benefit on stop-signal performance also comes from human and animal studies of methylphenidate, a reuptake inhibitor of both noradrenaline and dopamine (24, 25, 26, 27, 28). The beneficial effect of methylphenidate on SSRT might, in principle, be due to either dopaminergic or noradrenergic mechanisms alone, but noradrenergic and dopaminergic systems may also act synergistically (27), which is of particular relevance to the possible use of noradrenergic drugs for adjunctive treatment of PD.
Using pharmacological functional magnetic resonance imaging (fMRI), we examined the effect of atomoxetine (40 mg, versus placebo) on response inhibition in PD. A favorable impact of atomoxetine on SSRT was identified in a preliminary double-blinded randomized crossover behavioral study (29). This study went further, using fMRI to reveal the mechanisms of atomoxetine’s effect and identifying potential predictors of treatment benefit. We studied the impact of atomoxetine on SSRT and NoGo inhibition, using a task incorporating both NoGo and stop-signal trials.
We predicted 1) that action restraint (NoGo) and cancellation (SSRT) are both impaired in PD (30, 31); 2) that atomoxetine selectively enhances activations of the RIFG on successful stop-signal trials; and 3) that the behavioral effects of atomoxetine would depend not only on the stop-signal RIFG activation but also on the RIFG-striatum connectivity that links the inhibition with motor systems.
Methods and Materials
Participants
Twenty-one patients with PD (United Kingdom PD Society Brain Bank Clinical Diagnostic Criteria) (32, 33) were recruited via the Cambridge University PD Research Clinic. Inclusion criteria were 1) Hoehn and Yahr Scale 1.5 to 3; 2) age 45 to 80; 3) English speaking; 4) right handed; and 5) nondemented (clinical impression and Mini Mental State Examination >26/30). Patients were excluded by 1) clinically significant current depression; and 2) contraindications to magnetic resonance imaging (MRI) or atomoxetine. None of our patients had impulse control disorders. All of them were tested on regular PD medication, including levodopa (n = 19), nonergot dopamine agonists (n = 17; 8 pramipexole, 8 ropinirole, 1 rotigotine), and other antiparkinsonian medications (n = 8; 7 amantadine, 1 rasagiline, 1 selegiline). All patients were tested in their “on” state. Levodopa equivalent dose (LED) was calculated by the formula of Tomlinson et al. (34). Demographic and clinical features of participants are given in Table 1.
Table 1.
Demographic and Clinical Features and Neuropsychological Measures (Means, Standard Deviations, and Group Differences)
| Features/Measures | Parkinson’s Disease | Control Subjects | Group Difference |
|---|---|---|---|
| Male:Female | 11:10 | 12:8 | ns |
| Age (Years) | 64.0 (8.1) | 65.3 (5.7) | ns |
| Education (Years) | 14.6 (3.8) | 15.1 (2.5) | ns |
| Mini Mental State Examination | 28.9 (1.2) | 29.3 (.9) | ns |
| Duration of Symptoms (Years) | 10.8 (4.9) | – | – |
| UPDRS I: Mentation, Behavior and Mood | 8.8 (4.9) | – | – |
| UPDRS III: Motor | 20.6 (7.7) | – | – |
| Hoehn and Yahr Scale | 1.9 (.4) | – | – |
| Schwab and England Activities of Daily Living Scale | 86.5 (5.9) | – | – |
| Levodopa Actual Dose (mg/day) | 393.4 (221.0) | – | – |
| Levodopa Equivalent Dose (mg/day) | 632.6 (310.6) | – | – |
| Beck Depression Inventory II | 9.9 (5.5) | 3.8 (3.9) | p < .001 |
| Epworth Sleep Scale | 9.3 (5.3) | 5.1 (2.9) | p < .01 |
| Insomnia Severity Index | 9.2 (5.8) | 3.1 (2.8) | p < .001 |
| REM Sleep Behaviour Disorder Screening Questionnaire | 6.4 (2.9) | 2.0 (1.5) | p < .001 |
| Spot-the-Word Test | 112.8 (13.6) | 121.2 (10.3) | p < .05 |
| Category Fluency Test | 20.6 (4.9) | 24.3 (6.7) | p < .05 |
| Letter Fluency Test | 17.0 (5.6) | 18.1 (4.5) | ns |
| Forward Digit Span | 7.0 (1.1) | 7.2 (.8) | ns |
| Backward Digit Span | 5.4 (1.5) | 5.7 (1.3) | ns |
| Simple Reaction Time (msec) | 293.7 (53.0) | 314.4 (71.8) | ns |
| Choice Reaction Time (msec) | 353.4 (47.3) | 392.2 (70.0) | p < .05 |
Group difference: p values of chi-squared or two-sample t tests as appropriate (two-tailed; ns, p > .1).
ns, not significant; UPDRS, Unified Parkinson’s Disease Rating Scale.
Twenty healthy control subjects with no history of significant neurologic or psychiatric disorder were recruited from the Cambridge University PD Research Clinic database and the Cognition and Brain Sciences Unit volunteer panel. This study was approved by the local research ethics committee and exempted from clinical trials status by the Medicines and Healthcare Products Regulatory Agency. Written informed consent was obtained from all participants.
Experimental Design
A double-blinded randomized crossover design was used, with separate sessions at least 6 days apart, including a neuropsychological battery and brain imaging, after either 40 mg oral atomoxetine or an identically overcoated placebo capsule. In humans, plasma concentration of atomoxetine peaks approximately 2 hours after a single oral dose (35). Blood samples, therefore, were collected 2 hours after administration, immediately before fMRI scanning in each session (plasma concentration after atomoxetine: mean 444 ng/mL, range 32–889 ng/mL; under placebo: 0 ng/mL). Control subjects underwent one testing session only without drug or placebo.
The inhibition task included randomly interleaved action restraint and cancellation trials, which were matched for drug levels, practice effects, and fatigue. There were 360 Go trials (75%), 40 NoGo trials (8%), and 80 stop-signal trials (SS) (17%). In Go trials, participants responded to a left/right black arrow (duration 1000 msec) by pressing left/right buttons with their right hand. In SS trials, the left/right black arrow changed color (from black to red) concurrent with a tone, after a short variable stop-signal delay, indicating the need to cancel the response. The stop-signal delay was varied from trial to trial in steps of 50 milliseconds by a tracking algorithm to maintain 50% successful inhibition (15). In NoGo trials, participants were required to make no response to a red left/right arrow (duration 1000 msec) and concurrent tone, equivalent to a stop-signal delay of zero. Preliminary studies in 20 healthy adults indicated that performances and activations were preserved in the combined task compared with separate NoGo and stop-signal tasks.
For this task, four key parameters of interest were measured: the rate of Go commission errors, mean reaction time of correct Go trials (Go RT), rate of NoGo commission errors, and SSRT. For Go trials, a commission error occurred when participants mistakenly pressed the opposite buttons. For NoGo trials, a commission error means participants pressed a button. Both error rates were arcsine transformed for further analysis. The SSRT was estimated by subtracting mean stop-signal delay from finishing time of the stop process (using the integration method) (36). The finishing time is the nth Go RT, where Go RTs are rank-ordered and n is determined by the probability of responding, p(respond|signal), and the number of correct Go trials, m, as n = m × p(respond|signal). We also monitored omission errors on Go trials. Patients showed higher rates of Go omission errors than control subjects (placebo 7%, atomoxetine 9%, control 4%, p < .01). Analysis of the Go RT distribution indicated that omission errors were not due to excessive response times but other impairments (e.g., slipping off the buttons or failure to press the button). Although the omissions can only be detected for Go trials, their occurrence in SS trials may affect SSRT estimation, as p(respond|signal) would be underestimated. The tracker algorithm may also elevate the stop-signal delay. We corrected the observed p(respond|signal) by individual subject’s Go omission rate using equation 1 below (written communication, G. Logan, Ph.D., 2013). Correction of NoGo error rate used equation 2.
| (1) |
| (2) |
Disease effects on behavioral indices were examined by two-sample t tests (PD-placebo > control). To investigate drug effects on SSRT and NoGo error rate, repeat-measures analyses of variance were conducted with drug (atomoxetine vs. placebo) as a within-subject factor and disease severity (Unified Parkinson’s Disease Rating Scale [UPDRS] motor), age, LED, and plasma concentration as covariates. Note that the disease effect refers to treated Parkinson’s disease (37, 38). In principle, differences between PD-placebo and control subjects could be due to the presence of PD, the use of medication, or in our design, the additional use of a placebo tablet in the PD-placebo group. However, the concurrent use of dopaminergic drugs is unlikely to fully explain the group differences (30).
Participants also completed the Beck Depression Inventory II, Epworth Sleepiness Scale, Insomnia Severity Index, REM Sleep Behaviour Disorder questionnaire, Spot-the-Word test, category and letter fluency tests, forward and backward digit spans, and simple and choice reaction times.
fMRI Data Acquisition and Analysis
Magnetic resonance imaging used a Siemens Trio 3T scanner (Siemens, München, Germany). Functional images were acquired using a quiet echo planar imaging sequence (2656-msec repetition time, 44-msec echo time, 78° flip angle, 32 sequential descending oblique axial slices, 192 × 192 mm2 field of view, 3-mm thickness, .75-mm gap, and 3 × 3 mm2 in-plane resolution) (39). High-resolution T1-weighted magnetization-prepared rapid-acquired gradient echo images were acquired (144 sequential sagittal slices, 240 × 240 mm2 field of view, 1.25-mm thickness, and 1.25 × 1.25 mm2 in-plane resolution).
Functional MRI analysis used SPM8 (Functional Imaging Laboratory, London, United Kingdom; www.fil.ion.ucl.ac.uk/spm). Eleven volumes were discarded to allow magnetization equilibration. Functional images were realigned to the first image, sinc-interpolated to correct for differences in slice acquisition time, and normalized to the Montreal Neurological Institute (MNI) template using iterative segmentation. Normalized images were spatially denoised using a wavelet-based three-dimensional denoising approach, which not only suppresses noise but also preserves essential spatial details (edge and shape of activation) that are often suppressed in Gaussian smoothing (40).
A univariate analysis examined the effects of disease and drug on activation. Subject-level general linear models convolved a design matrix with the canonical hemodynamic response function. Event types included in the design matrix were correct SS, NoGo, and Go trials and commission and omission error trials. Six movement parameters were included as nuisance regressors. Classical parameter estimation was applied with a one-lag autoregressive model and high-pass filter of 128 seconds. Contrasts of interest, SS > Go and NoGo > Go, were entered into one-sample t tests for stop-related and NoGo-related activations of each group (voxel-level p < .001 uncorrected, cluster-level p < .05 familywise error [FWE]-corrected).
In view of our anatomically constrained hypotheses regarding disease and drug effects, a study-specific RIFG region of interest (ROI) was built. This ROI is the intersection of the anatomical RIFG (Automated Anatomical Labeling based [Automated Anatomical Labeling, AAL, Caen University, France]) and the stop-related (SS > Go) or NoGo-related (NoGo > Go) activations in control subjects. Disease effects on stop-related and NoGo-related RIFG activations were examined by two-sample t tests with small volume correction (PD-placebo < control, voxel-level p < .05 FWE-corrected). To investigate drug effects, betas (parameter estimates) of the RIFG ROI were pooled in repeated-measures analyses of variance with drug as a within-subject factor and UPDRS motor, age, LED, and plasma concentration as covariates.
A functional connectivity analysis examined whether the interaction between the RIFG and striatum was modulated by the disease and/or atomoxetine. Physiological fMRI signals were extracted from a spherical RIFG ROI (unbiased ROI with 6-mm radius, centered at the RIFG peak of SS > Go in all subjects, i.e., a one-sample t test combining control subjects and patients) and included in another subject-level general linear model (physiological regressor). Estimated betas indicated the degree to which the time course of a voxel correlated with the RIFG time course. One-sample t tests showed regions positively correlated with the RIFG in each group (voxel-level p < .001 uncorrected, cluster-level p < .05 FWE-corrected). The effects of disease (PD-placebo < control) and drug (PD-atomoxetine > placebo) were examined by two-sample and paired-sample t tests, respectively (small volume correction, voxel-level p < .05 FWE-corrected).
Diffusion MRI
Diffusion-weighted images were collected along 63 gradient directions (single acquisition, 63 sequential interleaved ascending axial slices, 192 × 192 mm2 field of view, 2-mm thickness, and 2 × 2 mm2 in-plane resolution) and analyzed with FSL4.1 (FMRIB software library, FMRIB, Oxford, United Kingdom; www.fmrib.ox.ac.uk/fsl). Images were corrected for head movements and eddy currents and smoothed with a 2.5-mm Gaussian kernel. Diffusion tensors were linearly fitted to the diffusion-weighted images, and fractional anisotropy (FA) images were calculated for tract-based spatial statistics (41). The FA images were corrected for outlier values, registered to the FMRIB58_FA template, and normalized to the MNI 152 space. A mean FA skeleton was derived and thresholded at FA > .2 to represent the center of the white matter tracts common to all subjects. Permutation tests with threshold-free cluster enhancement (42) examined whether the effects of atomoxetine on SSRT varied with the strength of frontostriatal connections. Based on our hypothesis of frontostriatal interactions between inhibition and motor systems, we focused on the right anterior internal capsule, which contains fibers connecting the frontal lobe, striatum, and thalamus (ROI based on the Johns Hopkins University diffusion tensor imaging-based white-matter atlas).
Results
Behavioral Results
Results of the neuropsychological battery are given in Table 1. Groups were well matched by age, sex, education, and Mini Mental State Examination. As is typical of PD, sleep and depression symptom scores were higher and category (semantic) fluency was reduced in patients (33).
Table 2 shows the SSRT, Go RT, Go error rate, and NoGo error rate in each group. Compared with control subjects, PD-placebo had longer SSRT and more NoGo errors and Go errors. The group difference in SSRT could not be attributed to a difference in Go RT. Although there was no groupwise drug effect, the behavioral benefit of atomoxetine was conditional on individual differences in brain activation and functional and anatomical connectivity (see below).
Table 2.
Performance on the Stop-Signal and NoGo Tasks
| Parameters | Control Subjects | PD-PLA | PD-ATO | Disease Effect | ATO Effect (Whole Group) |
|---|---|---|---|---|---|
| SSRT (msec) | 142 (44) | 167 (50) | 181 (85) | p < .05 | ns |
| Go RT (msec) | 532 (129) | 554 (108) | 562 (104) | ns | ns |
| NoGo Error (rad) | .06 (.13) | .14 (.13) | .13 (.15) | p < .05 | ns |
| Go Error (rad) | .08 (.05) | .14 (.06) | .15 (.07) | p < .001 | ns |
Values are group means (standard deviations). Disease effect refers to the contrast PD-PLA vs. control subjects (two-sample t tests), which differs by the presence of Parkinson’s disease and concurrent dopaminergic/placebo medication in patients. ATO effect refers to the contrast PD-ATO vs. PD-PLA (repeated-measures analyses of variance with age, Unified Parkinson’s Disease Rating Scale, levodopa equivalent dose, and plasma concentration as covariates). ns, p > .1.
ATO, atomoxetine; ns, not significant; PD, Parkinson’s disease; PLA, placebo; rad, arcsin transformed to radians; RT, reaction time; SSRT, stop-signal reaction time.
fMRI Results
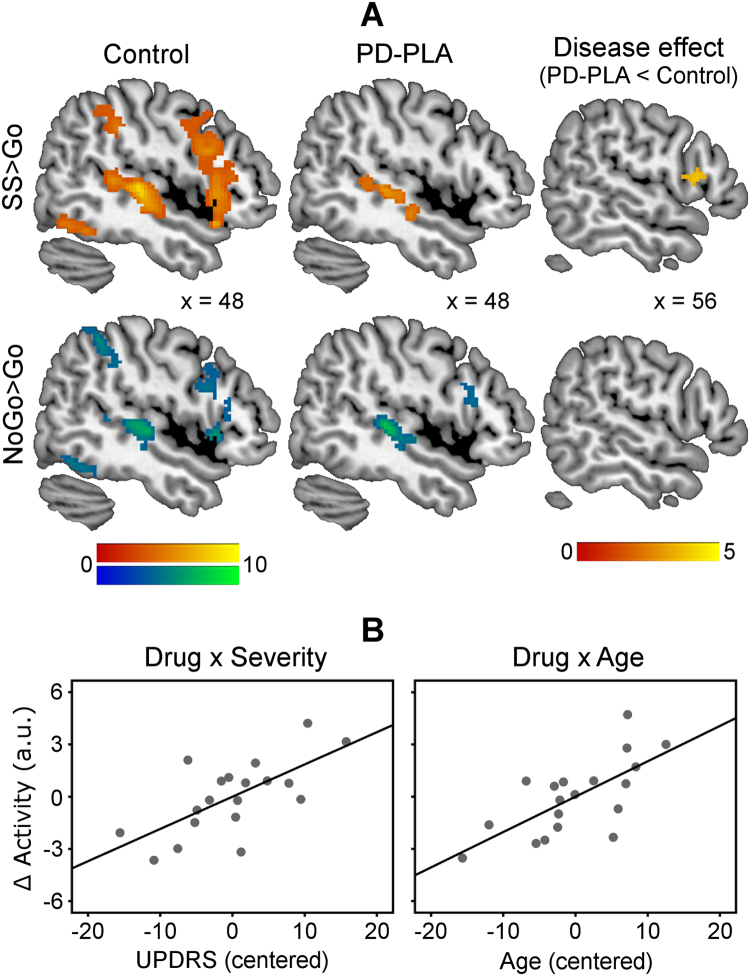
Figure 1 presents inhibition-related activations, including the RIFG, in each group. Control subjects showed RIFG activations for SS > Go (peak coordinates in MNI space [54 18 8], t = 7.17, 1621 voxels) and for NoGo > Go (peak [48 18 −2], t = 6.00, 452 voxels; Figure 1A). The stop-related RIFG activation (SS > Go), not the NoGo-related activation (NoGo > Go), was significantly reduced in PD-placebo compared with control subjects (disease effect, peak [56 16 12], t = 3.88, 14 voxels; Figure 1A).
Figure 1.
(A) Control subjects showed greater stop-related (stop-signal [SS] > Go, warm colors) and NoGo-related activations (NoGo > Go, cool colors) in the right inferior frontal gyrus (RIFG). The stop-related RIFG activation was significantly weaker in Parkinson’s disease-placebo (PD-PLA) than in control subjects (disease effect, p < .05 small-volume corrected). Color scales indicate t values. Coordinates are in Montreal Neurological Institute space. (B) Atomoxetine selectively enhanced the stop-related RIFG activation in more advanced disease (drug × severity) and in older patients (drug × age). The atomoxetine-induced change of RIFG activity (∆Activity) was positively correlated with Unified Parkinson’s Disease Rating Scale (UPDRS) and age (mean-corrected data).
The atomoxetine effect on stop-related RIFG activation showed an interaction between drug and UPDRS motor (F = 11.78, p < .005), as well as an interaction between drug and age (F = 12.56, p < .005). This indicates that atomoxetine enhanced RIFG activations in more advanced disease and in older patients (Figure 1B). There was no effect of LED or plasma concentration in the multiple regression model (see also Figure S2 in Supplement 1). No drug effect was obtained on NoGo-related RIFG activation.
The NoGo and SSRT tasks are associated with widespread activations beyond the RIFG (43, 44). The RIFG is the focus of this study, but we also analyzed the drug effects on other areas including the left inferior frontal gyrus, supplementary motor area, striatum, and thalamus. The results are presented in the details in Supplement 1. In brief, we observed an effect of atomoxetine in none of these areas except the thalamus (Figure S1 in Supplement 1).
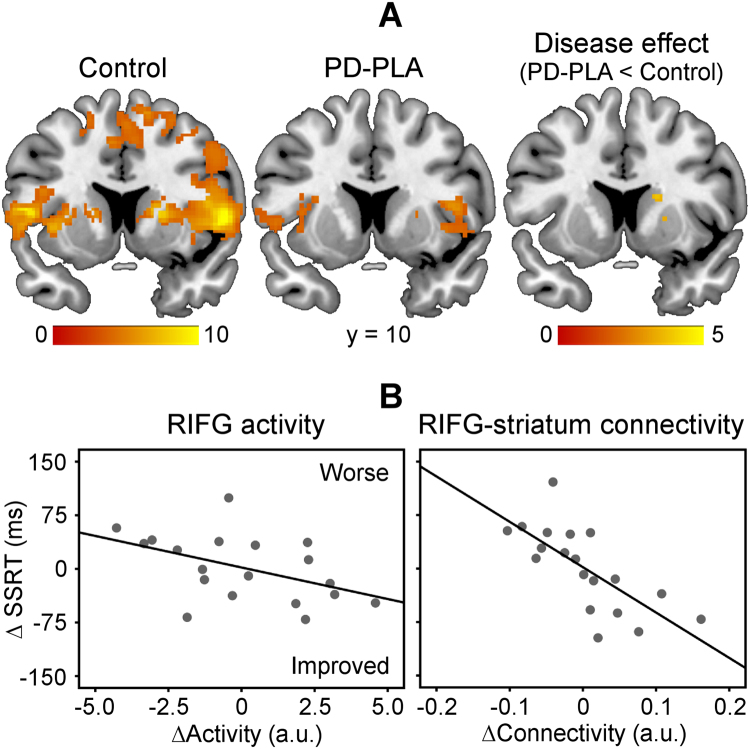
Figure 2A presents areas functionally correlated positively with the RIFG, including the bilateral striatum. Control subjects showed strong coupling between the RIFG and right striatum (peak [20 8 8], t = 9.16, 537 voxels). The interregional interaction was significantly reduced in PD-placebo compared with control subjects (disease effect, peak [18 10 16], t = 3.87, 13 voxels). Atomoxetine did not restore the frontostriatal connectivity at the group level.
Figure 2.
(A) Control subjects showed strong functional coupling between the right inferior frontal gyrus (RIFG) and striatum. The RIFG-striatum connectivity was significantly weaker in Parkinson’s disease-placebo (PD-PLA) than in control subjects (disease effect, p < .05 small-volume corrected). Color scales indicate t values. Coordinates are in Montreal Neurological Institute space. (B) The atomoxetine-induced change of stop-signal reaction time (SSRT) (∆SSRT) was related not only to the change of RIFG activity (∆Activity, p < .05) but also to the change of RIFG-striatum connectivity (∆Connectivity, mean-corrected data, p < .005).
To determine whether change of behavioral performance was related to atomoxetine’s enhancement of RIFG activity and/or RIFG-striatum connectivity, a linear regression model (equation 3) used the change of SSRT as a dependent variable (∆SSRT: SSRT-atomoxetine vs. SSRT-placebo). Independent variables were the change of RIFG activity in stop-signal trials (∆Activity, activity betas from the study-specific RIFG ROI), the change of RIFG-striatum connectivity (∆Connectivity, connectivity betas from the significant cluster of right striatum in control subjects), and the baseline SSRT under placebo (SSRT-placebo).
| (3) |
The model was significant overall (F = 8.97, p = .001). Figure 2B illustrates that ∆SSRT was related to both ∆Activity (β1 = −.37, t = −2.14, p < .05) and ∆Connectivity (β2 = −.63, t = −4.11, p = .001), in addition to the SSRT-placebo (β3 = .61, t = 3.49, p < .005). It suggests that the behavioral effect of atomoxetine depends not only on changes in frontal activity but also on changes in frontostriatal connectivity.
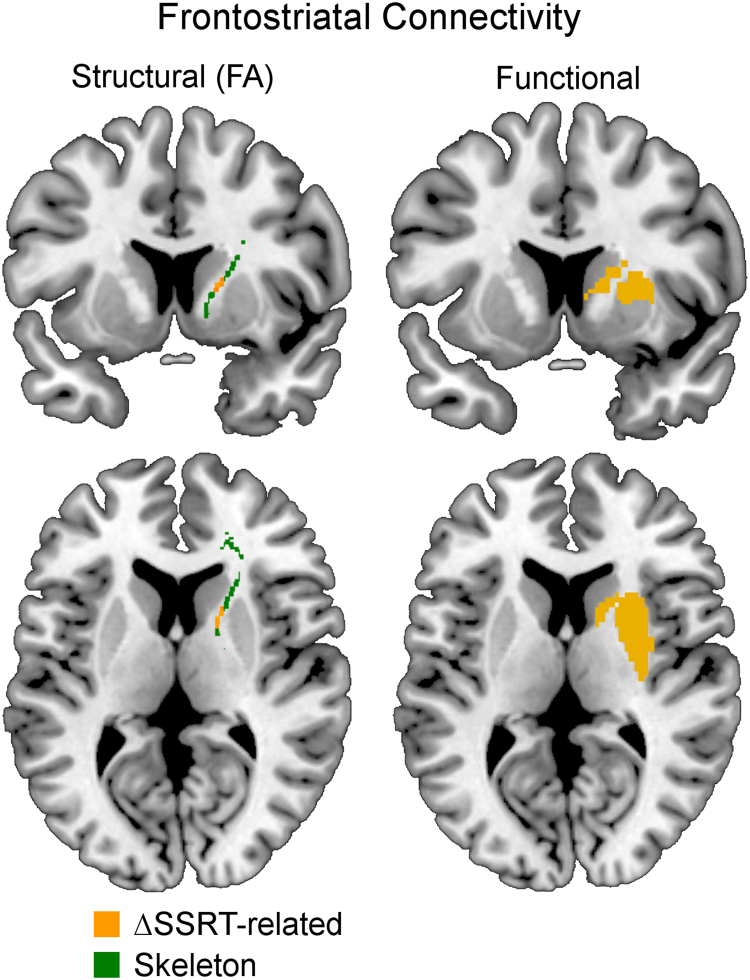
In addition to changes of functional connectivity, the strength of structural connectivity between the frontal cortex and striatum predicted atomoxetine’s effect on performance (Figure 3). The SSRT change (∆SSRT) was negatively correlated with the fractional anisotropy of white matter in the anterior limb of the internal capsule: a reduction in SSRT was observed in patients with greater anatomical frontostriatal connectivity.
Figure 3.
The behavioral benefit of atomoxetine (reduction in stop-signal reaction time, ΔSSRT) was related not only to the functional connectivity but also to the structural connectivity between the frontal cortex and striatum (fractional anisotropy [FA] of white matter, p < .05 threshold-free cluster enhancement-corrected within anterior-internal capsule skeleton).
Discussion
This study investigated effects of the selective noradrenaline reuptake inhibitor atomoxetine on response inhibition in patients with PD. We confirmed that patients with PD and on dopaminergic medication were impaired at response inhibition, even in the absence of impulse control disorders, with longer SSRT and more NoGo errors than healthy control subjects. Behavioral impairments were associated with reduced RIFG activations on successful stop-signal trials and weaker functional connectivity between the RIFG and striatum. The group differences might arise from the presence of neuropathology or concurrent optimized motor therapy by dopaminergic drugs. Atomoxetine modulated the stop-related RIFG activation in PD, similar to its enhancement of RIFG activation in another impulsive disorder, attention-deficit/hyperactivity disorder (ADHD) (11). Enhanced activation emerged in patients with more severe disease. Moreover, improvements in stop-signal performance correlated not only with atomoxetine’s effect on frontal activation but also with its effect on frontostriatal connectivity.
These observations build on studies of atomoxetine in healthy adults and those with ADHD (15, 19), confirming that noradrenaline plays an important role in regulating response inhibition. However, we propose that enhancement of prefrontal activation is not sufficient to improve behavioral inhibition, unless frontostriatal functional connectivity is also enhanced. This would explain why the behavioral benefits of atomoxetine were marked in healthy adults (19) with intact corticostriatal connectivity but not at the group level with PD or ADHD (11), where frontostriatal connectivity is often impaired (7). Moreover, patients vary in their responses to noradrenergic or other treatments. The effectiveness of treatment may be predicted if impairments of regional function and network communication are considered jointly, including anatomical frontostriatal connectivity. Functional and/or structural connectivity might therefore be used in the stratification of patients for treatment and in prediction of efficacy.
It might at first seem paradoxical that atomoxetine enhanced stop-related RIFG activation in patients with relatively more advanced disease, while reducing SSRT in patients with stronger pretreatment frontostriatal structural connectivity. However, these results are consistent. First, responses to atomoxetine may be strongly influenced by the baseline noradrenergic state. A Yerkes-Dodson model (of an inverted U-shaped function) has been proposed to account for the nonlinear relation between noradrenaline levels and task performances in animal studies (45, 46). In this model, the performance is optimal with intermediate noradrenaline levels but impaired at excess noradrenaline levels. In a previous study of an independent PD cohort, we observed this pattern of responses to atomoxetine in PD: low plasma concentration improved response inhibition, whereas high concentration impaired response inhibition (29). We propose that our dose of atomoxetine represents a replacement therapy for patients who have lost a significant proportion of noradrenergic capacity (5). But for patients with mild disease and minor changes in intrinsic noradrenaline transmission, the same atomoxetine dose represents in effect an overdose. Second, for atomoxetine to be able to exert a behavioral benefit, the RIFG must be effectively connected with the motor systems that ultimately generate behavioral outputs. Because the striatum modulates the execution of actions in response to the cortical motor systems (47), the relative preservation of frontostriatal connectivity improves the ability to relay inhibition commands. It is worth noting that neither UPDRS nor frontostriatal connectivity represents a gold standard biomarker for the stage of disease. While both would be expected to change when disease progresses, they might not be driven by the same biological factors. For example, UPDRS might be higher in some patients because of signs that are not directly related to the frontostriatal connectivity (e.g., tremor or balance). In our cohort, UPDRS motor scores and diffusion tensor imaging measures of frontostriatal connectivity were not significantly correlated.
Atomoxetine did not significantly affect NoGo activation or behavior. While this could be due to type II error, it is also consistent with preclinical studies (20, 21) reporting stop-signal modulation by noradrenaline (15, 19) and NoGo modulation by serotonin (48). This neuropsychopharmacologic specificity provides a reassuring internal control for the observed effects of atomoxetine.
The role of noradrenaline extends beyond response inhibition (8). Previous studies have linked the noradrenergic system with other executive functions, such as cognitive flexibility in response to a changing environment (10). For example, attentional set-shifting in the Wisconsin Card Sorting Test and Cambridge Neuropsychological Test Automated Battery intradimensional/extradimensional set-shifting task are impaired by PD (49, 50) and in animals with selective depletion of cortical noradrenaline (51, 52) but can be relieved by atomoxetine (53, 54). Noradrenergic systems may therefore support multiple executive processes, including control of motor and nonmotor impulsivity, response inhibition, and cognitive flexibility (21, 55), which requires further evaluation for PD.
There are clinical, methodological, and pharmacologic limitations with this study. First, our investigation was limited to response inhibition rather than other dimensions of impulsivity, such as reflection impulsivity or impairment of decisions based on risk and rewards. The latter have been strongly associated with dopaminergic dysfunction in treated PD (1, 2, 3). Second, we relied on clinical diagnostic criteria because the United Kingdom PD Society Brain Bank Criteria have high sensitivity and specificity. Clinically, none of our patients had impulse control disorders (ICDs). But, we note that PD causes impulsivity and response inhibition deficits even without ICDs (30, 31, 56). Further studies are needed to test whether atomoxetine can improve impulsivity in patients with ICDs. Third, fMRI measures a hemodynamic response to neural activity and in principle atomoxetine might alter fMRI signals through affecting regional cerebral blood flow. However, the frontal regions and striatum retain normal blood flow under atomoxetine (57). Moreover, the cognitive and anatomical specificity of the effects we observed make a significant direct or generic effect of atomoxetine on the neurovascular response unlikely.
Finally, we must consider the neuropharmacologic complexity of patients and atomoxetine. Our data alone cannot determine whether the effects of atomoxetine are via cortical reuptake inhibition (most likely) (23, 55), from changes in phasic-to-tonic firing ratios in the locus coeruleus (45), or through antagonism of glutamate receptors (58). We recognize that atomoxetine can increase extracellular dopamine levels (59) and our patients took levodopa and/or dopamine agonists. But the atomoxetine effect is unlikely mediated by indirect dopaminergic mechanisms. First, as we have shown with multiple regression models, the atomoxetine effect on activation was independent of LED. Second, the specific effect of dopamine on stop-signal inhibition is unclear. Some studies of SSRT have used methylphenidate, which increases extracellular levels of both dopamine and noradrenaline, leaving it ambiguous as to whether dopamine or noradrenaline is responsible for the beneficial effect (11, 24, 60, 61). Studies that used more selective dopaminergic agents, such as levodopa and dopamine transporter blocker, have observed minor effects on SSRT, and patients with PD have poor response inhibition both on and off medication (12, 30, 55, 62). The advent of more selective noradrenergic drugs will further address these issues in the future.
In conclusion, the noradrenergic reuptake inhibitor atomoxetine can improve response inhibition in a subgroup of patients, especially when it is associated with enhanced stop-signal activation in the inferior frontal cortex (which is more likely with relatively more advanced disease). The behavioral measure or response inhibition efficacy was improved (i.e., shorter SSRT) for patients in whom frontostriatal functional connectivity was also enhanced by atomoxetine or in those with relatively preserved frontostriatal structural connectivity. These findings contribute to the broader understanding of the importance of noradrenergic systems for executive functions and point the way to new stratified clinical trials of noradrenergic therapies in selected patients with Parkinson’s disease.
Acknowledgments and Disclosures
This work was primarily funded by the Wellcome Trust (088324) with additional support from the Medical Research Council (MC_US_A060_0016, and RG62761), the National Institute for Health Research Cambridge Biomedical Research Centre, and Parkinson’s UK. The Behavioural and Clinical Neurosciences Institute is supported by the Wellcome Trust and Medical Research Council. We are grateful to Dr. G. Logan for advice on stop-signal reaction time estimation and to Dr. Marta Correia for advice on diffusion tensor imaging analysis.
Dr. Housden reported being employed by the Cambridge Cognition.
Mr. Rittman disclosed grants from the Medical Research Council (G1100464) and Raymond and Beverley Sackler scholarship and financial support for travel to meeting from the Association of British Neurologists. He also reported being employed by the Norfolk and Norwich University Hospitals National Health Service Trust and by the Addenbrooke’s Hospital National Health Service Trust.
Ms. Rae reported receiving the Guarantors of Brain Travel Award.
Dr. Sahakian reported as receiving consulting fees and lecture honoraria from Lundbeck, lecture honorarium from Servier, consulting fees from Cambridge Cognition, and a grant from Janssen/Johnson & Johnson. She receives honoraria from Psychological Medicine as Associate Editor and fees from the Foresight, Government Office for Science for a commissioned article. She also receives royalties from Oxford University Press for co-editing The Oxford Handbook of Neuroethics and co-authoring “Bad moves. How decision making goes wrong and the ethics of smart drugs,” and from Wiley Blackwell as a co-editor of Mental Capital and Wellbeing.
Dr. Barker disclosed grants from National Institute for Health Research and payment from Wiley for a basic textbook for Neuroscience.
Dr. Robbins reported receiving consulting fees from Cambridge Cognition, Lilly, Merck, Lundbeck, GlaxoSmithKline, Teva, and Shire; grants from GlaxoSmithKline, Lilly and Lundbeck; payments from Cambridge Cognition for Cambridge Neuropsychological Test Automated Battery, CANTAB; and honorarium from Springer-Verlag for editorial duties for Psychopharmacology.
Dr. Rowe disclosed grants from the Wellcome Trust, Medical Research Council, National Institute for Health Research, McDonnell Foundation, and Parkinson’s UK.
All other authors reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at doi:10.1016/j.biopsych.2014.01.024.
Appendix A. Supplementary Materials
Supplementary Material
References
- 1.Cools R., Barker R.A., Sahakian B.J., Robbins T.W. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 2.Voon V., Hassan K., Zurowski M., Duff-Canning S., de Souza M., Fox S., et al. Prospective prevalence pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub D., Siderowf A.D., Potenza M.N., Goveas J., Morales K.H., Duda J.E., et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politis M., Wu K., Loane C., Quinn N.P., Brooks D.J., Oertel W.H., et al. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003391. 128ra141. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein D.S., Sullivan P., Holmes C., Kopin I.J., Basile M.J., Mash D.C. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Politis M., Wu K., Loane C., Kiferle L., Molloy S., Brooks D.J., Piccini P. Staging of serotonergic dysfunction in Parkinson’s disease: An in vivo 11C-DASB PET study. Neurobiol Dis. 2010;40:216–221. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Rae C.L., Correia M.M., Altena E., Hughes L., Barker R.A., Rowe J.B. White matter pathology in Parkinson’s disease: The effect of imaging protocol differences and relevance to executive function. Neuroimage. 2012;62:1675–1684. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh L., Biglan K., Gerstenhaber M., Williams J.R. Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: A pilot open-label study. Mov Disord. 2009;24:277–282. doi: 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weintraub D., Mavandadi S., Mamikonyan E., Siderowf A.D., Duda J.E., Hurtig H.I., et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75:448–455. doi: 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazey E.M., Aston-Jones G. The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front Behav Neurosci. 2012;6:48. doi: 10.3389/fnbeh.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubillo A., Smith A.B., Barrett N., Giampietro V., Brammer M.J., Simmons A., Rubia K. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb Cortex. 2014;24:174–185. doi: 10.1093/cercor/bhs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bari A., Eagle D.M., Mar A.C., Robinson E.S., Robbins T.W. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bari A., Mar A.C., Theobald D.E., Elands S.A., Oganya K.C., Eagle D.M., Robbins T.W. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson E.S., Eagle D.M., Mar A.C., Bari A., Banerjee G., Jiang X., et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain S.R., Del Campo N., Dowson J., Müller U., Clark L., Robbins T.W., Sahakian B.J. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain S.R., Müller U., Blackwell A.D., Clark L., Robbins T.W., Sahakian B.J. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baarendse P.J., Winstanley C.A., Vanderschuren L.J. Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology (Berl) 2013;225:719–731. doi: 10.1007/s00213-012-2857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broos N., Schmaal L., Wiskerke J., Kostelijk L., Lam T., Stoop N., et al. The relationship between impulsive choice and impulsive action: A cross-species translational study. PLoS One. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain S.R., Hampshire A., Müller U., Rubia K., Del Campo N., Craig K., et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Graf H., Abler B., Freudenmann R., Beschoner P., Schaeffeler E., Spitzer M., et al. Neural correlates of error monitoring modulated by atomoxetine in healthy volunteers. Biol Psychiatry. 2011;69:890–897. doi: 10.1016/j.biopsych.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Hester R., Nandam L.S., O’Connell R.G., Wagner J., Strudwick M., Nathan P.J., et al. Neurochemical enhancement of conscious error awareness. J Neurosci. 2012;32:2619–2627. doi: 10.1523/JNEUROSCI.4052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., et al. Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 23.Eagle D.M., Bari A., Robbins T.W. The neuropsychopharmacology of action inhibition: Cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 24.Nandam L.S., Hester R., Wagner J., Cummins T.D., Garner K., Dean A.J., et al. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2011;69:902–904. doi: 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Potter A.S., Newhouse P.A. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- 26.Boonstra A.M., Kooij J.J., Oosterlaan J., Sergeant J.A., Buitelaar J.K. Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? J Clin Exp Neuropsychol. 2005;27:278–298. doi: 10.1080/13803390490515757. [DOI] [PubMed] [Google Scholar]
- 27.Li C.S., Morgan P.T., Matuskey D., Abdelghany O., Luo X., Chang J.L., et al. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci U S A. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eagle D.M., Tufft M.R., Goodchild H.L., Robbins T.W. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- 29.Housden C, Kehagia AA, Barker RA, Rittman T, Faluyi Y, Muller U, et al. (2011): The effects of atomoxetine on impulsivity in Parkinson’s Disease. Presented at the Summer Meeting of the British Association for Psychopharmacology, July 24–27, Harrogate, United Kingdom.
- 30.Obeso I., Wilkinson L., Casabona E., Bringas M.L., Álvarez M., Álvarez L., et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp Brain Res. 2011;212:371–384. doi: 10.1007/s00221-011-2736-6. [DOI] [PubMed] [Google Scholar]
- 31.Nombela C., Rittman T., Robbins T.W., Rowe J.B. Multiple modes of impulsivity in Parkinson’s disease. PLoS One. 2014;9:e85747. doi: 10.1371/journal.pone.0085747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams-Gray C.H., Foltynie T., Brayne C.E., Robbins T.W., Barker R.A. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 34.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 35.Sauer J.M., Ring B.J., Witcher J.W. Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet. 2005;44:571–590. doi: 10.2165/00003088-200544060-00002. [DOI] [PubMed] [Google Scholar]
- 36.Logan G.D., Cowan W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 37.Lewis S.J., Dove A., Robbins T.W., Barker R.A., Owen A.M. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weintraub D., Doshi J., Koka D., Davatzikos C., Siderowf A.D., Duda J.E., et al. Neurodegeneration across stages of cognitive decline in Parkinson’s disease. Arch Neurol. 2011;68:1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitter S., Diesch E., Amann M., Kroll A., Moayer M., Schad L.R. Silent echo-planar imaging for auditory FMRI. MAGMA. 2008;21:317–325. doi: 10.1007/s10334-008-0132-4. [DOI] [PubMed] [Google Scholar]
- 40.Khullar S., Michael A., Correa N., Adali T., Baum S.A., Calhoun V.D. Wavelet-based fMRI analysis: 3-D denoising, signal separation, and validation metrics. Neuroimage. 2011;54:2867–2884. doi: 10.1016/j.neuroimage.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Smith S.M., Nichols T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 43.Duann J.R., Ide J.S., Luo X., Li C.S. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S., Li C.S. Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp. 2012;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bari A., Aston-Jones G. Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology. 2013;64:53–64. doi: 10.1016/j.neuropharm.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 47.Redgrave P., Vautrelle N., Reynolds J.N. Functional properties of the basal ganglia’s re-entrant loop architecture: Selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 48.Macoveanu J., Hornboll B., Elliott R., Erritzoe D., Paulson O.B., Siebner H., et al. Serotonin 2A receptors, citalopram and tryptophan-depletion: A multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology. 2013;38:996–1005. doi: 10.1038/npp.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owen A.M., James M., Leigh P.N., Summers B.A., Marsden C.D., Quinn N.P., et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 50.Downes J.J., Roberts A.C., Sahakian B.J., Evenden J.L., Morris R.G., Robbins T.W. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: Evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 51.Tait D.S., Brown V.J., Farovik A., Theobald D.E., Dalley J.W., Robbins T.W. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- 52.McGaughy J., Ross R.S., Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman L.A., Darling J., McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl) 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cain R.E., Wasserman M.C., Waterhouse B.D., McGaughy J.A. Atomoxetine facilitates attentional set shifting in adolescent rats. Dev Cogn Neurosci. 2011;1:552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robbins T.W. Shifting and stopping: Fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bentivoqlio A.R., Baldonero E., Ricciardi L., De Niqris F., Daniele A. Neuropsychological features of patients with Parkinson’s disease and impulse control disorders. Neurol Sci. 2013;34:1207–1213. doi: 10.1007/s10072-012-1224-5. [DOI] [PubMed] [Google Scholar]
- 57.Marquand A.F., O’Daly O.G., De Simoni S., Alsop D.C., Maquire R.P., Williams S.C., et al. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: A multi-class pattern recognition approach. Neuroimage. 2012;60:1015–1024. doi: 10.1016/j.neuroimage.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludolph A.G., Udvardi P.T., Schaz U., Henes C., Adolph O., Weiqt H.U., et al. Atomoxetine acts as an NMDA receptor blocker in clinically relevant concentrations. Br J Pharmacol. 2010;160:283–291. doi: 10.1111/j.1476-5381.2010.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bymaster F.P., Katner J.S., Nelson D.L., Hemrick-Luecke S.K., Threlkeld P.G., Heiligenstein J.H., et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 60.Rubia K., Halari R., Mohammad A.M., Taylor E., Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheres A., Oosterlaan J., Swanson J., Morein-Zamir S., Meiran N., Schut H., et al. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- 62.Bari A., Robbins T.W. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: Possible relevance to ADHD. Psychopharmacology (Berl) 2013;230:89–111. doi: 10.1007/s00213-013-3141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material