Abstract
Background
Fatalistic beliefs may be implicated in longer help-seeking intervals, and consequently, greater risk of advanced stage at cancer diagnosis.
Methods
We examined associations between fatalism and stage at diagnosis in a population-based cohort of 4,319 U.S. patients with newly-diagnosed lung or colorectal cancer participating in the Cancer Care Outcomes and Research Surveillance (CanCORS) study. Fatalistic beliefs were assessed with an established measure. A fatalism score (range 4-16) was created by summing Likert-scale responses to four items. Cancer stage at diagnosis was abstracted from medical records by trained staff. Logistic regression was used to assess the association between fatalism score and advanced stage at diagnosis (IV vs I-III), adjusting for socio-demographic and clinical characteristics.
Results
Overall, 917 (21%) patients had stage IV cancers (lung: 28%, colorectal: 16%). The mean fatalism score was 10.7 (median=11, inter-quartile range 9-12). In adjusted analyses, a higher fatalism score was associated with greater odds of stage IV diagnosis (odds ratio per unit increase in fatalism=1.05, 95% confidence interval 1.02-1.08, p=0.003). Patients with the highest fatalism score had an adjusted 8.9% higher frequency of stage IV diagnosis compared with patients with the lowest score (25.4% vs. 16.5%).
Discussion
In this large and socioeconomically, geographically and ethnically diverse population of patients with lung and colorectal cancer, fatalistic beliefs were associated with higher risk of advanced stage at diagnosis. Longitudinal studies are needed to confirm causation.
Impact
These findings support the value of incorporating information about the curability of early-stage cancers in public education campaigns.
Keywords: Stage, Diagnosis, Fatalism, Colorectal, Lung
INTRODUCTION
Many cancer patients are diagnosed after a symptomatic presentation, because effective screening tests exist for few cancer sites and participation rates are sub-optimal. For example, in England, more than 90% of all cancer patients are diagnosed following symptomatic presentations (1). As stage at diagnosis is a key determinant of cancer survival, interventions to ensure that symptomatic patients are diagnosed at the earliest possible stage can help to reduce cancer mortality.
Psychosocial factors are important candidates for influencing the length of the period from symptom onset to presentation to a doctor (i.e. the ‘patient interval’), and, consequently, the stage at diagnosis (2,3). These factors encompass both cognitive (e.g. awareness of potential associations of symptoms with cancer) and emotional processes (e.g. fear of cancer or embarrassment about symptoms) (4).
Fatalism, whether it relates to a general belief that life events are pre-determined and inevitable, or ‘cancer fatalism’, defined by Powe as ‘the belief that death is inevitable when cancer is present’ (5), has been implicated in longer patient intervals in a study of intended help-seeking for breast cancer among asymptomatic women (6). Individuals with more fatalistic beliefs may be both more fearful of a cancer diagnosis and more sceptical about the value of early detection of cancer, and therefore, may delay seeking medical help (7). Several studies have shown that cancer fatalism influences cancer screening uptake (8-10). Direct evidence that fatalism is associated with longer patient intervals in symptomatic patients is limited, although two small single-centre studies including a few hundred patients each and primarily examining the potential determinants of ethnic disparities in cancer outcomes have shown that fatalistic beliefs were associated with advanced stage of diagnosis of breast and lung cancer (11,12). Specifically, an association between endorsing the fatalistic belief ‘if it’s meant to be, I will stay healthy’ and advanced stage at diagnosis was reported in a sample of 540 women with breast cancer (11). Further, in 357 patients with lung cancer; there was an independent association between one of three fatalism items (‘bad things are meant to be’) and advanced stage at diagnosis (12). It is therefore important to extend the focus of prior inquiries about the potential influence of fatalism on stage at diagnosis to larger and more representative samples of patients and encompass cancers other than those previously studied.
Against this background, we hypothesised that cancer patients with higher scores on measures of fatalistic beliefs will be more likely to be diagnosed with cancer at a more advanced stage. We have subsequently examined this hypothesis association in a large population-based survey of newly-diagnosed patients with lung and colorectal cancer.
MATERIALS AND METHODS
Data
Study design and participants
The CanCORS (Cancer Care Outcomes Research and Surveillance) study prospectively enrolled U.S. adults diagnosed with lung or colorectal cancer in 2003-2005 who lived in certain geographic areas (northern California; Los Angeles County, California; North Carolina; Iowa; or Alabama) or received care in one of five health maintenance organizations (HMOs) or fifteen Veterans Affairs medical centers (13,14). Participants were representative of U.S. patients with these cancers (15). Patients were identified within weeks of diagnosis and interviews (with the patient or a surrogate –if the patient was deceased or too ill to participate) conducted approximately 4-6 months after diagnosis. Interviews were conducted by telephone; trained interviewers used computer-assisted telephone interviewing software to navigate complex skip patterns. The study was approved by human subjects committees at all participating institutions.
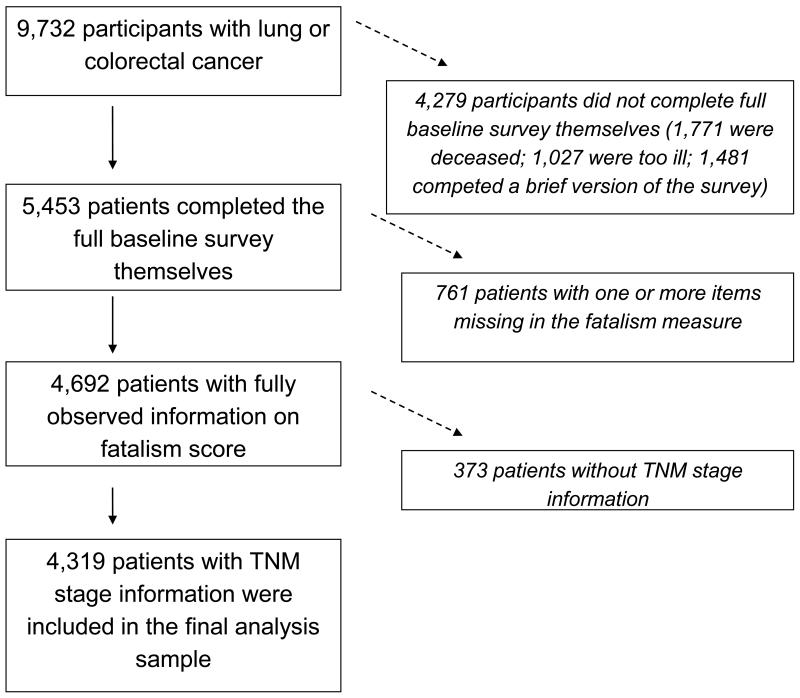
Among 9,732 CanCORS participants, we studied the 5,453 for whom interviews were conducted with the patients themselves. We then excluded 761 patients who did not respond to all four fatalism items and 373 patients for whom data on stage at diagnosis were not available, resulting in an analysis sample of 4,319 patients (Figure 1).
Figure 1. Analysis sample derivation.
Data items
A four-item measure assessing general fatalism that had been developed for the Americans’ Changing Lives Panel Study (House JS, http://sodapop.pop.psu.edu/codebooks/hwboa/acl.pdf) and reported by Jacobson was included in the questionnaire for patients who completed the survey themselves (16). Patients were asked how strongly they agreed or disagreed with the following four statements:
When bad things happen, we are not supposed to know why, we are just supposed to accept them.
People die when it is their time to die and nothing can change that.
Everything that happens is a part of God’s plan.
If bad things happen, it is because they were meant to be.
Responses were on a 4-point Likert scale (strongly agree/agree/disagree/strongly disagree; coded as 4, 3, 2, and 1). Jacobson reported results of a factor analysis showing that the four items formed a single scale with high internal reliability (Alpha=0.77) (16), whereas in our analysis sample we have observed a slightly higher value (Alpha=0.79).
Trained abstractors documented stage at diagnosis from medical records based on the criteria of the American Joint Committee on Cancer, 6th edition. Medical records were available for 87% of patients; for other patients, stage data were obtained from cancer registries. Information was also available on cancer site (colorectal or lung), age, sex, race/ethnicity, education, income, marital status, smoking status, count of self-reported co-morbid conditions using the Katz et al. questionnaire (17), self-reported health status a year before the interview (0-100 on a visual analogue scale, 100=perfect health), symptom status at diagnosis (yes/no), and CanCORS study site. Variables were categorized as in Table 1.
Table 1. Distribution of the fatalism scores (n=4,319 patients who completed a post-diagnosis interview themselves, with fully observed information on fatalism score and stage at diagnosis).
| Patient characteristic | N | Median | Mean | P | |
|---|---|---|---|---|---|
| Sex * | Men | 2372 | 11 | 10.5 | <0.001 |
| Women | 1947 | 11 | 10.9 | ||
|
| |||||
| Age at diagnosis * | <55 | 950 | 11 | 10.8 | <0.001 |
| 55-59 | 553 | 10 | 10.4 | ||
| 60-64 | 604 | 11 | 10.4 | ||
| 65-69 | 671 | 11 | 10.7 | ||
| 70-74 | 588 | 11 | 10.7 | ||
| 75-80 | 525 | 11 | 10.9 | ||
| 80+ | 428 | 11 | 10.9 | ||
|
| |||||
| Race/ethnicity | White | 3115 | 11 | 10.5 | <0.001 |
| Hispanic | 310 | 12 | 11.3 | ||
| Black | 577 | 11 | 11.3 | ||
| Asian | 200 | 11 | 10.9 | ||
| Other | 117 | 11 | 11.3 | ||
|
| |||||
| Educational attainment | <High school | 730 | 12 | 11.8 | <0.001 |
| High school/some college | 2514 | 11 | 10.9 | ||
| ≥College degree | 1070 | 9 | 9.5 | ||
| Missing | 5 | 12 | 12.4 | ||
|
| |||||
| Income in past year (US $) | <20,000 | 1181 | 12 | 11.3 | <0.001 |
| 20,000 - 39,999 | 1133 | 11 | 10.8 | ||
| 40,000 - 59,999 | 677 | 10 | 10.4 | ||
| ≥60,000 | 1000 | 10 | 9.8 | ||
| Missing | 328 | 12 | 11.2 | ||
|
| |||||
| Study site | 5 Integrated health-care delivery systems | 606 | 10 | 10.3 | <0.001 |
| 8 counties in Northern California | 905 | 11 | 10.4 | ||
| Los Angeles County | 962 | 11 | 10.5 | ||
| State of Alabama | 486 | 12 | 11.4 | ||
| 22 counties in North Carolina | 446 | 11 | 11.1 | ||
| State of Iowa | 409 | 11 | 11.0 | ||
| 15 Veterans Affairs Medical Centers | 505 | 11 | 10.9 | ||
|
| |||||
| Married/living with partner | Yes | 2686 | 11 | 10.6 | 0.01 |
| No | 1630 | 11 | 10.8 | ||
| Missing | 3 | 9 | 9.7 | ||
|
| |||||
| Smoking status | Never smoker | 1242 | 11 | 10.8 | <0.001 |
| Former smoker | 2583 | 11 | 10.6 | ||
| Current smoker | 487 | 11 | 11.0 | ||
| Missing | 7 | 11 | 11.1 | ||
|
| |||||
| Co-morbidities (count of) | 0** | 1724 | 11 | 10.7 | 0.24 |
| 1 | 1441 | 11 | 10.6 | ||
| 2 | 718 | 11 | 10.8 | ||
| ≥3 | 436 | 11 | 10.9 | ||
|
| |||||
| Symptom status at diagnosis | ‘Yes, symptomatic’ | 3541 | 11 | 10.7 | 0.23 |
| ‘No, diagnosed without symptoms’ | 777 | 11 | 10.6 | ||
| Missing | 1 | 12 | 12.0 | ||
|
| |||||
| Cancer site * | Colorectal | 2396 | 11 | 10.6 | 0.06 |
| Lung | 1923 | 11 | 10.8 | ||
|
| |||||
| Stage at diagnosis * | I | 1266 | 11 | 10.7 | 0.007 |
| II | 849 | 11 | 10.5 | ||
| III | 1287 | 11 | 10.6 | ||
| IV | 917 | 11 | 10.9 | ||
|
| |||||
| Fatalism scores * | 4 | 98 | |||
| 5 | 47 | ||||
| 6 | 108 | ||||
| 7 | 151 | ||||
| 8 | 443 | ||||
| 9 | 505 | ||||
| 10 | 544 | ||||
| 11 | 664 | ||||
| 12 | 923 | ||||
| 13 | 322 | ||||
| 14 | 179 | ||||
| 15 | 133 | ||||
| 16 | 202 | ||||
|
| |||||
| Health status a year before interview | Median 90 (inter-quartile range 75-100) | 4,286 | |||
|
| |||||
| Total | 4,319 | ||||
Information on these variables was fully observed (no ‘missing’ values) in the analysis sample
Included missing observations
Statistical analysis
We compared patients included in the analysis with those excluded (because of not completing an interview themselves, missing responses to all four fatalism items or missing stage at diagnosis, see Figure 1) using logistic regression. After examining Spearman’s rank correlation coefficients for pair-wise associations between the four fatalism items, to maximize power, replies to each of the four items were summed and the total score was used in the main analysis; higher scores reflected higher levels of fatalistic beliefs. We categorized advanced stage at diagnosis as stage IV (vs. I-III) and examined crude and adjusted associations between advanced stage at diagnosis and fatalism using logistic regression. We initially tested whether there was evidence that the association between fatalism and stage at diagnosis varied by cancer site by an interaction term fatalism*cancer site, but found no such evidence (p=0.40). We therefore included all patients in the same model, adjusting for cancer site, and sex, age, race/ethnicity, education, income, marital status, smoking status, number of co-morbidities, health status a year before interview, and study site. Subsequently, to aid interpretation, we predicted the proportion of patients diagnosed in stage IV for each score of the fatalism scale, by direct standardisation using the regression model (18). The fully observed dataset was used for sample descriptions (Table 1) and 5 imputed datasets, produced through multiple imputation as previously described (19), were used in the logistic regression models, and outputs were combined with the SAS MIANALYZE procedure. In the present analysis imputed data only relate to the small proportion of records with missing income (8%) and education, marital and smoking status information (<1% for all three), as all other variables were complete in the analysis sample (a priori restricted to patients with fully observed fatalism and stage at diagnosis, Text Box).
Sensitivity and supplementary analyses
In sensitivity analyses we examined alternative parameterizations of stage at diagnosis and fatalism score, i.e. by categorising advanced stage at diagnosis as stage III/IV vs. I/II; by analysing scores for each fatalism item individually; and by excluding the religious beliefs (‘God’s plan’) item from the total fatalism score. In supplementary analysis we also examined the potential moderating effect of symptom status at diagnosis by repeating the main analysis model, also adjusting for symptom presence at the time of diagnosis (yes/no) and an interaction between symptom presence and fatalism score. Lastly, in a subgroup of patients with known grade and/or lung cancer tumour type (non-small cell / small cell) we have additionally adjusted for tumour grade and/or type.
RESULTS
Sample description
Compared with the final sample (Figure 1), excluded patients (who were predominantly deceased, or too ill to participate at the time of the baseline survey and thus were not asked the fatalism items) were more likely to have had lung than colorectal cancer and to be male, older, Asian and smokers, and with lower educational attainment and income, higher count of comorbidities and more advanced stage at diagnosis (p<0.01 for all, Supplementary Table 1). Among patients included in the analysis the median age was 65 (inter-quartile range: 56-73) and there was a slight preponderance of colorectal cancer and male patients (56% and 55% of the sample, respectively). Just under three quarters of patients where White (72%) and 7%, 13% and 5% were Hispanic, Black and Asian, respectively. Most patients (82%) had symptoms at the time of diagnosis. Further sample details are provided in Table 1.
Stage at diagnosis
Among all patients 1,266 (29%), 849 (20%), 1287 (30%), and 917 (21%) patients were diagnosed in stages I to IV, respectively. The proportion diagnosed with stage IV cancers was greater among patients with lung than colorectal cancer (28% vs 16%, p<0.001).
Fatalism
The mean fatalism score was 10.7 (median score: 11, inter-quartile range: 9-12). Responses to the four individual items were moderately correlated (pair-wise Spearman rank correlation coefficients range: 0.34 to 0.55, p<0.001 for all). There were differences in fatalism scores by race/ethnicity, with mean scores of 10.5 for White, and 11.3, 11.3 and 10.9 for Hispanic, Black and Asian patients, respectively (p<0.001). On average, women had higher mean fatalism scores than men (10.9 vs 10.5, p<0.001) and the same was true for current smokers compared with former smokers and non-smokers (11.0, 10.6, 10.8 respectively, p<0.001). Patients with lower educational attainment had higher fatalism scores than patients with higher education (11.8, 10.9 and 9.5 respectively for non-high school graduates, high school graduates and college graduates, p<0.001). Patients with lower income also had higher fatalism scores than patients with higher income (11.3 and 9.8 respectively for patients with annual income <$20,000 and >60,000, p<0.001). There were no notable associations of fatalism with count of comorbid conditions, symptomatic detection status, or cancer site.
Crude associations
In crude analysis (logistic regression), higher fatalism scores were associated with advanced stage at diagnosis (odds ratio per unit increase in fatalism=1.05, 95% confidence interval (CI): 1.02-1.08, p=0.001). Other factors associated with stage IV diagnosis included lung cancer, younger age, lower count of co-morbidities, being a current smoker, and symptomatic detection (p<0.01 for all, Table 2).
Table 2. Factors associated with diagnosis of cancer at stage IV*.
| Variable | Stage IV (%) | Crude odds ratios for stage IV | 95% confidence intervals | p* | Adjusted odds ratios for stage IV | 95% confidence intervals | p* | |
|---|---|---|---|---|---|---|---|---|
| Fatalism (per one unit increase 4-16 scale) | 1.05 | 1.02-1.08 | 0.001 | 1.05 | 1.02-1.08 | 0.003 | ||
|
| ||||||||
| Sex | Men | 22.6 | - | 0.015 | - | <0.001 | ||
| Women | 19.6 | 0.83 | 0.72-0.97 | 0.73 | 0.61-0.86 | |||
|
| ||||||||
| Age at diagnosis | <55 | 24.6 | - | <0.001 | - | <0.001 | ||
| 55-59 | 24.1 | 0.97 | 0.76-1.24 | 0.93 | 0.72-1.20 | |||
| 60-64 | 23.7 | 0.95 | 0.75-1.21 | 0.88 | 0.69-1.14 | |||
| 65-69 | 20.0 | 0.76 | 0.60-0.97 | 0.72 | 0.56-0.93 | |||
| 70-74 | 21.8 | 0.85 | 0.67-1.09 | 0.83 | 0.63-1.08 | |||
| 75-80 | 16.8 | 0.62 | 0.47-0.81 | 0.60 | 0.44-0.80 | |||
| 80 or more | 13.3 | 0.47 | 0.34-0.64 | 0.48 | 0.34-0.67 | |||
|
| ||||||||
| Race/ethnicity | White | 20.3 | - | 0.204 | - | 0.87 | ||
| Hispanic | 23.6 | 1.20 | 0.91-1.59 | 1.18 | 0.86-1.62 | |||
| Black | 23.5 | 1.20 | 0.97-1.48 | 1.03 | 0.82-1.30 | |||
| Asian | 24.5 | 1.27 | 0.91-1.78 | 1.11 | 0.77-1.60 | |||
| Other | 23.9 | 1.24 | 0.80-1.91 | 1.09 | 0.70-1.72 | |||
|
| ||||||||
| Education | <High school | 20.4 | - | 0.019 | - | 0.099 | ||
| High school/some college | 22.6 | 1.14 | 0.93-1.40 | 1.23 | 0.98-1.53 | |||
| ≥College degree | 18.5 | 0.89 | 0.70-1.13 | 1.06 | 0.80-1.41 | |||
|
| ||||||||
| Income (US $) | <20,000 | 23.0 | - | 0.50 | - | 0.82 | ||
| 20,000 - 39,999 | 21.9 | 0.92 | 0.75-1.12 | 0.97 | 0.78-1.20 | |||
| 40,000 - 59,999 | 20.4 | 0.86 | 0.69-1.08 | 0.89 | 0.68-1.15 | |||
| ≥60,000 | 20.3 | 0.88 | 0.71-1.07 | 0.91 | 0.70-1.19 | |||
|
| ||||||||
| Study site | 5 Integrated health-care delivery systems | 20.1 | - | 0.001 | - | 0.134 | ||
| 8 counties in Northern California | 23.1 | 1.19 | 0.93-1.53 | 1.20 | 0.93-1.56 | |||
| Los Angeles County | 20.6 | 1.03 | 0.80-1.32 | 0.97 | 0.74-1.26 | |||
| State of Alabama | 23.3 | 1.20 | 0.90-1.61 | 1.11 | 0.82-1.51 | |||
| 22 counties in North Carolina | 13.5 | 0.62 | 0.44-0.86 | 0.85 | 0.59-1.21 | |||
| State of Iowa | 23.2 | 1.20 | 0.89-1.63 | 0.82 | 0.59-1.13 | |||
| 15 Veterans Affairs Medical Centers | 23.8 | 1.24 | 0.93-1.65 | 1.00 | 0.73-1.37 | |||
|
| ||||||||
| Married/living with partner | Yes | 20.7 | - | 0.28 | - | 0.134 | ||
| No | 22.1 | 1.09 | 0.94-1.26 | 1.13 | 0.95-1.34 | |||
|
| ||||||||
| Smoking status | Never smoker | 18.8 | - | <0.001 | - | 0.109 | ||
| Ex-smoker | 21.2 | 1.16 | 0.98-1.37 | 0.89 | 0.73-1.09 | |||
| Current smoker | 27.5 | 1.63 | 1.28-2.09 | 1.13 | 0.85-1.49 | |||
|
| ||||||||
| Co-morbidities | 0 | 23.9 | - | 0.004 | - | 0.003 | ||
| 1 | 20.2 | 0.81 | 0.68-0.97 | 0.78 | 0.65-0.94 | |||
| 2 | 18.8 | 0.74 | 0.59-0.92 | 0.70 | 0.56-0.89 | |||
| ≥3 | 17.9 | 0.69 | 0.53-0.90 | 0.65 | 0.48-0.87 | |||
|
| ||||||||
| Cancer site | Lung | 27.6 | - | <0.001 | - | <0.001 | ||
| Colorectal | 16.2 | 0.51 | 0.44-0.59 | 0.44 | 0.36-0.52 | |||
|
| ||||||||
| Symptoms at diagnosis | Yes | 23.4 | 2.33 | 1.85-2.94 | <0.001 | |||
| No | 11.6 | - | ||||||
|
| ||||||||
| Health status a year before interview (0-100) | 1.00 | 1.00-1.01 | 0.028 | 1.00 | 1.00-1.01 | 0.223 | ||
From univariable or multivariable regression, as applicable. Multivariable regression models adjusted for all variables in the table except symptom status at diagnosis.
Adjusted associations
Adjusted analysis (logistic regression) revealed a very similar association between fatalism and stage IV diagnosis (odds ratio per unit increase in fatalism=1.05, 95% CI: 1.02-1.08, p=0.003, Table 2). Patients with the highest fatalism score (16) had an odds ratio of 1.77 (95% CI 1.21-2.57) for advanced stage at diagnosis compared with those with the lowest score (4) – equivalent to an absolute adjusted difference of 8.9% in the proportion of patients diagnosed with stage IV cancers (Table 3). Similarly, patients with the highest score (16) had an odds ratio of 1.34 (95% CI 1.10-1.60) for stage IV diagnosis compared with those in mid-scale (10) – equivalent to 4.7% increase in the proportion of patients diagnosed with stage IV cancers. There was also evidence that lung cancer, male sex, younger age, and lower count of co-morbid conditions were associated with higher odds of stage IV diagnosis (p<0.01 for all, Table 2).
Table 3. Proportion of patients diagnosed with stage IV cancers (crude and case-mix adjusted).
| Fatalism score | n | Observed (crude) rate | Adjusted percentage* |
|---|---|---|---|
| 4 | 98 | 18.4% | 16.5% |
| 5 | 47 | 14.9% | 17.2% |
| 6 | 108 | 18.5% | 17.8% |
| 7 | 151 | 17.9% | 18.5% |
| 8 | 443 | 16.7% | 19.2% |
| 9 | 505 | 21.4% | 19.9% |
| 10 | 544 | 23.7% | 20.7% |
| 11 | 664 | 21.8% | 21.4% |
| 12 | 923 | 19.2% | 22.2% |
| 13 | 322 | 27.0% | 23.0% |
| 14 | 179 | 16.2% | 23.8% |
| 15 | 133 | 27.8% | 24.6% |
| 16 | 202 | 29.2% | 25.4% |
Adjusted proportions calculated from the logistic regression model – this is the expected percentage that would have been observed if the case-mix of patients in each fatalism score category was the same as in the total sample
Sensitivity analysis
When we examined the association of fatalism with stage III/IV diagnosis, the direction of association remained but its strength was attenuated, such that there was only weak evidence for an association (OR=1.03, 95% CI: 1.00-1.05, p=0.055, Supplementary Table 2). When running logistic regression models that included each individual fatalism item separately instead of the total score (four separate models) the observed findings were concordant with those of the main analysis, with respective odds ratio >1.0 and some significant associations. Repeating the analysis using a total score but excluding the ‘God’s plan’ individual item also produced concordant results (OR=1.06, 95% CI: 1.02 to 1.10, p=0.004). Similarly, inclusion of symptomatic detection status and an interaction variable symptomatic detection*fatalism score produced concordant findings to those observed in the main analysis with no evidence for an interaction (p=0.68). Adjustment for tumour grade and/or lung cancer type made no material difference to the findings of the main analysis model.
DISCUSSION
In a large population based survey of patients with lung and colorectal cancer, we observed an independent association between higher fatalism scores and advanced stage at diagnosis of lung and colorectal cancer. Sensitivity analyses provided concordant findings.
Although previous research has documented associations between fatalism and lower participation in cancer screening (8-10), evidence linking fatalism with clinical outcomes such as stage at diagnosis is limited. Our inquiry is methodologically similar to two previous studies, and a smaller qualitative study of breast cancer patients (11,12,20), but has a substantially larger and socio-demographically and geographically more diverse sample that includes patients with both lung and colorectal cancer.
Powe and Finnie suggest that the two factors that shape fatalism are angst, defined as the perceived collapse of meaning in the presence of despair about the future, and nihilism, defined as the lived experiences of coping with feelings of meaningless, hopelessness, and despair (5). Accordingly, cancer fatalism takes shape as individuals experience others being diagnosed with cancer at an advanced stage, leading to poor outcomes and death. This can lead to scepticism about the value of cancer screening or prompt symptomatic presentation, leading to prolonged intervals to presentation and increasing the risk of advanced stage at diagnosis (21). In addition, cultural or religious values emphasising acceptance may accentuate fatalistic attitudes among some groups (22). Modifying entrenched fatalistic beliefs is not likely to be easy, but evidence that an understanding of the value of prevention or early diagnosis can be held in parallel with fatalism suggests that exposure to culturally appropriate information on the value of early detection might be beneficial (23). If fatalistic beliefs can be modified, campaigns to promote symptom awareness are also likely to contribute to more rapid symptomatic presentation (24). In a recent development of a new scale, Shen et al. propose that fatalism is a multidimensional construct, encompassing ‘predestination’, ‘luck’ and ‘pessimism’ (25). The Fatalism scale used in the present study more closely resembles the predestination dimension, and it will be important in future work to examine the differential effects of these three elements on screening and help-seeking behaviours.
Strengths of our study include its relatively large and diverse patient population and high data quality, including information on stage at diagnosis abstracted from medical records and the use of validated instruments for assessing fatalism. We were also able to adjust our analysis for a large number of potential confounders, including age, sex, race/ethnicity, education, income, co-morbidity, smoking status and prior health status.
Our study has two principal limitations. First, our analysis included only patients who were alive and able to complete the baseline survey, resulting in relative under-representation of older and more co-morbid patients and those with more advanced stage at diagnosis (Supplementary Table 1). However, sample attrition and non-response patterns are a necessary but not sufficient condition for non-response bias in estimates of associations. After appropriate adjustment for case-mix the effect of such bias in surveys with an appropriately defined sampling frame is small (26,27). Achieving more representative patient samples might be possible if interviews are conducted sooner after diagnosis; however, this is rarely feasible in large population-based studies of cancer patients.
Second, as for any cross-sectional study, the causal direction of the observed association cannot be proven. Fatalism could lead to advanced stage at diagnosis, but it is also possible that advanced stage at diagnosis could lead to higher fatalism scores, although Powe has reported unpublished data indicating that cancer fatalism has declined from pre- to post-diagnosis (28). Nevertheless, a causal association of fatalism with advanced stage at diagnosis is plausible. If people believe that cancer is inevitably fatal, then they might delay or avoid seeking medical help after symptom onset or participation in cancer screening, and thus have a higher risk of advanced cancer at diagnosis. Although we did not observe statistically significant differences in fatalism scores based on presence or absence of symptoms, patients may have differed in how long they waited to seek care after developing symptoms. Other studies of asymptomatic individuals have documented associations between fatalism and both intended help-seeking and participation in cancer screening, further supporting a causal effect of fatalism on the length of the patient interval and stage at diagnosis (6-10). Prospective cohort studies involving fatalism measurement in individuals who are free of cancer at study entry would be ideal, but would require large populations and adequate follow-up to achieve sufficient numbers of cancer cases. Critically, new prospective studies would be associated with ethical challenges (i.e. leaving fatalistic beliefs about cancer curability unchallenged during follow-up could be considered unethical). This may explain why the few previous studies have also measured fatalism after diagnosis (11,12).
When fatalism items form part of surveys of patients after a cancer diagnosis, there may be ethical concerns about use of cancer-specific fatalism items, and this was the rationale for using a general fatalism item in the CanCORS study. A qualitative study confirmed low acceptability of cancer fatalism questions among recently diagnosed breast cancer patients (29). General (as opposed to cancer-specific) fatalism items may have both merits (e.g. less likely to be prone to bias resulting from knowledge of cancer diagnosis) and limitations (it makes the argument for public health interventions aimed at reducing ‘cancer fatalism’ less direct). Subject to research ethics considerations, future surveys of cancer patients may be able to investigate differences in results from use of general and cancer-specific fatalism items.
In conclusion, we identified an association between fatalism and advanced stage at diagnosis for patients with newly-diagnosed lung and colorectal cancer, most of whom had presented with symptoms. The consistency of these findings with those from other qualitative and quantitative studies, the plausibility of the association and its large size suggest that the findings should be considered in the context of public health policy initiatives and education campaigns designed to shorten the patient interval of newly diagnosed cancer cases (30,31). Traditionally such strategies have focused on cognitive aspects (i.e. ‘awareness’ of cancer symptoms) but including ‘anti-fatalism’ components could have a powerful impact. Such approaches may include factual information about the high probability of long-term survival when the diagnosis occurs at a non-advanced stage and the availability of effective treatments that offer good health-related quality of life.
Supplementary Material
Acknowledgements
Financial support: This work of the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA093339, University of North Carolina U01 CA093326) and by a Department of Veteran’s Affairs grant to the Durham VA Medical Center CRS 02-164. Dr. Keating’s effort was also supported by 1R01CA164021-01A1 and K24CA18151 from the NCI. Professor Wardle’s contribution is supported by Cancer Research UK Programme grant C1418/A14134. Dr. Lyratzopoulos is supported by a Post-Doctoral Fellowship award by the (UK’s) National Institute for Health Research (PDF-2011-04-047) 2012-2014 and a Cancer Research UK Clinician Scientist Fellowship award (A18180) from 2015. The views expressed in this publication are those of the authors and not necessarily those of the National Cancer Institute, the Department of Veterans Affairs, the NHS (National Health Service), the National Institute for Health Research (NIHR), the (UK) Department of Health, or any other funder.
Footnotes
Ethics approval: The CanCORS study was approved by human subjects committees at all participating institutions.
Potential conflict of interests: None.
REFERENCES
- 1.Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, et al. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220–6. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyratzopoulos G. Measures and markers of timeliness of diagnosis in symptomatic patients. A conceptual framework and its implications. Cancer Epidemiol. 2014;38:211–3. doi: 10.1016/j.canep.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106:1262–7. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon AE, Forbes LJ, Boniface D, Warburton F, Brain KE, Dessaix A, et al. An international measure of awareness and beliefs about cancer: development and testing of the ABC. BMJ Open. 2012;2:pii, e001758. doi: 10.1136/bmjopen-2012-001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454–65. doi: 10.1097/00002820-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Facione NC, Miaskowski C, Dodd MJ, Paul SM. The self-reported likelihood of patient delay in breast cancer: new thoughts for early detection. Prev Med. 2002;34:397–407. doi: 10.1006/pmed.2001.0998. [DOI] [PubMed] [Google Scholar]
- 7.Beeken RJ, Simon AE, von Wagner C, Whitaker KL, Wardle J. Cancer fatalism: deterring early presentation and increasing social inequalities? Cancer Epidemiol Biomarkers Prev. 2011;20:2127–31. doi: 10.1158/1055-9965.EPI-11-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995;18:385–92. [PubMed] [Google Scholar]
- 9.Lostao L, Joiner TE, Pettit JW, Chorot P, Sandín B. Health beliefs and illness attitudes as predictors of breast cancer screening attendance. Eur J Public Health. 2001;11:274–9. doi: 10.1093/eurpub/11.3.274. [DOI] [PubMed] [Google Scholar]
- 10.Peek ME, Sayad JV, Markwardt R. Fear, fatalism and breast cancer screening in low-income African-American women: the role of clinicians and the health care system. J Gen Intern Med. 2008;23:1847–53. doi: 10.1007/s11606-008-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279:1801–7. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 12.Bergamo C, Lin JJ, Smith C, Lurslurchachai L, Halm EA, Powell CA, et al. Evaluating beliefs associated with late-stage lung cancer presentation in minorities. J Thorac Oncol. 2013;8:12–8. doi: 10.1097/JTO.0b013e3182762ce4. [DOI] [PubMed] [Google Scholar]
- 13.Malin JL, Ko C, Ayanian JZ, Harrington D, Nerenz DR, Kahn KL, et al. Understanding cancer patients’ experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–48. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 14.Ayanian JZ, Chrischilles EA, Fletcher RH, Fouad MN, Harrington DP, Kahn KL, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–6. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Catalano PJ, Ayanian JZ, Weeks JC, Kahn KL, Landrum MB, Zaslavsky AM, et al. Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the surveillance, epidemiology, and end results program. Med Care. 2013;51:e9–15. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson CK. Denominational and Racial and Ethnic Differences in Fatalism. Rev Relig Research. 1999;41(1):9–20. [Google Scholar]
- 17.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Little RJ. Direct standardization: a tool for teaching linear models for unbalanced data. Am Stat. 1982;36:38–43. [Google Scholar]
- 19.He Y, Zaslavsky AM, Landrum MB, Harrington DP, Catalano P. Multiple imputation in a large-scale complex survey: a practical guide. Stat Methods Med Res. 2010;19:653–70. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed IE, Skeel Williams K, Tamburrino M, Wryobeck J, Carter S. Understanding locally advanced breast cancer: what influences a woman’s decision to delay treatment? Prev Med. 2005;41:399–405. doi: 10.1016/j.ypmed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Cohen M. Cancer Fatalism. In: Carr BI, Steel J, editors. Attitudes Toward Screening and Care. 1st ed Psychological Aspects of Cancer; Springer US: 2013. [Google Scholar]
- 22.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 23.Keeley B, Wright L, Condit CM. Functions of health fatalism: fatalistic talk as face saving, uncertainty management, stress relief and sense making. Sociol Health Illn. 2009;31:734–47. doi: 10.1111/j.1467-9566.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 24.Quaife SL, Forbes LJ, Ramirez AJ, Brain KE, Donnelly C, Simon AE, et al. Recognition of cancer warning signs and anticipated delay in help-seeking in a population sample of adults in the UK. Br J Cancer. 2014;110:12–8. doi: 10.1038/bjc.2013.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen L, Condit CM, Wright L. The psychometric property and validation of a fatalism scale. Psychol Health. 2009;24:597–613. doi: 10.1080/08870440801902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groves R. Nonresponse rates and nonresponse bias in household surveys. Public Opin Q. 2006;70:646–75. [Google Scholar]
- 27.Groves R, Peytcheva E. The impact of nonresponse rates on nonresponse bias: a meta-analysis. Public Opin Q. 2008;72:167–89. [Google Scholar]
- 28.Powe BD. Do recently diagnosed black breast cancer patients find questions about cancer fatalism acceptable? A preliminary report. J Cancer Educ. 2011;26:3–4. doi: 10.1007/s13187-010-0165-z. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard VB, Davis K, Boisvert M, Jennings Y, Montalvo B. Do recently diagnosed black breast cancer patients find questions about cancer fatalism acceptable? A preliminary report. J Cancer Educ. 2011;26:5–10. doi: 10.1007/s13187-010-0134-6. [DOI] [PubMed] [Google Scholar]
- 30.NHS England Knowing the signs of cancer could save your life. Be Clear on Cancer. http://www.nhs.uk/be-clear-on-cancer/
- 31.American Cancer Society Signs and Symptoms of Cancer. http://www.cancer.org/cancer/cancerbasics/signs-and-symptoms-of-cancer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.