Abstract
Background
Radiation therapy for head, neck, and esophageal cancer can result in esophageal strictures that may be difficult to manage. Radiation-induced esophageal strictures often require repeat dilation to obtain relief of dysphagia. This study aimed to determine the long-term clinical success and rates of recurrent and refractory stenosis in patients with radiation-induced strictures undergoing dilation.
Methods
Retrospective cohort study of patients with radiation-induced strictures who underwent endoscopic dilation by a single provider from October 2007– October 2012. Outcomes measured included long-term clinical efficacy, interval between sessions, number of dilations, and proportion of radiation strictures that were recurrent or refractory. Risk factors for refractory strictures were assessed.
Results
63 patients underwent 303 dilations. All presented with a stricture > 30 days after last radiation session. Clinical success to target diameter was achieved in 52 patients (83%). A mean of 3.3 (+/− 2.6) dilations over a median period of 4 weeks was needed to achieve initial patency. Recurrence occurred in 17 (33%) at a median of 22 weeks. Twenty-seven strictures (43%) were refractory to dilation therapy. Fluoroscopy during dilation (OR, 22.88; 95% CI, 3.19 – 164.07), severe esophageal stenosis (lumen <9 mm) (OR, 10.51; 95% CI, 1.94 – 56.88), and proximal location with prior malignancy extrinsic to the lumen (OR, 6.96; 95% CI, 1.33 – 36.29) were independent predictors of refractory strictures in multivariate analysis.
Conclusions
1. Radiation-induced strictures have a delayed onset (>30 days) from time of radiation injury. 2. Endoscopic dilation can achieve medium-term luminal remediation but the strictures have a high long-term recurrence rate of up to 33%. 3. Remediation of radiation strictures following laryngectomy can be achieved but require frequent dilations. 4. Clinical and procedural predictors may identify patients at high risk of refractory strictures. 5. The optimal strategy in highly selected refractory patients is not clear.
Keywords: esophageal stricture, recurrent, refractory, endoscopic dilation, radiation, risk factors
INTRODUCTION
External beam radiation is a common adjuvant therapy for esophageal, head and neck, and thoracic (breast and lung) malignancies. Dysphagia is commonly encountered after radiotherapy, and although enteral access feeding can typically preserve nutrition, dysphagia significantly decreases quality of life.1,2 The esophagus can be particularly sensitive to radiotherapy, and thus susceptible to injury that can lead to stenosis following treatment for tumors within or near to the esophagus.3,4 A recent meta-analysis had a stricture incidence as high as 16.7% in patients treated with intensity-modulated radiation therapy and chemotherapy for head and neck cancer (HNC).5
Dilation therapy has been the mainstay of treatment for these benign strictures, but due to anatomic distortion, non-surgical endoscopic management can often be technically challenging.6 Most patients have only partial occlusion that is amenable to anterograde endoscopic dilation with either wire-guided rigid polyvinyl dilators (e.g. Savary-Galliard) or through-the-scope balloon dilators with or without guidewire assistance.7 Fluoroscopic guidance can be utilized for complex strictures both to monitor guidewire and dilator passage as well as to control and assess the device during dilation. In patients with tight stenosis that does not permit guidewire passage, retrograde endoscopic dilation via gastric access can be used. Complex strictures, such as those that are longer (>2 cm), angulated, irregular, or have a severely narrowed diameter, may require multiple dilation sessions, and may be simultaneously treated with supplemental therapies, including injection of steroids and temporary endoprosthetic placement.8
Despite these treatment modalities, radiation strictures, along with anastomotic and caustic strictures, remain a common cause of recurrent and refractory strictures in the Western World.9 A recurrent or refractory esophageal stricture has been previously defined as an anatomic restriction secondary to cicatricial luminal compromise or fibrosis that result in dysphagia without endoscopic evidence of inflammation. If the stricture cannot be successfully remediated to a diameter of 14 mm over 5 sessions at 2-week intervals it is considered refractory; if one cannot maintain a satisfactory luminal diameter for 4 weeks once 14 mm has been achieved it is considered recurrent.10
We set out to analyze the prevalence of recurrent and refractory strictures for radiation-induced esophageal stenosis referred to a single provider at a tertiary care center. Our secondary aims were to evaluate the safety, initial efficacy to achieve remediation, and identify factors associated with lack of durable response to endoscopic dilation.
METHODS
A retrospective analysis was performed on patients that underwent endoscopic dilation for esophageal stenosis at the University of Pennsylvania Health System by a single provider (MLK) from October 2007 to October 2012. Patients were identified through a prospective database kept by the provider. The study population consisted of patients who had primary tumors of the head and neck, esophagus, breast, and lung. Patients underwent dilation after primary chemoradiotherapy (CRT) or radiotherapy (XRT), or primary surgery with adjuvant CRT/XRT. Only patients who had prior radiotherapy, clinical dysphagia (as defined by patient reporting difficulty swallowing both solids and liquids), and at least one month of follow-up after dilation in the electronic medical record were included in the study. Several patients with an anastomosis following treatment of head and neck cancer were included for analysis. Patients who had undergone a prior esophagectomy for primary esophageal cancer were excluded to avoid confounding of the primary and secondary outcomes (recurrence/refractory/dilation efficacy for radiation-related strictures). The protocol was approved by the institutional review board.
Dilation Procedures
Patients with partial stenosis underwent anterograde dilation (Figure 1). If the patient had a complete stenosis, i.e. a guidewire was unable to be passed through the residual lumen or a residual lumen was not visualizable either via endoscope or fluoroscopy, retrograde dilation was performed as previously described by Lew et al.8 Dilation technique was at the discretion of the operator after estimating the initial diameter of the stenosis and using an appropriate dilator sized to apply moderate resistance to the stenosis. Dilations following intervals of 2 to 4 weeks were performed until the stricture was remediated to a diameter of 12 to 14 mm, and repeat dilations were performed based on clinical symptoms of dysphagia.10 Patients underwent serial dilation until successful stricture remediation was achieved, or re-intervention if they developed recurrent dysphagia following initial remediation. Further descriptions of our standard approach to alleviating complex benign strictures, dilation techniques using Savary or balloon dilators, and details on adjunctive therapies have been described previously.9,11
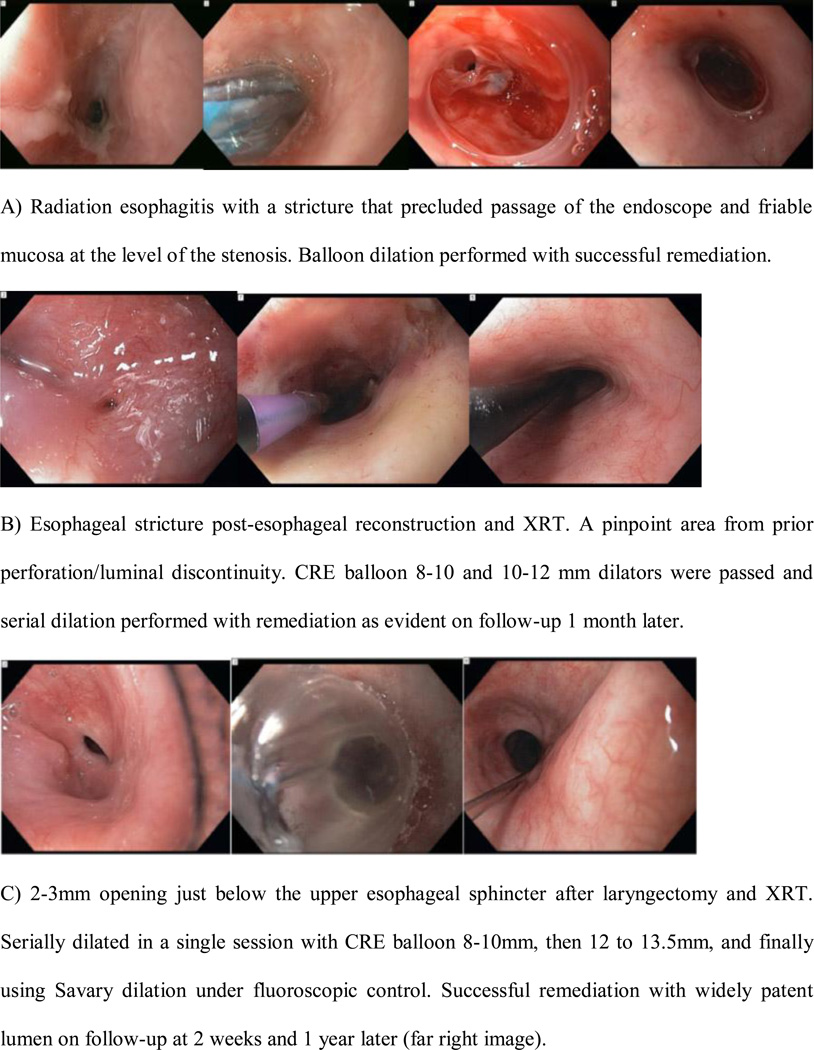
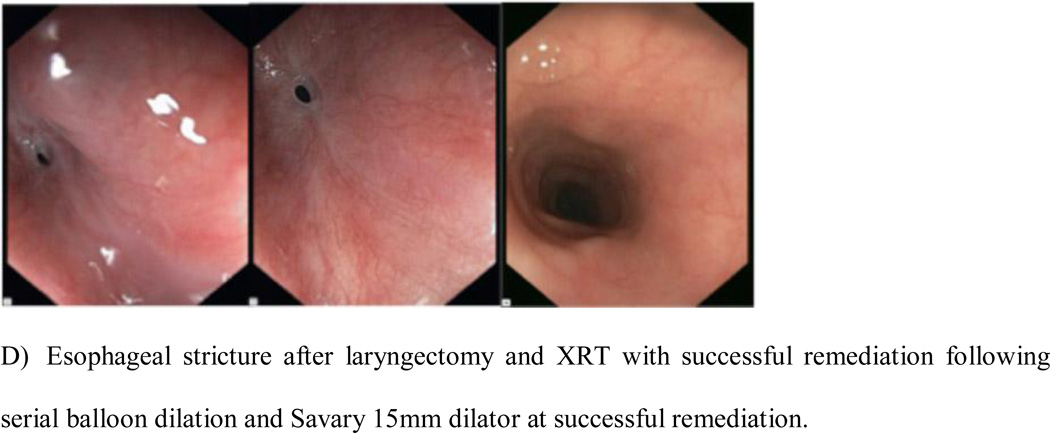
Figure 1.
Radiation-induced strictures remediated.
Stricture Characteristics
Stricture lumen was evaluated based on procedure reports, as were any intraprocedural complications and supplemental therapies such as steroid injection. Technical success was defined as the ability of the endoscopist to traverse the stricture with the chosen dilator and subsequent completion of dilation (effectively increasing luminal diameter by at least 3 mm). Clinical success (resolution of dysphagia) and post-procedure complications were identified using available electronic medical records. A stricture was considered refractory if luminal patency greater than 14 mm could not be achieved after at least 5 dilation sessions over a 10-week period. A stricture was considered recurrent if a luminal patency of 14 mm was achieved, but the stricture was then found to be less than 14 mm on repeat endoscopic evaluation for dysphagia within 4 weeks. Patients who required endoprosthesis or operative revision after failure to achieve sustained luminal patency from repeated dilations were considered refractory.
Covariates and Analysis
Baseline patient and stricture characteristics were identified and recorded. Factors included in the analyses were age, sex, race, presence of a surgical anastomosis, exposure to chemotherapy, type of cancer (split into the following categories: esophageal, HNC, other [metastases from breast and lung], both HNC and esophageal cancer, or both HNC and other), time from last radiation dose to first dilation, size of stenosis (<9 mm or >9 mm based on the diameter of the adult upper endoscope [9.8 mm, GIF-H180, Olympus Corporation]), intra- and post-procedural complications (mild self-limited bleeding, hemorrhage requiring intervention or transfusion, aspiration, perforation, and death), and number of dilations to achieve luminal remediation. Results were either expressed as median with interquartile ranges if data did not follow normal distribution or mean with standard deviation if data was normally distributed. Wilcoxon rank-sum tests were used to compare the differences between two groups of a priori interest for non-parametric data. A two-sided P <0.05 was considered statistically significant. Potential risk factors for refractory strictures were assessed by univariate analysis with the chi-square statistic in the case of categorical variables and simple logistic regression in the case of continuous variables. Significant predictors (P <0.20) in the univariate analysis were then included in a backward, stepwise elimination multiple-regression model to identify the most important risk factors for a stricture to be refractory to endoscopic dilation. Variables that no longer had a P <0.20 in the regression model were excluded to make the model parsimonious. Patients for whom relevant data were missing were excluded from the multivariate analyses. Data analysis was performed using STATA version 12.1 (Stata Corp, College Station, TX).
RESULTS
Patient Characteristics
63 patients met criteria for inclusion in the study, and underwent a total of 303 dilations for remediation (Table 1). Eleven patients had only one month of follow-up following initial dilation. Of the 52 patients with greater than one month of follow-up, the median follow-up was 7 months (interquartile range, IQR 2, 31.5). The overall median follow-up (including those with only one month of follow-up) was 4 months (IQR 1, 21).
Table 1.
Patient Characteristics.
| Characteristics | |
|---|---|
| Total Number of Patients | 63 |
| Follow-up, median in months (IQR) | 4 (1, 21) |
| Sex, no. (%) | |
| Male | 29 (46%) |
| Female | 34 (54%) |
| Age, yr., median (range) | 63 (20–86) |
| Primary tumor site, no. (%) | |
| Esophageal (distal esophagus) | 12 (19%) |
| Head and Neck (HNC) (proximal) | 32 (51%) |
| Other (including breast and lung) (mid- to distal) | 12 (19%) |
| Both Esophageal and HNC (diffuse- proximal and distal) | 3 (5%) |
| Both HNC and other (diffuse) | 4 (6%) |
| Treatment, no. (%) | |
| Received XRT | 63 (100%) |
| Received Chemotherapy | 42 (67%) |
| Received Surgery- Laryngectomy#, no. (%*) | 14 (22%) |
| Time to 1st dilation§, median (IQR) in months | 14 (6, 47.5) |
| Time to 1st dilation§, in pts. with no surgery, median (IQR) in mo. | 20 (6, 71) |
| Size of stenosis at first dilation, no. (%) | |
| <9 mm | 25 (40%) |
| >9 mm | 38 (60%) |
Of those receiving surgery
Stenosis in setting of radiation-induced injury and concurrent proximal anastomosis
From date of last radiation treatment to first dilation
Half of the patients had radiation therapy for a primary head and neck cancer. Seven patients had more than one primary cancer yet required radiotherapy that included the esophagus as part of the XRT window. Almost one-fourth of patients included were treated with surgical laryngectomy prior to XRT. Two-thirds of all patients received neoadjuvant chemotherapy in addition to XRT. The median time from last date of XRT to first dilation was 14 months (IQR 6, 47.5). In patients with no prior surgery, the time from the last date of XRT to first dilation was 20 months (IQR 6, 71).
Dilation Techniques
Patients were dilated using Savary dilators (9 patients), through-the-scope balloons (37 patients), or both (17 patients) (Table 2). Patients required a median of 3 dilations before achieving clinical success, with an interquartile range of 2 to 8. Intralesional steroids were injected in 5 patients, two of whom had a prior anastomosis. An adult upper endoscope was unable to pass in all patients with a lumen diameter less than 9 mm (25 patients), and retrograde dilation was performed in 9 of these patients to achieve remediation. All others received anterograde dilation using the combination of ERCP catheters and guidewires, fluoroscopy, and pediatric or ultra-thin endoscopes.
Table 2.
Dilations
| Factors | |
|---|---|
| Total Dilations, no. | 303 |
| Dilations per patient, median (IQR) | 3 (2, 8) |
| Types of dilators, no. (%) | |
| Savary | 9 (14%) |
| TTS Balloon* | 37 (59%) |
| Both | 17 (27%) |
| Fluoroscopy utilized, no. (%) | 48 (76%) |
| Intralesional Steroids, no. (%) | 5 (8%) |
| Retrograde Dilation, no. (% of patients) | 9 (14%) |
TTS = Through-the-scope
Clinical Outcomes
Technical success was defined as achieving luminal patency after dilation was attempted, and accomplished in all patients (Table 3). Clinical success was defined by endoscopic dilation up to 14 mm and subsequent relief of dysphagia, and was achieved in 52 patients (83%). A mean of 3.3 (+/− 2.6) dilations over a median period of 4 (IQR 0,12) weeks was needed to achieve patency during the initial treatment phase.
Table 3.
Clinical Outcomes
| Factors | number (% or std dev) |
|---|---|
| Technical Successi | 63 (100%) |
| Clinical Successii | 52 (83%) |
| Dilations to achieve initial patencyiii, mean | 3.3 (std dev 2.6) |
| Weeks to achieve initial patencyiii, median (IQR) | 4 weeks (0,12) |
| Complications | 3 (4.8%)v |
| Recurrenceiv | 17 (33%) |
| Time to recurrence, median (IQR) | 22 weeks (17.3, 49.3) |
| Dilations to reestablish patency, mean | 2 (std dev 1.9) |
Stricture was able to be dilated by at least 3 mm during initial procedure
Dilation to 14 mm and relief of dysphagia maintained for at least 4 weeks
Patency defined as 14 mm, before any recurrence
Need for delayed repeat dilation among those with clinical success
All 3 had self-limited bleeding not requiring transfusion
Complications
Complications occurred in 3 patients (4.8%). All 3 complications were self-limited bleeding near the site of a transesophageal prosthesis (after laryngectomy) that did not require transfusion. There were no perforations. There was a single patient who had a prior operative repair for esophageal rupture preceding dilation therapy; he then underwent a gastrojejunostomy for pyloric stenosis and gastroparesis to effectively reduce his reflux symptoms following multiple uncomplicated dilation sessions for esophageal stricture management.
Recurrence
Among patients with clinical success, recurrence – need for delayed repeat dilation with an inability to maintain luminal patency of 14 mm after successful dilation - occurred in a third (17 patients). Recurrence occurred at a median of 22 weeks (IQR 17.3, 49.3). In patients with recurrent strictures, a mean of 2 (+/− 1.9) dilations was needed to establish patency.
Refractory Strictures
Twenty-seven strictures (43%) were considered refractory (Table 4). Patients with a prior anastomosis had a higher proportion of refractory strictures versus those who had radiation alone (50% vs. 41%, p= 0.4), although this finding was not statistically significant. Overall, patients with a prior anastomosis required more frequent dilations (median 3 vs. 2, p=0.02) over a longer period of time (median 8 weeks vs. 2 weeks, p=0.03) to achieve initial patency compared to patients with radiation alone.
Table 4.
Refractory Strictures
| Characteristics | |||
|---|---|---|---|
| Number of refractory strictures | 27 (43%) | ||
| Anastomosis + XRT (n=14) | XRT alone (n=49) | P-value | |
| Refractory strictures, no. (%) | 7 (50%) | 20 (41%) | 0.4 |
| Dilations to initial patency, median (IQR) | 3 (2,8) | 2 (1,4) | 0.02 |
| Weeks to initial patency, median (IQR) | 8 (4, 19) | 2 (0, 11) | 0.03 |
Intraoperative Steroid Injection
Five patients received endoscopic injection of Kenalog (four 1-mL aliquots of 10 mg/mL triamcinolone acetonide in a four-quadrant pattern, for a total of 40 mg) using a standard sclerotherapy needle. The provider elected to use adjunct steroid therapy for these strictures based on clinical history of these patients who were expected to be more difficult to remediate. When compared to patients who required more than one dilation and did not receive steroids (n=40), the injection of steroids was not associated with fewer dilations (3 dilations vs. 8 dilations, p=0.0008) or treatment length (median 9 weeks vs. 30 weeks, p=0.03) when compared to patients who did not receive injected steroids (Table 5).
Table 5.
Steroid Injections
| Steroid Injection (N=5) |
No steroids (N=40) |
p-value | |
|---|---|---|---|
| Dilations to initial patency, median (IQR) | 8 (6, 8) | 3 (2, 5.5) | <0.001 |
| Weeks to initial patency, median (IQR) | 30 (12, 36) | 9 (2.6, 13) | 0.03 |
Risk Factors for Refractory Stricture
The results of our analysis of risk factors associated with refractory strictures following endoscopic dilation are shown in Table 6. We defined extrinsic malignancy as having a history of a head and neck cancer or metastases from breast or lung cancer in contra-distinction to intrinsic malignancy as a patient with a history of esophageal cancer. Of the 8 hypothesized risk factors, 4 factors remained significant by univariate analysis and 3 remained significant in multivariate analysis (P <0.05). These factors included use of fluoroscopy during the initial dilation (adjusted OR, 22.88; 95% CI, 3.19 – 164.07), severe esophageal stenosis with a luminal diameter <9 mm (adjusted OR, 10.51; 95% CI, 1.94 – 56.88) and radiation-induced strictures from prior head and neck cancer or metastatic disease (adjusted OR, 6.96; 95% CI, 1.33 – 36.29). Female sex was significantly associated with refractory strictures in univariate analysis (OR, 2.9; 95% CI, 1.03 – 8.17) but was not statistically significant in the multivariate model (adjusted OR, 3.76; 95% CI, 0.82 – 17.25). Presence of anastomosis, prior chemotherapy, and age were not found to be significant risk factors associated with radiation-induced refractory strictures.
Table 6.
Risk Factors for Radiation-Induced Refractory Stricture After Dilation in the Univariate and Multivariate Analyses*
| Risk Factor | Unadjusted Odds Ratio (95% CI)¶ |
Univariat e P-Value |
Adjusted Odds Ratio (95% CI)** |
Multi- variate P-Value |
|---|---|---|---|---|
| Significant in univariate and multivariate analyses | ||||
| Female Sex@ | 2.9 (1.03 – 8.17) | 0.04 | 3.76 (0.82 – 17.25) | 0.09 |
| Use of Fluoroscopy | 23.75 (4.74 – 119) | < 0.001 | 22.88 (3.19 – 164.07) | 0.002 |
| Severe Stenosis | 8.75 (2.52 – 30.42) | 0.001 | 10.51 (1.94 – 56.88) | 0.006 |
| History of Extrinsic Cancer (Proximal Location) | 3.9 (1.22 – 12.4) | 0.02 | 6.96 (1.33 – 36.29) | 0.02 |
| Not significant in univariate or multivariate analysis | ||||
| Presence of anastomosis (Head/neck cancer only) | 1.14 (0.40 – 3.30) | 0.80 | ||
| Prior chemotherapy | 0.65 (0.22 – 1.91) | 0.43 | ||
| Middle age (45–64 years old)# | 0.71 (0.1 – 4.93) | 0.73 | ||
| Older Age (>64 years old)# | 1.33 (0.19 – 9.19) | 0.77 |
Only candidate factors with p values below 0.05 in the univariate analysis were included in the multivariate analysis. There were no candidate predictors with missing data on subjects included in this study. Steroid injection only occurred in refractory strictures thus was excluded from analysis. Race was excluded since all subjects but one were Caucasian. CI denotes confidence interval.
Unadjusted odds ratio as compared to respective referent: male, no use of fluoroscopy during procedure, proximal lesion from extrinsic malignancy (head and neck cancer or metastases from breast or lung cancer) as compared to distal lesion from intrinsic malignancy (esophageal cancer), severe stenosis (luminal diameter <9 mm with an inability to pass upper adult endoscope) as compared to diameter >9 mm, no prior resection/anastomosis for head and neck cancer, no exposure to neoadjuvant chemotherapy.
Odds ratio as compared with a patient age 44 or younger.
Odds ratios have been adjusted for the effect of the other variables in the model.
CI was statistically significant in univariate analysis but not in multivariate model.
DISCUSSION
Radiotherapy is integral therapy for esophageal, head and neck, and chest (breast, lung) malignancies. The esophagus may be particularly radiosensitive. Dysphagia can result from a variety of mechanisms including mucositis, radiation dermatitis, xerostomia, and edema in the acute setting, with fibrosis, neuromuscular damage, lymphedema, and mechanical obstruction as late sequelae to radiation injury.12 Resultant dysphagia can be difficult to manage, unlike gastro-esophageal reflux-induced strictures in the PPI era.9 The baseline features of a refractory or recurrent stricture have been previously defined.10 We now set out to establish the prevalence of these strictures within patients with radiation-related esophageal stenosis. A secondary aim was to evaluate the long-term efficacy of endoscopic dilation and factors associated with lack of response to endoscopic therapy.
First, our study validates what has been previously suggested: radiation induced strictures can present several months after the cessation of radiotherapy.9 However, while this delayed phenomenon has been typically described as 4–8 months from the last session of radiotherapy, the patients in our cohort developed dysphagia much later, at a median of a year and a half from their last radiation session and a few developed a symptomatic stenosis after as many as 10 years following radiation. Thus, radiotherapy appears to have clinically significant delayed, long-term side effects to the esophagus within the radiation field.
The clinical and procedural factors that affect the success rate of endoscopic dilation for non-peptic strictures are not well understood. A Turkish group investigated factors affecting endoscopic bougie dilation of radiation strictures, and found that the total prescribed dosage of radiation seems to have minimal effect on the result of dilation.13 The same group found that the time of onset of stenosis since radiation therapy affects the success rate of endoscopic bougie dilation; the later the onset, the higher the success rate.13 We did not find this same association in our study that consisted predominantly of patients who underwent endoscopic balloon dilation as primary therapy; given our long median time to initial dilation following radiation therapy of 14 months (IQR 6, 47.5) and high rate of refractory strictures in our cohort, it is impossible to definitively identify the reason for this discordance. It may be because our cohort excluded patients who underwent previous resection for primary esophageal malignancy (excluding those with a primary esophago-gastric anastomosis from partial or total esophagectomy). Perhaps the discrepancy is accounted for by the inclusion of only five non-responders to dilation in the Turkish study; a sample size that may preclude statistical detection of reported true association.
We believe that balloon dilation has favorable advantages for radiation-related strictures and other stenosis resulting from chronic inflammation. Balloon dilation offers direct visualization and controlled application of expansile radial forces at the level of the stricture. Additionally, any bougie technique may have an inherently higher complication risk due to its method of dilation: the longitudinal forces that impact on the stricture are not as uniformly predictable and ‘hot’ spots potentially lead to perforation within the stricture or just proximal if the longitudinal shear strength of the tissue is less than that of the stricture itself. Studies to date have not shown a clear advantage between these two dilator types, although a prior study is suggestive.14 However most studies did not stratify endpoints based on different etiologies for the benign stricture.15,16,17,18
The literature on the efficacy of intralesional steroids to facilitate remediation of benign esophageal strictures had initially been promising.9,19,20,21,22 Older studies have shown limited value in its use for refractory strictures.23,24 In a recent randomized, controlled trial, the addition of endoscopic injection of corticosteroids to Savary dilation therapy did not decrease the number of repeat dilations needed nor prolong the dysphagia-free intervals in patients with anastomotic esophagogastric strictures.25 Similarly, in our cohort, endoscopically injected steroids did not promote remediation of radiation-induced strictures. All patients who underwent targeted injection of steroids had focal strictures and had prior anastomosis for head and neck cancer. This data is difficult to interpret, however, as the patients who received adjunct intralesional steroid injections were expected to have extremely refractory strictures and would have likely failed to achieve sustained remediation regardless. Ramage et al demonstrated that steroids might be of benefit for peptic strictures in a randomized controlled trial, albeit in a small number of patients.26 Steroids may not effectively resolve radiation-related stenosis once the ischemia and inflammation has resulted in permanent collagen deposition. Clearly, a prospective, randomized controlled trial with an adequate sample size may better address the utility of an adjunctive agent within these complex radiation-induced strictures.
Overall, the complication rate was 4.8%, but nil when excluding patients who had self-limited bleeding who did not require transfusion or intervention. It has been postulated that prior radiation may be associated with increased risk of perforation or clinically significant bleeding in the setting of esophageal stent placement.27,28 We did not observe these same risks for balloon dilation in post-radiation strictures; even the patient with a prior esophageal perforation preceding dilation did not have a complicated post-procedural course despite multiple balloon and bougie dilations. Thus, endoscopic dilation remains a safe alternative for initial radiation associated stricture management, and our findings are consistent with the overall complication incidence of 0.4% we previously reported in a series of 1000 dilation procedures and as published in other respective series.9,29
Fourth, our cohort demonstrated that while dilation can achieve technical and clinical success in most patients with radiation-induced strictures, many of them will require frequent repeat dilations for recurrent symptoms within 6 months following initial remediation. In addition to this high recurrence rate, there was a high prevalence of refractory strictures. The high prevalence of both recurrent and refractory strictures may be due to the following: 1] the mechanism of injury from radiation is from a transmural process and results in tissue fibrosis and irreversible deposition of collagen; 2] the high proportion of patients with near complete obstruction/obliteration of esophageal patency (39.6%); 3] the selection bias inherent from the referral pattern of a tertiary academic center with a provider focused on complex stricture management. This bias likely resulted in patients with more co-morbidities that were not measured as part of this study (as evidenced by the frequency of two primary cancers in each patient [11%]); there may be additional unmeasured clinical factors that affected response to dilation in this cohort.
Lastly, we identified certain patient and intraprocedural characteristics as independent risk factors associated with radiation-induced strictures refractory to endoscopic dilation. Two were clinical predictors, namely female sex and prior malignancy that had resulted in extrinsic compression of the proximal esophageal lumen. Women seemed to have a three-fold increased risk for refractory strictures as compared to men for unclear reasons, although this observation lost statistical significance in the multivariate model. Similarly, radiotherapy for head and neck cancer or metastases from breast or lung cancer had a seven-fold risk as compared to patients who developed radiation strictures as part of therapy for esophageal cancer. One explanation could be that these strictures resulted from radiation injury and extrinsic luminal compression, often in the proximal upper third of the esophagus, thereby leading to more complex, fibrotic, angulated, and potentially multi-focal stenoses, especially for cases in which dilation was performed as palliative therapy for metastatic disease.
Two intraprocedural risk factors were identified. The use of fluoroscopy during the initial dilation session heralded a high likelihood that the stricture would be refractory with an almost 22-fold risk as compared to strictures dilated without fluoroscopic guidance. Fluoroscopy is often reserved for dilation of complex strictures to facilitate guidewire and dilator passage through longer, angulated, and severely narrowed strictures, sometimes with near total obstruction. For this study, the endoscopist’s choice of when to utilize contrast to guide therapy is probably linked to the degree of complexity of the stricture and thus more prone to be applied in patients who will require frequent dilations at short intervals. Furthermore, the use of fluoroscopy was not only a surrogate for procedural difficulty but was often employed for complex non-traversable strictures to ensure procedural safety. Safe passage of dilators or guidewires can be facilitated where endoscopic visualization can be difficult. Proximally located strictures and a severely compromised lumen carry a high risk of refractory stenosis (as identified in our results), and are more challenging to dilate without fluoroscopic guidance. While it seems intuitive that the greater the degree of stenosis, the more likely the stricture may be refractory to dilation therapy, there are no studies to date that have validated this association. Several series including our own have described reestablishing luminal patency using retrograde endoscopy by way of gastrostomy tube tracts or using a combined antegrade and retrograde approach in patients with complete, or near complete, esophageal stenosis.8,30,31,32,33 Our results suggest that the degree of stricture severity confers a higher likelihood of refractoriness. Patients who had near complete stenosis (16 patients), or complete stenosis requiring retrograde luminal reconstitution and dilation (9 patients), were ten times more likely to have a refractory stricture than those with an initial larger diameter (>9 mm) allowing passage of the upper endoscope before dilation.
Several limitations must be taken into account. First, this data may be difficult to generalize to general practice as it was based on experiences from a single expert provider at a single tertiary care center. However, patients with radiation-induced strictures often have multiple co-morbidities and are typically managed under a multidisciplinary team at large referral centers. Furthermore, while the experiences of a single endoscopist may also make the data difficult to generalize, it does have the advantage of confounders related to provider selection or therapeutic algorithms. Second, standardized dysphagia scores were not utilized in this study. Repeat dilation after remediation was based on the persistence or recurrence of solid food dysphagia. Patients with chronic dysphagia can easily accommodate and often underreport symptoms. Many of these patients have other factors that contribute to their dysphagia including radiation exposure resulting in neuromuscular problems and a decrease in salivation that is not accurately reflected with the extent of luminal restoration. Nonetheless, there is no good objective scoring system for chronic dysphagia. Thus, our results are based on the scenario that is encountered in clinical practice and primarily looking at correction of the anatomic problem.
Furthermore, a step-wise approach was undertaken for dilating severe stenosis. While a more aggressive approach to dilation may have resulted in fewer endoscopic sessions to reestablish patency, our approach resulted in minimal adverse events including no perforations. While there is little objective data to support the "rule of three" for esophageal dilation (i.e. no greater than three consecutive dilators passed after moderate resistance is encountered), this rule has been arbitrarily proposed to prevent overaggressive dilation and resultant perforation. Recent evidence suggests that non-adherence to the "rule of three" does not increase the risk of adverse events, however stricture etiology, adjunct therapy, and efficacy outcomes were not evaluated in this study.34 For select patients in our study, dilation above the "rule of three" was performed in the same treatment session but this approach did not seem to result in a lower rate of refractory strictures.
Finally, this was a retrospective cohort study, which carries certain additional limitations. Decisions regarding interventions were made by the provider, including the use of fluoroscopy, use of intralesional steroids, and frequency of dilations. Although a uniform and consistent approach was adopted for endoscopic management of esophageal strictures over the course of the study period, it is difficult to assess the inter-variability of these risk factors among patients retrospectively. Additionally, patients may have been lost to follow-up or sought care at another institution as they were not all prospectively followed longitudinally.
While these are inherent limitations to conclusions drawn from this and other cohort studies, and though the study may be underpowered to achieve precise estimates of effect for significance in our model (i.e. female sex), there are several strengths to our study. First, we report one of the largest studies to date on endoscopic therapy of radiation-induced strictures; our study encompasses the largest number of refractory/recurrent strictures in the literature.33,35,36,37 Second, our selected cohort had comprehensive long-term follow-up data to investigate detailed safety and efficacy factors. Lastly, this is the first study to categorize outcomes of dilation of esophageal stenosis based on standardized definitions for recurrent and refractory strictures and identify risk factors of refractory radiation-related strictures.
Our series demonstrates the following: 1] radiation-induced strictures have a delayed onset (>30 days) from time of radiation injury; 2] endoscopic dilation can achieve medium-term luminal remediation but the strictures have a high long-term recurrence rate of up to 33%; 3] endoscopically injected steroids do not promote remediation of radiation strictures; 4] remediation of radiation strictures following laryngectomy can be achieved but require frequent dilations; 5] certain clinical and procedural predictors may identify patients at high risk of refractory strictures. Operative revision or earlier placement of long-term removable endoprosthesis may be preferred strategies in select patients.38
Acknowledgments
#This work was supported by the NIH/NIDDK T32 DK007740 (Clinical Epidemiology Training in Gastroenterology) and the NIH/NIDDK P30DK050306 Center for Molecular Studies in Digestive and Liver Diseases.
This work was also supported by the Wilmott Center for Endoscopic Innovation, Research, and Training.
Footnotes
Disclosures:
Dr. Kochman is a consultant for Boston Scientific Company, Cook Medical Inc., and Olympus Inc. Drs. Agarwalla, Small, Mendelson, and Scott have no financial interest in any products mentioned in this manuscript.
REFERENCES
- 1.Luarell G, Kraepelien T, Mavroidis P, et al. Stricture of the Proximal Esophagus in Head and Neck Carcinoma Patients after Radiotherapy. Cancer. 2003;97:1693–1700. doi: 10.1002/cncr.11236. [DOI] [PubMed] [Google Scholar]
- 2.De Boer MF, Pruyn JFA, van den Borne HW, et al. Rehabilitation outcomes of long-term survivors treated for head and neck cancer. Head Neck. 1995;17:503–515. doi: 10.1002/hed.2880170608. [DOI] [PubMed] [Google Scholar]
- 3.Roswit B. Complications of radiation therapy: the alimentary tract. Semin Roentgenol. 1974;9:51–63. doi: 10.1016/0037-198x(74)90009-1. [DOI] [PubMed] [Google Scholar]
- 4.Lepke RA, Libshitz HI. Radiation-induced injury of the esophagus. Radiology. 1983;148:375–378. doi: 10.1148/radiology.148.2.6867327. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Goldsmith TA, Holman AS, et al. Pharyngoesophageal stricture after treatment for head and neck cancer. Head & Neck. 2012;34:967–973. doi: 10.1002/hed.21842. [DOI] [PubMed] [Google Scholar]
- 6.de Wijkerslooth LRH, Vleggar FP, Siersema PD. Am J Gastroenterol. 2011;106:2080–2091. doi: 10.1038/ajg.2011.348. [DOI] [PubMed] [Google Scholar]
- 7.Silvain C, Barrioz T, Besson I, et al. Treatment and long- term outcome of chronic radiation esophagitis after radiation therapy for head and neck tumors. A report of 13 cases. Dig Dis Sci. 1993;38:927–931. doi: 10.1007/BF01295922. [DOI] [PubMed] [Google Scholar]
- 8.Lew RJ, Shah JN, Chalian A, et al. Technique of endoscopic retrograde puncture and dilatation of total esophageal stenosis in patients with radiation-induced strictures. Head & Neck. 2004;26:179–183. doi: 10.1002/hed.10365. [DOI] [PubMed] [Google Scholar]
- 9.Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilatation. J Clin Gastroenterol. 2002;35:117–126. doi: 10.1097/00004836-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointest Endosc. 2005;62:474–475. doi: 10.1016/j.gie.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Shah PM, Kochman ML. Alternative techniques for treating benign esophageal strictures. Tech Gastrointest Endosc. 2010;12:225–230. [Google Scholar]
- 12.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;29:35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Tuna Y, Koçak E, Dinçer D, Köklü S. Factors affecting the success of endoscopic bougie dilatation of radiation-induced esophageal stricture. Dig Dis Sci. 2012;57:424–428. doi: 10.1007/s10620-011-1875-8. [DOI] [PubMed] [Google Scholar]
- 14.Lew RJ, Ginsberg GG, Long WB, et al. Esophageal dilation is safe despite increasing complexity of strictures [abstract] Gastrointest Endosc. 2001;53:AB77. [Google Scholar]
- 15.Saeed ZA, Winchester CB, Ferro PS, et al. Prospective randomized comparison of polyvinyl bougies and through-the-scope balloons for dilation of peptic strictures of the esophagus. Gastrointest Endosc. 1995;41:189–195. doi: 10.1016/s0016-5107(95)70336-5. [DOI] [PubMed] [Google Scholar]
- 16.Cox JG, Winter RK, Maslin SC, et al. Balloon or bougie for dilatation of benign oesophageal stricture? An interim report of a randomised controlled trial. Gut. 1988;29:1741–1747. doi: 10.1136/gut.29.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scolapio JS, Pasha TM, Gostout CJ, et al. A randomized prospective study comparing rigid to balloon dilators for benign esophageal strictures and rings. Gastrointest Endosc. 1999;50:13–17. doi: 10.1016/s0016-5107(99)70337-8. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H, Hughes RW, Jr, Schroeder KW, et al. Treatment of benign esophageal stricture by Eder-Puestow or balloon dilators: a comparison between randomized and prospective nonrandomized trials. Mayo Clin Proc. 1992;67:228–236. doi: 10.1016/s0025-6196(12)60097-4. [DOI] [PubMed] [Google Scholar]
- 19.Zein NN, Greseth JM, Perrault J. Endoscopic intralesional steroid injections in the management of refractory esophageal strictures. Gastrointest Endosc. 1995;41:596–598. doi: 10.1016/s0016-5107(95)70198-2. [DOI] [PubMed] [Google Scholar]
- 20.Berenson GA, Wyllie R, Caulfield M, et al. Intralesional steroids in the treatment of refractory esophageal strictures. J Pediatr Gastroenterol Nutr. 1994;18:250–252. doi: 10.1097/00005176-199402000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Kochhar R, Ray JD, Sriram PV, et al. Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49:509–513. doi: 10.1016/s0016-5107(99)70052-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Kubik CM, Polhamus CD, et al. Preliminary experience with endoscopic intralesional steroid injection therapy for refractory upper gastrointestinal strictures. Gastrointest Endosc. 1995;41:598–601. doi: 10.1016/s0016-5107(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 23.Holder TM, Ashcraft KW, Leape L. The treatment of patients with esophageal strictures by local steroid injections. J Pediatr Surg. 1969;4:646–653. doi: 10.1016/0022-3468(69)90492-8. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn HJ, Maloney WH. The treatment of benign strictures of the esophagus with cortisone injection. Ann Otol Rhinol Laryngol. 1970;79:900–904. doi: 10.1177/000348947007900504. [DOI] [PubMed] [Google Scholar]
- 25.Hirdes MM, van Hooft JE, Koornstra JJ, et al. Endoscopic corticosteroid injections do not reduce dysphagia after endoscopic dilation therapy in patients with benign esophagogastric anastomotic strictures. Clin Gastroenterol Hepatol. 2013;11:795–801. doi: 10.1016/j.cgh.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Ramage JI, Jr, Rumalla A, Baron TH, et al. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol. 2005;100:2419–2425. doi: 10.1111/j.1572-0241.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Kozarek R. Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258–273. doi: 10.1038/ajg.2009.684. [DOI] [PubMed] [Google Scholar]
- 28.de Wijkerslooth LR, Vleggaar FP, Siersema PD. Endoscopic management of difficult or recurrent esophageal strictures. Am J Gastroenterol. 2011;106:2080–2091. doi: 10.1038/ajg.2011.348. [DOI] [PubMed] [Google Scholar]
- 29.Kochman ML. Minimization of risks of esophageal dilation. Gastrointest Endosc Clin N Am. 2007;17:47–58. doi: 10.1016/j.giec.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822–828. doi: 10.1016/j.gie.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Steele NP, Tokayer A, Smith RV. Retrograde endoscopic balloon dilation of chemotherapy-and radiation-induced esophageal stenosis under direct visualization. Am J Otolaryngol. 2007;28:98–102. doi: 10.1016/j.amjoto.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Garcia A, Flores RM, Schattner M, et al. Endoscopic retrograde dilation of completely occlusive esophageal strictures. Ann Thorac Surg. 2006;82:1240–1243. doi: 10.1016/j.athoracsur.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 33.Dellon ES, Cullen NR, Madanick RD, et al. Outcomes of a combined antegrade and retrograde approach for dilatation of radiation-induced esophageal strictures (with video) Gastrointest Endosc. 2010;71:1122–1129. doi: 10.1016/j.gie.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 34.Grooteman KV, Vleggaar FP, Siersema PD, Baron TH. Violation of the "rule of three" does not increase the risk of perforation following esophageal dilation. Poster presented at: United European Gastroenterology Week; October 2013; Berlin, Germany. [Google Scholar]
- 35.Hu HT, Shin JH, Kim JH, et al. Fluoroscopically guided balloon dilation for pharyngoesophageal stricture after radiation therapy in patients with head and neck cancer. AJR Am J Roentgenol. 2010;194:1131–1136. doi: 10.2214/AJR.09.3345. [DOI] [PubMed] [Google Scholar]
- 36.Polese L, Angriman I, Bonello E, et al. Endoscopic dilation of benign esophageal strictures in a surgical unit: a report on 95 cases. Surg Laparosc Endosc Percutan Tech. 2007;17:477–481. doi: 10.1097/SLE.0b013e3181514217. [DOI] [PubMed] [Google Scholar]
- 37.Swaroop VS, Desai DC, Mohandas KM, et al. Dilation of esophageal strictures induced by radiation therapy for cancer of the esophagus. Gastrointest Endosc. 1994;40:311–315. doi: 10.1016/s0016-5107(94)70062-1. [DOI] [PubMed] [Google Scholar]
- 38.Kochman ML. Removable endoprosthetics in the management of esophageal pathology: all strictures and fistulae are not created equal. Gastrointest Endosc. 2008;67:26–27. doi: 10.1016/j.gie.2007.08.033. [DOI] [PubMed] [Google Scholar]