Abstract
Nanotechnology is a field of science that is nowadays developing in a dynamic way. It seems to offer almost endless opportunities of contribution to many areas of economy and human activity, in general. Thanks to nanotechnology, the so-called nanomaterials can be designed. They present structurally altered materials, with their physical, chemical and biological properties entirely differing from properties of the same materials manufactured in microtechnology. Nanotechnology creates a unique opportunity to modify the matter at the level of atoms and particles. Therefore, it has become possible to obtain items displaying new, useful properties, i.e. self-disinfecting and self-cleaning surfaces. Those surfaces are usually covered by a thin layer of a photocatalyst. The role of the photocatalyst is most of the time performed by the nanosized titanium dioxide (nano-TiO2). Excitation of nano-TiO2 by ultraviolet radiation initiates advanced oxidation processes and reactions leading to the creation of oxygen vacancies that bind water particles. As a result, photocatalytic surfaces are given new properties. Those properties can then be applied in a variety of disciplines, such as medicine, food hygiene, environmental protection or building industry. Practically, the applications include inactivation of microorganisms, degradation of toxins, removing pollutants from buildings and manufacturing of fog-free windows or mirrors.
Keywords: Nanotechnology, Photocatalysis, Titanium dioxide, Reactive oxygen species, Self-disinfecting and self-cleaning surfaces, Bacteria
Review
Introduction
The past decade redounded the discovery that many materials used in a number of industries - e.g. titanium white (TiO2) and zinc white (ZnO) applied in the manufacturing of paints and varnishes, refractory magnesia (MgO) added to cement, or silica (SiO2) applied in the fabrication of glass products - after they have been powdered to nanoparticles (NPs) (1 < φ ≤ 100 nm), significantly alter their properties, i.e. they exhibit increased hardness, tensile strength, plasticity [1], higher resistance to chemical agents [2], greater hydrophilicity [3] or (photo)catalytic properties [4]. Materials powdered to NPs, called nanomaterials (NMs), have found many applications, e.g. in the production of cosmetics [5], including sunscreens [6], fabrication of ceramics [7] and ingredients of photocatalytic coatings applied on various work surfaces [4]. On surfaces with a thin layer of photocatalyst, i.e. nanosized titanium dioxide (nano-TiO2), the inactivation of microorganisms [8,9] and the mineralization of organic matter [10,11] have been observed. It is a result of advanced oxidation processes (AOPs), initiated by ultraviolet (UV) radiation [12]. Photocatalytic surfaces, also referred to as self-disinfecting and self-cleaning surfaces, are applied in many fields of industry, such as the building sector (i.e. in the manufacturing of concrete blocks, plasters, windows and ceramic tiles) [13] or road transport (i.e. in the production of asphalt and road signs) [14]. Due to their antibacterial [15-17], antifungal [18,19] and deodorizing [20] properties, photocatalytic films are more and more frequently applied to coat surfaces of sanitary products, laboratory tables, air filters, as well as in hospital rooms, canteens, production halls and rooms exposed to onerous odours, e.g. animal stables. NMs can also assist in keeping clean various textile materials, such as garment, bed sheets and carpets [21,22]. Increasingly frequent are the opinions that NMs are very soon going to play a crucial role in medicine [23] as well as in a number of industries, including the pharmaceutical industry [24] and food processing [25], as weapons to destroy cancer cells and fight pathogens, as carriers of drugs in organisms or as agents modifying organoleptic parameters of food. It is assumed that the introduction of NMs in food industry, animal production, or medicine will contribute towards a more efficient prevention of food poisonings and food infections and will increase the animal welfare by creation of better living conditions for animals. It will also enhance the efficiency of antibiotic-based therapies.
There are different NMs applied to create photocatalytic coatings that confer on the surfaces self-cleaning and self-disinfecting properties. Among them, there are oxides of some metals, e.g. titanium dioxide (TiO2), powdered to NPs [26-32]. Its undeniable attributes are low production costs, insolubility in most of the reaction environments and high photochemical stability [33]. This oxide exists in three polymorphic forms: anatase, brookite and rutile, whereby only two of them, anatase and rutile, are widely used to obtain NMs [34].
The number and variety of goods on the market that contain nanosized metal oxides, particularly nano-TiO2, are immense [35]. Therefore, the level of human exposure to their effects is varied. The omnipresence of nano-TiO2 in the human environment may evoke justified concerns as to the potential impact of its catalytic properties on human health [36-38].
Toxicity of titanium dioxide to people and natural environment
Since the beginning of the twentieth century, there has been a greater industrial interest in TiO2. Then, this pigment started to be used, replacing toxic lead compounds applied in manufacturing of paints and varnishes. As the report of the Institute for Market Research Ceresana [39] indicates, the world production of TiO2 - which, powdered to microparticles (MPs) (0.1 < φ ≤ 100 μm), is an odourless amorphic powder with a pristine white colour - amounted to 6.2 million tonnes in 2013. Its application as a white pigment is quite varied. This compound is applied in the production of paints [40], plastics [41], sunscreen cosmetics [42] and foods (i.e. as pigment E-171) [43].
The issue of TiO2 toxicity has been a research subject in many research centres around the world for many years. It is assumed that the microsized titanium dioxide (micro-TiO2) is harmless to people and animals [36,44]. As the dynamic development of nanotechnology goes on, however, there has been an increased concern that this compound might be toxic in the NP form, though. There are studies proving that NPs of many other metal oxides, such as ZnO or MgO, are more harmful to people and animals than MPs of the same compounds [45]. One of the main differences between micro-TiO2 and nano-TiO2 is a much bigger active surface of nano-TiO2, a feature resulting in a higher absorption rate of UV and a greater photocatalytic activity. Hence, nano-TiO2, contrary to micro-TiO2, was classified in the 2B group by the International Agency for Research on Cancer (IARC) [46]. The 2B group assembles compounds that might be carcinogenic for humans (to compare: in the same group, there are also chloroform, nitrobenzene, fumonisins B1 or ochratoxins A).
Yamamoto et al. [47] suggest that the nano-TiO2 present in sunscreen cosmetics (with UV filter) enhances the creation of reactive oxygen species (ROS) in skin cells, a phenomenon that may result in DNA damages and mutations and consequently induce the development of cancer diseases. Dunford et al. [48] and Subrahmanyam et al. [49] share that opinion. In view of those findings, a question as to the safety of products containing nano-TiO2 must be thoroughly answered [50].
In line with the Regulation (WE) 1223/2009 of the European Parliament and the Council [51], the maximum allowed amount of TiO2 in toiletries may not exceed 25%. As Hansen [37] calculated, the consumers' exposure to nano-TiO2 in face moisturizer creams, body care lotions or antiperspirants amounts annually to 26, 15 and 44 μg kg−1 body weight, respectively. A number of cosmetics manufacturers started to respond to consumers' fears and launched products containing nano-TiO2 in a new, safer formula. Effectively, the surface of nano-TiO2 particles is coated by a thin layer of ethylene glycol, and the particles are subsequently heated up to 300°C. In this temperature, the carbonization of ethylene glycol takes place. Therefore, the surface of nano-TiO2 particles becomes enfolded by a thin layer with a high carbon share that nearly completely stops their photocatalytic properties [5], without simultaneously altering their other physicochemical properties. It means that nano-TiO2 modified in this way is safe for the human skin. For example, when added to sunscreens, it can effectively protect the skin from negative effects of sun radiation, without generating any ROS at the same time. It seems, therefore, that the real risk of health loss due to cosmetics containing nano-TiO2 is scarce [50].
There are still very few studies available that would handle the issue of natural environment infiltration by nano-TiO2, its accumulation in water and soil, and its impact on organisms living in those miscellaneous environments [52]. According to the report by the United States Environmental Protection Agency (USEPA) [53], TiO2 is freed to the atmosphere, surface waters and soil, i.e. from such sources as already mentioned sunscreens, sun protecting textiles, plasters, paints or food packaging. Those data are confirmed by many authors [54-58]. Gottschalk et al. [59] present the opinion that nano-TiO2 occurs in a higher concentration in benthic sediments than in water. The conclusions are based on the water ecosystems studies with regard to their pollution by nanosized metal oxides. Mueller and Nowack [60] were the first to determine the quantitative risk for water animals coming from the presence of nano-TiO2 in their environment. By comparing the predicted effect concentration (PEC) of nano-TiO2 in various water habitats (0.70 to 9.60 ppb) with the predicted no effect concentration (PNEC) (less than 1.00 ppb), the authors discovered that the danger of nano-TiO2 for water biotope animals, including pollution-sensitive water purity indicator species (such as Anodonta anatina, Heloecius cordiformis and Limanda limanda), is non-existent (PEC/PNEC ≤ 1) or at most barely present (1 < PEC/PNEC ≤ 10). Having conducted their own studies, Gottschalk et al. [61] reached similar conclusions with respect to soil fauna, i.e. soil purity bioindicators (such as Lumbricus terrestris and Orchesella cincta).
Effective immobilization of nano-TiO2 on surfaces covered by photocatalytic films remains a crucial problem. The application of nano-TiO2 onto surfaces by means of magnetron sputtering deposition [62], chemical vapour deposition [63] or sol-gel deposition [64] results in an inseparably base-fixed, resistant to mechanical factors, thin layer of photocatalyst. The application of those technologies allows to significantly reduce the health damage risk, resulting from the transmission of nano-TiO2 from photocatalytic coatings to the environment and from the contact of free NPs, i.e. with human or animal bronchial epithelial cells.
Photocatalytic properties of titanium dioxide
The interest in nano-TiO2 started with the discovery of its catalytic properties induced by UV radiation, in the beginning of the 1970s of the twentieth century [65] - and it is still growing [12,66,67]. There is already a lot of literature data presenting the mechanism of the conversion of solar energy into chemical energy and on the possibilities of increasing the photocatalysis efficiency, both homogeneous (e.g. O3/UV) and heterogeneous (e.g. TiO2/UV), in case when the catalyst is located in the same and in the other thermodynamic phase, respectively, as the substrates and products of reaction [33,68,69]. The catalytic properties of semiconductors, e.g. of TiO2, can be explained by their electron structure. They have a valence band (VB) full with electrons and an electron-free conduction band (CB). The energy difference (ΔE) between those bands, defined as band gap, also referred to as energy gap, presents the amount of energy which must be delivered in order to excite an electron from VB to CB. For three polymorphic TiO2 forms, brookite, anatase and rutile, the width of the energy gap amounts to 2.9, 3.0 and 3.2 eV, respectively, an equivalent of the electromagnetic radiation photon energy with a wavelength of λ < 400 nm. In biological experiments, to excite semiconducting metal oxides (e.g. TiO2), the UV radiation is used, mainly in the near-ultraviolet range (UV-A, λ = 315 to 400 nm) [70-73], and to a lesser extent, the UV radiation with a wavelength in the indirect ultraviolet range (UV-B, λ = 280 to 315 nm) [70,72]. The application of far ultraviolet (UV-C, λ = 100 to 280 nm) as an agent initiating AOPs poses a serious danger to human health [72].
As a result of the semiconductor excitation, an electron (e−) excites from VB to CB, leaving behind a positively charged electron hole (h+) hence generating a specific ‘hole-electron’ pair (h+ + e−) [33,69] (Figure 1).
Figure 1.
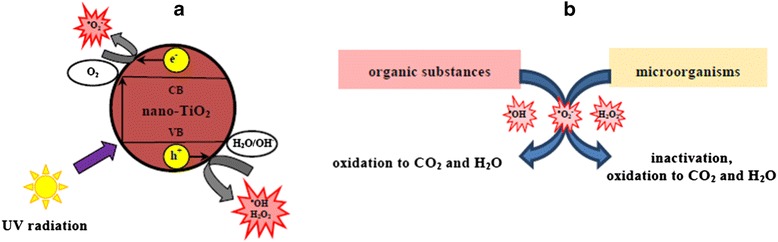
ROS generation and its effects. Mechanism of reactive oxygen species (ROS) generation on the surface of titanium dioxide nanoparticles (a) and the effects of ROS activity on organic substances and microorganisms (b). On the surface of the nano-TiO2 particles, exposed to UV radiation, ROS (•O2 −, •OH, H2O2) are formed (a) that have the ability to inactivate microorganisms and to oxidize organic matter (b).
This highly instable condition, called exciton, exhibits strong redox properties. The electron holes (h+) that condition oxidation processes, together with electrons (e−) that determine reduction processes, react with molecular oxygen (O2), water molecules (H2O) or hydroxyl ions (OH−), generating thereby ROS, such as superoxide anion radicals (•O2−), hydroxyl radicals (•OH) or hydrogen peroxide molecules (H2O2) [33,69] (Figure 1).
ROS that emerge on the photocatalytic surfaces, such as hydroxyl radicals (•OH), superoxide anion radicals (•O2−) and hydrogen peroxide (H2O2), not only inactivate but also oxidize bacteria, yeasts and moulds to CO2 and H2O [18,19,74,75], similarly as molecules of nearly all organic compounds [11,76] (Figure 1). Among the mineralized compounds, there are hydrocarbons, alcohols, aldehydes, ketones, and aromatic compounds [33,69] which are dangerous to human health, as well as dioxins and polychlorinated biphenyls (PCBs) [77,78] which are present in air and wastewater. Oxidized organic compounds are often onerous and hard to remove from air [79], water [80] and solid surfaces (e.g. windows, building exteriors and machines) [75], whereby inactivated microorganisms are frequently pathogens which are dangerous for humans and animals [81]. Due to photocatalytic properties, TiO2 is applied, after powdering to NPs, in AOPs and UV radiation-based methods for pathogen inactivation and organic pollutant decomposition [82].
Superhydrophilicity and superhydrophobicity of titanium dioxide
Apart from photocatalytic properties, another unique feature of nano-TiO2 is strong hydrophilicity, induced by UV radiation, and called superhydrophilicity [33,67]. This mechanism provides that CB excited electrons (e−) reduce ions Ti4+ to ions Ti3+ (see Equation 1), while electron holes (h+) oxidize oxide anions (O2−) to molecular oxygen (O2) (see Equation 2). Generated oxygen molecules are removed from the nano-TiO2 surface. The remaining oxygen vacancies react with water molecules (H-OH). As a result, oxygen vacancies bind to the surface covered by a thin layer of nano-TiO2 hydroxyl ions (OH−) that are responsible for the surface's superhydrophilicity.
| 1 |
| 2 |
The longer such surface is exposed to UV irradiation, the smaller the contact angle becomes, which is defined as an angle between the solid matter plane and the plane tangent to liquid drop placed on that solid matter (in the tangent point of liquid and solid matter) (Figure 2).
Figure 2.
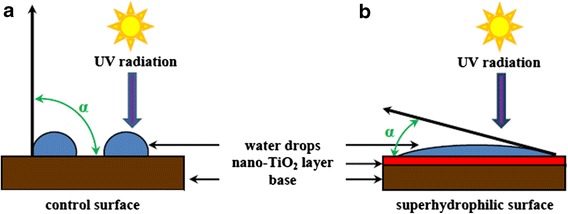
Comparison of contact angle ( α ) on control surface (a) and surface covered by a thin layer of nano-TiO 2 (b). Both surfaces are exposed to UV radiation. The surface covered by a thin layer of nano-TiO2 and exposed to UV radiation exhibits superhydrophilic properties; the contact angle is an acute angle, 0° < α < 90° (b).
Thirty minutes after the exposure of the photocatalytic surface to UV radiation, the contact angle is close to zero. It means that water shows a tendency to ideally spread over the surface. It allows to obtain a thin, homogeneous, quickly evaporating, invisible and pollutant-removing film of water [83]. That is why glass surfaces covered by a thin layer of nano-TiO2 are applied in manufacturing of fog-free mirrors [84] or self-cleaning windows [66]. It is not the only example of the practical application of the nano-TiO2 strong hydrophilicity. Thanks to its superhydrophilicity, nano-TiO2 is also widely used in production of self-cleaning plasters [10], paints [40] and plastics [6,41].
Surfaces covered by a thin layer of nano-TiO2 do not become polluted even in the dark. Its particles are responsive also to water or pollutants and act as little ‘piles’. They push water drops and dust particles off the surface, hence preventing the surface from becoming wet or dirty. For the first time, this phenomenon was observed with Nelumbo nucifera, an angiosperm plant, and it was given the name lotus effect [85]. Surfaces displaying identical properties can also be found in animals, for example, the chitin armour of some insects, such as Stenocara gracilipes [86]. This phenomenon is referred to as superhydrophobicity [87] (Figure 3).
Figure 3.
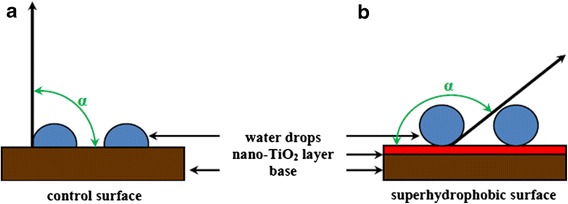
Comparison of contact angle ( α ) on control surface (a) and surface covered by a thin layer of nano-TiO 2 (b) in darkness. The surface covered by a thin layer of nano-TiO2 exhibits in darkness superhydrophobic properties; the contact angle is an obtuse angle, 90° < α < 180° (b).
To summarize, surfaces covered by photocatalytic coatings that contain nanosized metal oxides, i.e. nano-TiO2, exhibit - when irradiated by UV - not only antistatic [33] or deodorizing [20] properties but, more importantly, they also have self-disinfecting [88,89] and self-cleaning abilities [13,66].
Examples of TiO2/UV photocatalysis application in bacteria eradication
The majority of bacteria examined in vitro are common also in the natural environment, i.e. in water and soil, on the skin and in the digestive tract of humans and animals. Therefore, they may contaminate foods and may also form opportunistic biota in hospitals [90-96]. In recent years, numerous studies have been focused on the aspect of using the photocatalysis phenomenon in eradication of pathogenic microorganisms in ventilation systems [82,97-99], water supplies [27,29,32,82,100-103], sewage systems [104,105] and on work surfaces (e.g. tables and floors) in medical centres [15,16,106] or in food processing plants [107,108]. Among the photocatalytic processes that have been most studied in the context of microorganism inactivation and degradation of toxic compounds to human and animals is the TiO2/UV process where titanium dioxide (TiO2), after it has been powdered to nanoparticles, performs as photocatalyst, and ultraviolet (UV) radiation is an agent generating reactive oxygen species [10].
Air purification
Catalytic properties of nano-TiO2 exhibited in the presence of UV radiation give rise to its application, i.e. in decomposition of volatile organic compounds (VOC) [79], as well as in inactivation of microorganisms [99] present in building interiors of houses, offices or manufacturing sites [109]. Bioaerosols are nowadays the main air pollutant in rooms [98]. Many bacteria have been identified as infectious agents disseminated through ventilation systems. Among them, there are pathogens causing tuberculosis (Mycobacterium tuberculosis) [110-112], pneumonia (Streptococcus pneumoniae) [113], scarlet fever (Streptococcus pyogenes) [112,113], diphtheria (Corynebacterium diphtheriae) [112] or whooping cough (Bordetella pertussis) [114]. To purify air in closed areas, high efficiency particulate air (HEPA) filters are applied [115]. They arrest the majority of mechanical and biological pollutants. According to Goswami et al. [116], an equally effective tool in fighting the pathogens can be photocatalysis. For the purposes of the studies evaluating the effectiveness of the TiO2/UV process in inactivation of pathogens in closed areas, the authors designed UV-transparent air recirculation systems, covered by a thin nano-TiO2 layer. In this pioneering experiment, the air was completely free from microorganisms, and bacteria cells were entirely mineralized within a 5-h period of the TiO2/UV process [97]. Later experiments were able to reduce this period down to less than 3 min [98]. Photocatalytic processes, including the TiO2/UV process, are also an efficient tool in the eradication of bacteria spores, e.g. endospores of Bacillus cereus [117], as well as in eradication of microorganisms applied in biological weapons, e.g. Bacillus anthracis [118].
Water treatment
The TiO2/UV process finds its practical application also in water treatment [101]. The rapid growth of the human population in recent decades induces the increase in demand for drinking water. Incorrect management of water resources results in a pollution upsurge of world water resources, reflected by the growing number of waterborne disease outbreaks (WBDO) [119]. It might be attributed to the ineffective inactivation of pathogens in water treatment and water distribution systems and common disinfectants, such as chlorine, chlorine dioxide, chloramine or ozone [73]. Moreover, some pathogenic microorganisms, such as Legionella pneumophila, show limited susceptibility to traditional disinfectants like chlorine [120]. Conventional water treatment methods generate around 600 harmful disinfection by-products (DBPs), such as trihalomethanes or chlorophenols [78,121]. Those compounds are formed in chemical reactions between natural organic substances and compounds of disinfectants with strong oxidizing properties. Many DBPs show carcinogenic, mutagenic and teratogenic properties [122]. An ideal disinfectant should have inactivating properties towards many species of microorganisms, should not induce forming of DBPs, must not negatively impact human health and should be low cost, easy to handle and to keep, non-invasive for work tools and harmless to the environment. Some NMs, e.g. nano-TiO2, fulfil those requirements and can, therefore, be used in both water treatment [105,123] and disinfection of solid surfaces [71,124,125]. Already in the beginning of the 1980s in the twentieth century, Heller et al. [68] reported that a small amount of catalytically active TiO2 powder added to wastewater exposed to solar light purifies that water after some time, and the organic pollutants are decomposed into simple non-organic compounds. Due to mineralization of a number of organic and biological pollutions that takes place on surfaces covered by a thin layer of nano-TiO2, the application of the TiO2/UV process in the treatment of much polluted wastewater, i.e. in the treatment of water coming from resin plants, paper mills, dye-works and refineries [100,103], is being discussed, as well as its application in the decomposition of toxins released to water by cyanobacteria [126]. The TiO2/UV process can also be rendered useful in hospitals as a tool to control the dissemination of Legionnaires disease, a disease induced by L. pneumophila, e.g. in hot water distribution systems [127].
Disinfection of work areas and food packaging
Frequent and thorough disinfection of surfaces with the purpose to reduce the amount of bacteria and to prevent their dissemination is a necessity, e.g. in food processing plants, microbiological laboratories, veterinary medicine clinics and hospitals. Conventional disinfection methods, such as cleaning with chemical disinfectants, are work and time consuming and not always sufficient [128]. Photocatalytic processes taking place on surfaces covered by a thin layer on nanosized metal oxides (i.e., nano-TiO2) present an increasingly important alternative to traditional disinfection methods [97,102,104,129,130]. A reason for the greater interest in the practical application of photocatalysis is its high bactericidal effectiveness in the treatment of microorganisms, such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus faecium [71,129,131-134], playing a crucial role in public health protection.
According to Szczawiński et al. [107,108] as well as Tomaszewski and Jach [62], the number of living cells of E. coli, P. aeruginosa, Salmonella Enteritidis and S. aureus on the floor and wall ceramic tiles (applied in production halls, hospital rooms or sanitary rooms) covered by a thin layer of nano-TiO2 and exposed to UV radiation for 2 min was reduced by 6 to 7 logarithmic units, depending on the method of photocatalytical layer deposition and on the kind of tiles (shiny or matt). On control surfaces, the bacteria reduction rate amounted to around 1 logarithmic cycle.
The TiO2/UV process can also be applied in disinfection of food packaging. Its high efficiency in eradication of E. coli (strain ATCC11775) has been repeatedly proven [135], similar to the eradication of Listeria monocytogenes [136] from plastic bags and containers used for food storage.
The importance of effective surface disinfection in food manufacturing can hardly be overestimated. Shortcomings in this field have frequently caused various ingestions and poisonings. After an epidemic caused by E. coli (strain O157:H7) in Japan, the influence of the TiO2/UV process on the decomposition of the toxin produced by this bacterial strain was examined. The results showed that after 120 min of the TiO2/UV process, a partial decomposition of the toxin occurred, and after a further 2 h, its entire decomposition followed [33]. The studies by Oza et al. [137] confirmed high effectiveness of the TiO2/UV process not only in the decomposition of the toxin, but also in the inactivation of the bacteria producing the toxin. Guillard et al. [82] observed in their experiments on E. coli (strain PHL1273 with curli, a sort of long, flaccid adhesive fibres, a proteinaceous component of an extra-cellular matrix, formed on the surface of some bacteria species, allowing the bacteria to cling to the bottom) a reduction in the TBC by 7 logarithmic units after no more than 3 min of the TiO2/UV process.
As Szczawiński et al. [107,108] report, the application of photocatalytic surfaces, such as of ceramic wall tiles covered by a thin layer of nano-TiO2, in food processing plants, veterinary clinics, hospitals, treatment rooms, laboratories and wherever the UV radiation to disinfect surfaces is put to use, should significantly increase the disinfection effectiveness and contribute to a dramatic improvement of hygiene conditions in those areas. It has been pointed out for many years that photocatalytic processes can become a tool of a permanent surface disinfection of those items that people are in frequent contact with, e.g. door handles, taps or toilet seats [138]. Thereby, the disinfection process using AOPs can be broadly applied in the public areas, such as in toilets, schools, railway and bus stations, hotels, public transport and airports.
The TiO2/UV process does not only inactivate microorganisms, but also induces the mineralization of organic matter such as dead microorganisms [10,11]. Jacoby et al. [11] observed the inactivation of all cells of E. coli on a test ceramic surface after 30 min of the TiO2/UV process. After the next 45 min, 54% of the dead bacteria cells were completely oxidized to CO2 and H2O. In other experiments, the prolonged TiO2/UV process caused a complete mineralization of four strains of L. pneumophila (997, 1004, 1009, ATCC33153) [120]. Those studies have confirmed both the bactericidal properties and the self-cleaning properties of surfaces covered by a thin layer of nano-TiO2 and exposed to the UV radiation.
Disinfection of medical appliances
The AOP-based disinfection process has been tested also with regard to its feasibility in medicine [139]. Here, nano-TiO2 is used as a component of photocatalytic films covering catheters [140], scalpels [141] and surgical masks [142]. Ohko et al. [98] stated that the effectiveness of the UV-based disinfection of catheters is three times higher if they were covered by a thin layer of nano-TiO2. A similar result of the TiO2/UV application was observed with infected dental implants [143,144]. High bactericidal efficiency of the TiO2/UV process was also reported in orthopaedics and cosmetic surgery in the case of S. aureus on implants coated by a thin layer of nano-TiO2 [94]. It seems, therefore, photocatalytic processes can present a valuable method to reduce bacterial infections resulting from the application of implants in medicine.
Conclusions
Nanotechnology allows modifying the properties of various materials through alteration of their structure at the level of atoms and molecules. Thus, products can be designed that are incomparably better than microtechnology products. Materials, components or devices designed using nanotechnology display a number of precious properties, i.e., they can be small, light, quick or efficient. Nanotechnology is regarded as the key technology of the twenty-first century. It appears as a source of new development opportunities for many economy sectors, and it may contribute to a better environmental protection. It can also assist in finding solutions to many problems in medicine, food hygiene and public health protection. TiO2 powdered to NPs is a material that, after it has been coated on surfaces and exposed to UV radiation, gives to those surfaces self-cleaning and self-disinfecting properties. This compound has, however, one important weakness: it absorbs UV radiation (λ < 400 nm), only that amounts to approximately 3% of the electromagnetic radiation spectrum that reaches the Earth. Thus, studies are already ongoing to examine the possibility of visible light (VL) (400 nm < λ < 700 nm) to excite nano-TiO2 that, if successful, would largely increase the effectiveness of photocatalysis and widen its application range. Photocatalytic processes, leading to inactivation of pathogens and to mineralization of organic pollutants on the surfaces coated by a thin layer of a photocatalyst, i.e. nano-TiO2, present a valid supplement of the traditional disinfecting methods. AOP-based disinfection is rapid and effective. Its application in the public space on a wide scale, especially where traditional disinfecting methods do not appear sufficient, can largely improve the sanitary and hygienic conditions, restrict the dissemination of pathogens, as well as reduce the number of food contaminations and poisonings. Photocatalytic processes help also keep the building walls clean for many years, road signs to not get dirty quickly and mirrors to not become covered by fog. It is supposed that nanomaterials - including nano-TiO2 - can play a crucial role, e.g. in medicine, food hygiene or public health protection. Will it be so, indeed? Let us wait and see. Nanotechnologists have not yet said their final ‘nano-word’.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JB performed the overall review and drafted the manuscript. AJT and JZ conducted the research work on the latest developments in the application of TiO2/UV photocatalysis in bacteria eradication, and helped draft the manuscript and sequence alignment. JPC is the main coordinator of this manuscript and prepared its revision. All authors read and approved the final manuscript.
Contributor Information
Janusz Bogdan, Email: janusz_bogdan@sggw.pl.
Agnieszka Jackowska-Tracz, Email: agnieszka_jackowska_tracz@sggw.pl.
Joanna Zarzyńska, Email: joanna_zarzynska@sggw.pl.
Joanna Pławińska-Czarnak, Email: joanna_plawinska@sggw.pl.
References
- 1.Heo SJ, Sinnott SB. Computational investigation of the mechanical properties of nanomaterials. Diam Relat Mater. 2009;18(2–3):438–42. doi: 10.1016/j.diamond.2008.10.041. [DOI] [Google Scholar]
- 2.Tjong SC, Chen H. Nanocrystalline materials and coatings. Mater Sci Eng R Rep. 2004;45(1):1–88. doi: 10.1016/j.mser.2004.07.001. [DOI] [Google Scholar]
- 3.Lee YC, Hong YP, Lee HY, Kim H, Jung YJ, Ko KH, et al. Photocatalysis and hydrophilicity of doped TiO2 thin films. J Colloid Interface Sci. 2003;267(1):127–31. doi: 10.1016/S0021-9797(03)00603-9. [DOI] [PubMed] [Google Scholar]
- 4.Costa AL, Ortelli S, Blosi M, Albonetti S, Vaccari A, Dondi M. TiO2 based photocatalytic coatings: from nanostructure to functional properties. Chem Eng J. 2013;225:880–6. doi: 10.1016/j.cej.2013.04.037. [DOI] [Google Scholar]
- 5.Mihranyan A, Ferraz N, Strømme M. Current status and future prospects of nanotechnology in cosmetics. Prog Mater Sci. 2012;57(5):875–910. doi: 10.1016/j.pmatsci.2011.10.001. [DOI] [Google Scholar]
- 6.Wakefield G, Green M, Lipscomb S, Flutter B. Modified titania nanomaterials for sunscreen applications - reducing free radical generation and DNA damage. Mater Sci Technol. 2004;20(8):985–8. doi: 10.1179/026708304225019803. [DOI] [Google Scholar]
- 7.Belyakov AV. Introduction of nanomaterials and nanotechnologies in ceramics plants. Glass Ceram. 2010;67(7–8):203–8. doi: 10.1007/s10717-010-9263-y. [DOI] [Google Scholar]
- 8.Cheng CL, Sun DS, Chu WC, Tseng YH, Ho HC, Wang JB, et al. The effects of the bacterial interaction with visible-light responsive titania photocatalyst on the bactericidal performance. J Biomed Sci. 2009;16(1):7. doi: 10.1186/1423-0127-16-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu C, Guo J, Qu J, Hu X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir. 2007;23(9):4982–7. doi: 10.1021/la063626x. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Yang X, Xu F, Wu Q, Zhang Y. Correlation of photocatalytic bactericidal effect and organic matter degradation of TiO2: part I: observation of phenomena. Environ Sci Technol. 2009;43(4):1180–4. doi: 10.1021/es802499t. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby WA, Maness PC, Wolfrum EJ, Blake DM, Fennell JA. Mineralization of bacteria cell mass on a photocatalytic surface in air. Environ Sci Technol. 1998;32(17):2650–3. doi: 10.1021/es980036f. [DOI] [Google Scholar]
- 12.Carp O, Huisman CL, Reller A. Photoinduced reactivity of titanium dioxide. Prog Solid State Chem. 2004;32(1):33–177. doi: 10.1016/j.progsolidstchem.2004.08.001. [DOI] [Google Scholar]
- 13.Khataee R, Heydari V, Moradkhannejhad L, Safarpour M, Joo SW. Self-cleaning and mechanical properties of modified white cement with nanostructured TiO2. J Nanosci Nanotechnol. 2013;13(7):5109–14. doi: 10.1166/jnn.2013.7586. [DOI] [PubMed] [Google Scholar]
- 14.Fang C, Yu R, Liu S, Li Y. Nanomaterials applied in asphalt modification: a review. J Mater Sci Technol. 2013;29(7):589–94. doi: 10.1016/j.jmst.2013.04.008. [DOI] [Google Scholar]
- 15.Chung CJ, Lin HI, Tsou HK, Shi ZY, He JL. An antimicrobial TiO2 coating for reducing hospital-acquired infection. J Biomed Mater Res B Appl Biomater. 2008;85(1):220–4. doi: 10.1002/jbm.b.30939. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Xu Z, Wang X, Long J, Su W, Fu X, et al. Photocatalytic and antibacterial properties of medical-grade PVC material coated with TiO2 film. J Biomed Mater Res B Appl Biomater. 2008;87(2):425–31. doi: 10.1002/jbm.b.31120. [DOI] [PubMed] [Google Scholar]
- 17.Muranyi P, Schraml C, Wunderlich J. Antimicrobial efficiency of titanium dioxide-coated surfaces. J Appl Microbiol. 2010;108(6):1966–73. doi: 10.1111/j.1365-2672.2009.04594.x. [DOI] [PubMed] [Google Scholar]
- 18.Akiba N, Hayakawa I, Keh ES, Watanabe A. Antifungal effects of a tissue conditioner coating agent with TiO2 photocatalyst. J Med Dent Sci. 2005;52(4):223–7. [PubMed] [Google Scholar]
- 19.Wolfrum EJ, Huang J, Blake DM, Maness PC, Huang Z, Fiest J, et al. Photocatalytic oxidation of bacteria, bacterial and fungal spores, and model biofilm components to carbon dioxide on titanium dioxide-coated surfaces. Environ Sci Technol. 2002;36(15):3412–9. doi: 10.1021/es011423j. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Ge C, Zhu C, Li Y, Tian W, Cheng D, et al. Deodorizing properties of photocatalyst textiles and its effect analysis. Phys Procedia. 2012;25:240–4. doi: 10.1016/j.phpro.2012.03.078. [DOI] [Google Scholar]
- 21.Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus of anti-microbial properties. Colloids Surf B: Biointerfaces. 2010;79(1):5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Kangwansupamonkon W, Lauruengtana V, Surassmo S, Ruktanonchai U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomedicine. 2009;5(2):240–9. doi: 10.1016/j.nano.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomedicine. 2007;3(1):20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Foldvari M, Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: I Pharmaceutical properties. Nanomedicine. 2008;4(3):173–82. doi: 10.1016/j.nano.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E. Nanotechnologies in the food industry - recent developments, risks and regulation. Trends Food Sci Technol. 2012;24(1):30–46. doi: 10.1016/j.tifs.2011.10.006. [DOI] [Google Scholar]
- 26.Choi H, Antoniou MG, de la Cruz AA, Stathatos E, Dionysiou DD. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination. 2007;202(1–3):199–206. doi: 10.1016/j.desal.2005.12.055. [DOI] [Google Scholar]
- 27.Dunlop PSM, Byrne JA, Manga N, Eggins BR. The photocatalytic removal of bacterial pollutants from drinking water. J Photochem Photobiol A Chem. 2002;148(1–3):355–63. doi: 10.1016/S1010-6030(02)00063-1. [DOI] [Google Scholar]
- 28.Friedmann D, Mendive C, Bahnemann D. TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis. Appl Catal B. 2010;99(3–4):398–406. doi: 10.1016/j.apcatb.2010.05.014. [DOI] [Google Scholar]
- 29.Obare SO, Mayer GJ. Nanostructured materials for environmental remediation of organic contaminants in water. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39(10):2549–82. doi: 10.1081/ESE-200027010. [DOI] [PubMed] [Google Scholar]
- 30.Pleskova SN, Golubeva IS, Verevkin IK, Pershin EA, Burenina VN, Korolikhin VV. Photoinduced bactericidal activity of TiO2 films. Prikl Biokhim Mikrobiol. 2011;47(1):28–32. [PubMed] [Google Scholar]
- 31.Reddy MP, Venugopal A, Subrahmanyam M. Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007;41(2):379–86. doi: 10.1016/j.watres.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Sun DD, Tay JH, Tan KM. Photocatalytic degradation of E. coli form in water. Water Res. 2003;37(14):3452–62. doi: 10.1016/S0043-1354(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 33.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev. 2000;1(1):1–21. doi: 10.1016/S1389-5567(00)00002-2. [DOI] [Google Scholar]
- 34.Hwang YK, Park SS, Lim JH, Won YS, Huh S. Preparation of anatase/rutile mixed-phase titania nanoparticles for dye-sensitized solar cells. J Nanosci Nanotechnol. 2013;13(3):2255–61. doi: 10.1166/jnn.2013.6897. [DOI] [PubMed] [Google Scholar]
- 35.Anpo M. Utilization of TiO2 photocatalyst in green chemistry. Pure Appl Chem. 2000;72(7):1265–70. doi: 10.1351/pac200072071265. [DOI] [Google Scholar]
- 36.Bystrzejewska B, Golimowski J, Urban PL. Nanoparticles: their potential toxicity, waste and environmental management. Waste Manage. 2009;29(9):2587–95. doi: 10.1016/j.wasman.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Hansen SF. Regulation and risk assessment of nanomaterials: too little, too late? PhD thesis. Department of Environmental Engineering, Technical University of Denmark; 2009.
- 38.Roszak J, Stępnik M, Nocuń M, Ferlińska M, Smok-Pieniążek A, Grobelny J, et al. A strategy for in vitro safety testing of nanotitania-modified textile products. J Hazard Mater. 2013;256–257:67–75. doi: 10.1016/j.jhazmat.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Ceresana. Market intelligence. Consulting. Market study: titanium dioxide. http://www.ceresana.com/en/market-studies/chemicals/titanium-dioxide/ (2014). Accessed 20 Nov 2014.
- 40.Colling JH, Dunderdale J. The durability of paint films containing titanium dioxide - contraction, erosion and clear layer theories. Prog Org Coat. 1981;9(1):47–84. doi: 10.1016/0033-0655(81)80015-5. [DOI] [Google Scholar]
- 41.Day RE. The role of titanium dioxide pigments in the degradation and stabilization of polymers in plastics industry. Polym Degrad Stab. 1990;21(1):73–92. doi: 10.1016/0141-3910(90)90023-Z. [DOI] [Google Scholar]
- 42.Jaroenworaluck A, Sunsaneeyametha W, Kosachan N, Stevens R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf Interface Anal. 2006;38(4):473–7. doi: 10.1002/sia.2313. [DOI] [Google Scholar]
- 43.Frazer L. Titanium dioxide: environmental white knight? Environ Health Perspect. 2001;109(4):174–7. doi: 10.1289/ehp.109-a174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard A, Drobne D, Jemec A. Ecotoxicity of nanosized TiO2: review of in vivo data. Environ Pollut. 2011;159(3):677–84. doi: 10.1016/j.envpol.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations-many questions, some answers. Mutat Res. 2009;681(2–3):241–58. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 46.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Carbon black, titanium dioxide and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1–413. [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto A, Honma M, Sumita T, Hanawa J. Cytotoxicity evaluation of ceramic particles of different sizes and shapes. J Biomed Mater Res A. 2004;68(2):244–56. doi: 10.1002/jbm.a.20020. [DOI] [PubMed] [Google Scholar]
- 48.Dunford R, Salinaro L, Cai L, Serpone N, Horikoshi S, Hidaka H, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418(1–2):87–90. doi: 10.1016/S0014-5793(97)01356-2. [DOI] [PubMed] [Google Scholar]
- 49.Subrahmanyam A, Arokiadoss T, Ramesh TP. Studies on the oxygenation of human blood by photocatalytic action. Artif Organs. 2007;31(11):819–25. doi: 10.1111/j.1525-1594.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 50.Schilling K, Bradford B, Castelli D, Dufor E, Nash JF, Pape W, et al. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci. 2010;9(4):495–509. doi: 10.1039/b9pp00180h. [DOI] [PubMed] [Google Scholar]
- 51.European Parliament and the Council of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. OJ. 342:59–209.
- 52.Gottschalk F, Sun TY, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut. 2013;181(38):287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Varner KE, Rindfusz K, Gaglione A, Viveiros E. Nano titanium dioxide environmental matters: state of the science literature review. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-10/089, 2010.
- 54.David H, Motabar D, Quiñones O, Stanford B, Vanderford B, Moss D. Titanium distribution in swimming pool water is dominated by dissolved species. Environ Pollut. 2013;181:68–74. doi: 10.1016/j.envpol.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 55.Kaiser JP, Zuin S, Wick P. Is nanotechnology revolutionizing the paint and lacquer industry? A critical option. Sci Total Environ. 2014;184:570–8. doi: 10.1016/j.scitotenv.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Rai M, Dave NG. Building materials for low-cost housing. Build Environ. 1991;26(3):295–300. doi: 10.1016/0360-1323(91)90053-E. [DOI] [Google Scholar]
- 57.Rhim JW, Park HM, Ha CS. Bio-nanocomposites for food packaging applications. Prog Polym Sci. 2013;38(10–11):1629–52. doi: 10.1016/j.progpolymsci.2013.05.008. [DOI] [Google Scholar]
- 58.Windler L, von Goetz N, Hungerbühler K, Amberg M, Heuberger M, Nowack B. Release of titanium dioxide from textiles during washing. Environ Sci Technol. 2012;46(15):8181–8. doi: 10.1021/es301633b. [DOI] [PubMed] [Google Scholar]
- 59.Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol. 2009;43(24):9216–22. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 60.Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol. 2008;42(12):4447–53. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- 61.Gottschalk F, Kost E, Nowack B. Engineered nanomaterials in waters and soils: a risk quantification based on probabilistic exposure and effect modeling. Environ Toxicol Chem. 2013;32(6):1278–87. doi: 10.1002/etc.2177. [DOI] [PubMed] [Google Scholar]
- 62.Tomaszewski H, Jach K. Influence of deposition conditions of titania thin films by magnetron sputtering on catalytic, hydrophilic and bactericidal properties of the layers. Ceram Mater. 2012;64(1):1–21. [Google Scholar]
- 63.Ding Z, Hu XJ, Yue PL, Lu G, Greenfield PF. Synthesis of anatase TiO2 supported on porous solids by chemical vapor deposition. Catal Today. 2001;68(1–3):173–82. doi: 10.1016/S0920-5861(01)00298-X. [DOI] [Google Scholar]
- 64.Su C, Hong BY, Tseng CM. Sol–gel preparation and photocatalysis of titanium dioxide. Catal Today. 2004;96(3):119–26. doi: 10.1016/j.cattod.2004.06.132. [DOI] [Google Scholar]
- 65.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238(5358):37–8. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 66.Li X, He J. Synthesis of raspberry-like SiO2-TiO2 nanoparticles toward antireflective and self-cleaning coatings. ACS Appl Mater Interfaces. 2013;5(11):5282–90. doi: 10.1021/am401124j. [DOI] [PubMed] [Google Scholar]
- 67.Yu JC, Ho W, Lin J, Yip H, Wong PK. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environ Sci Technol. 2003;37(10):2296–301. doi: 10.1021/es0259483. [DOI] [PubMed] [Google Scholar]
- 68.Heller A. Conversion of sunlight into electrical power and photoassisted electrolysis of water in photoelectrochemical cells. Acc Chem Res. 1981;14(5):154–62. doi: 10.1021/ar00065a004. [DOI] [Google Scholar]
- 69.Le Mills A, Hunte S. An overview of semiconductor photocatalysis. J Photochem Photobiol A Chem. 1997;108(1):1–35. doi: 10.1016/S1010-6030(97)00118-4. [DOI] [Google Scholar]
- 70.Kim S, An YJ. Effect of ZnO and TiO2 nanoparticles preilluminated with UVA and UVB light on Escherichia coli and Bacillus subtilis. Appl Microbiol Biotechnol. 2012;95(1):243–53. doi: 10.1007/s00253-012-4153-6. [DOI] [PubMed] [Google Scholar]
- 71.Kühn KP, Chaberny IF, Massholder K, Stickler M, Benz VW, Sonntag HG, et al. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere. 2003;53(1):71–7. doi: 10.1016/S0045-6535(03)00362-X. [DOI] [PubMed] [Google Scholar]
- 72.Lee JE, Ko G. Norovirus and MS2 inactivation kinetics of UV-A and UV-B with and without TiO2. Water Res. 2013;47(15):5607–13. doi: 10.1016/j.watres.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 73.Pablos C, Marugán J, van Grieken R, Serrano E. Emerging micropollutant oxidation during disinfection processes using UV-C, UV-C/H2O2, UV-A/TiO2 and UV-A/TiO2/H2O2. Water Res. 2013;47(3):1237–45. doi: 10.1016/j.watres.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 74.Pan D, Zhan Z, Chen D, Wu Z, Pang A, Wang Y, et al. Photocatalytic bactericidal mechanism of nanoscale TiO2 films on Escherichia coli. J Nanosci Nanotechnol. 2011;11(9):7621–6. doi: 10.1166/jnn.2011.4757. [DOI] [PubMed] [Google Scholar]
- 75.Sunada K, Kikuchi Y, Hashimoto K, Fujishima A. Bactericidal and detoxification effects of TiO2 thin film photocatalyst. Environ Sci Technol. 1998;32(5):726–8. doi: 10.1021/es970860o. [DOI] [Google Scholar]
- 76.Herrmann JM. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal Today. 1999;53(1):115–29. doi: 10.1016/S0920-5861(99)00107-8. [DOI] [Google Scholar]
- 77.Buechler KJ, Noble RD, Koval CA, Jacoby WA. Investigation of the effects of controlled periodic illumination on the oxidation of gaseous trichloroethylene using a thin film of TiO2. Ind Eng Chem Res. 1999;38(3):892–6. doi: 10.1021/ie9804374. [DOI] [Google Scholar]
- 78.Guillard C, Disdier J, Herrmann JM, Lehaut C, Chopin T, Malato S, et al. Comparison of various titania samples of industrial origin in the solar photocatalytic detoxification of water containing 4-chlorophenol. Catal Today. 1999;54(2–3):217–28. doi: 10.1016/S0920-5861(99)00184-4. [DOI] [Google Scholar]
- 79.Alberici RM, Jardim WF. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl Catal B. 1997;14(1–2):55–68. doi: 10.1016/S0926-3373(97)00012-X. [DOI] [Google Scholar]
- 80.Chong MN, Jin B, Chow CW, Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010;44(10):2997–3027. doi: 10.1016/j.watres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 81.Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, et al. Occurrence of a new generation of disinfection byproducts. Environ Sci Technol. 2006;40(23):7175–85. doi: 10.1021/es060353j. [DOI] [PubMed] [Google Scholar]
- 82.Guillard C, Bui TH, Felix C, Moules V, Lina B, Lejeune P. Microbiological disinfection of water and air by photocatalysis. C R Chim. 2008;11(1–2):107–13. doi: 10.1016/j.crci.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng B, Tan L, Chen D, Meng X, Tang F. Programming surface morphology of TiO2 hollow spheres and their superhydrophilic films. ACS Appl Mater Interfaces. 2012;4(1):96–101. doi: 10.1021/am2009986. [DOI] [PubMed] [Google Scholar]
- 84.Ohdaira T, Nagai H, Kayano S, Kazuhito H. Antifogging effects of a socket-type device with the superhydrophilic, titanium dioxide-coated glass for the laparoscope. Surg Endosc. 2007;21(2):333–8. doi: 10.1007/s00464-006-0795-8. [DOI] [PubMed] [Google Scholar]
- 85.Bartholtt W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 1997;202(1):1–8. doi: 10.1007/s004250050096. [DOI] [Google Scholar]
- 86.Parker AR, Lawrence CR. Water capture by a desert beetle. Nature. 2001;414(6859):33–4. doi: 10.1038/35102108. [DOI] [PubMed] [Google Scholar]
- 87.Fujishima A, Zhang X, Tryk DA. TiO2 photocatalysis and related surface phenomena. Surf Sci Rep. 2008;63(12):515–82. doi: 10.1016/j.surfrep.2008.10.001. [DOI] [Google Scholar]
- 88.Fujishima A, Zhang X. Titanium dioxide photocatalysis: present situation and future approaches. C R Chim. 2005;9(5–6):750–60. [Google Scholar]
- 89.Ohko Y, Utsumi Y, Niwa C, Tatsuma T, Kobayakawa K, Satoh Y, et al. Self-sterilizing and self-cleaning of silicone catheters coated with TiO2 photocatalyst thin films: a preclinical work. J Biomed Mater Res B Appl Biomater. 2001;58(1):97–101. doi: 10.1002/1097-4636(2001)58:1<97::AID-JBM140>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 90.Correa L, Martino MD, Siqueira I, Pasternak J, Gales AC, Silva CV, et al. A hospital-based matched case–control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis. 2013;13:80. doi: 10.1186/1471-2334-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gouveia EL, Reis JN, Flannery B, Cordeiro SM, Lima JB, Pinheiro RM, et al. Clinical outcome of pneumococcal meningitis during the emergence of pencillin-resistant Streptococcus pneumoniae: an observational study. BMC Infect Dis. 2011;11:323. doi: 10.1186/1471-2334-11-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haenle M, Fritsche A, Zietz C, Bader R, Heidenau F, Mittelmeier W, et al. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: in vitro effectiveness against MRSA and mechanical properties. J Mater Sci Mater Med. 2011;22(2):381–7. doi: 10.1007/s10856-010-4204-4. [DOI] [PubMed] [Google Scholar]
- 93.Mohanty S, Maurya V, Gaind R, Deb M. Phenotypic characterization and colistin susceptibilities of carbapenem-resistant of Pseudomonas aeruginosa and Acinetobacter spp. J Infect Dev Ctries. 2013;7(11):880–7. doi: 10.3855/jidc.2924. [DOI] [PubMed] [Google Scholar]
- 94.Singer J, Merz A, Frommelt L, Fink B. High rate of infection control with one-stage revision of septic knee prostheses excluding MRSA and MRSE. Clin Orthop Relat Res. 2012;470(5):1461–71. doi: 10.1007/s11999-011-2174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yahdi M, Abdelmageed S, Lowden J, Tannenbaum L. Vancomycin-resistant enterococci colonization-infection model: parameter impacts and outbreak risks. J Biol Dyn. 2012;6(2):645–62. doi: 10.1080/17513758.2012.670733. [DOI] [PubMed] [Google Scholar]
- 96.Yang YS, Lee YT, Tsai WC, Kuo SC, Sun JR, Yang CH, et al. Comparison between bacteremia caused by carbapenem resistant Acinetobacter baumannii and Acinetobacter nosocomialis. BMC Infect Dis. 2013;13:311. doi: 10.1186/1471-2334-13-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goswami DY, Trivedi DM, Block SS. Photocatalytic disinfection of indoor air. J Sol Energy Eng. 1997;119(1):92–6. doi: 10.1115/1.2871871. [DOI] [Google Scholar]
- 98.Goswami TK, Hingorani SK, Griest H, Goswami DY, Block SS. Photocatalytic system to destroy bioaerosols in air. J Adv Oxid Technol. 1999;4(2):185–8. [Google Scholar]
- 99.Yao Y, Ochiai T, Ishiguro H, Nakano R, Kubota Y. Antibacterial performance of a novel photocatalytic-coated cordierite foam for use in air cleaners. Appl Catal B. 2011;106(9):592–9. doi: 10.1016/j.apcatb.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blanco-Galvez J, Fernández-Ibáñnez S, Malato-Rodriguez J. Solar photocatalytic detoxification and disinfection of water: recent overviews. J Sol Energy Eng. 2007;129(1):4–15. doi: 10.1115/1.2390948. [DOI] [Google Scholar]
- 101.Cooper AT, Goswami DY, Block SS. Solar photochemical detoxification and disinfection for water treatment in tropical developing countries. J Adv Oxid Technol. 1998;3(2):151–4. [Google Scholar]
- 102.Lonnen J, Kilvington LJ, Kehoe SC, Al-Toutai F, McGuigan KG. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005;39(5):877–83. doi: 10.1016/j.watres.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 103.Malato S, Blanco J, Alarcón DC, Maldonaldo MI, Fernández-Ibáñez P, Gernjak W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal Today. 2007;122(1–2):137–49. doi: 10.1016/j.cattod.2007.01.034. [DOI] [Google Scholar]
- 104.Herrera-Melián JA, Doña-Rodríguez JM, Viera-Suárez A, Rendón ET, Valdés-do-Campo C, Arana J, et al. The photocatalytic disinfection of urban waste waters. Chemosphere. 2000;41(3):323–7. doi: 10.1016/S0045-6535(99)00502-0. [DOI] [PubMed] [Google Scholar]
- 105.Watts RJ, Kong S, Orr MP, Miller GC, Henry BE. Photocatalytic inactivation of coliform bacteria in secondary wastewater effluent. Water Res. 1995;29(1):95–100. doi: 10.1016/0043-1354(94)E0122-M. [DOI] [Google Scholar]
- 106.Aboelzahab A, Azad AM, Dolan S, Goel V. Mitigation of Staphylococcus aureus-mediated surgical site infections with photoactivated TiO2 coatings on Ti implants. Adv Healthc Mater. 2012;1(3):285–91. doi: 10.1002/adhm.201100032. [DOI] [PubMed] [Google Scholar]
- 107.Szczawiński J, Tomaszewski H, Jackowska-Tracz A, Szczawińska ME. Effect of UV radiation on survival of Salmonella Enteritidis on the surface of ceramic tiles coated with TiO2. Bull Vet Inst Pulawy. 2010;54:479–83. [Google Scholar]
- 108.Szczawiński J, Tomaszewski H, Jackowska-Tracz A, Szczawińska ME. Survival of Staphylococcus aureus exposed to UV radiation on the surface of ceramic tiles coated with TiO2. Pol J Vet Sci. 2011;14(1):41–6. doi: 10.2478/v10181-011-0006-y. [DOI] [PubMed] [Google Scholar]
- 109.Zhao J, Yang X. Photocatalytic oxidation for indoor air purification: a literature review. Build Environ. 2003;38(5):645–54. doi: 10.1016/S0360-1323(02)00212-3. [DOI] [Google Scholar]
- 110.Chen SC, Liao CM, Li SS, You SH. A probabilistic transmission model to assess infection risk from Mycobacterium tuberculosis in commercial passenger trains. Risk Anal. 2011;31(6):930–9. doi: 10.1111/j.1539-6924.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 111.Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection: theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144(2):302–6. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 112.Schaal KP. Medical and microbiological problems arising from airborne infection in hospitals. J Hosp Infect. 1991;18(A):451–9. doi: 10.1016/0195-6701(91)90056-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cappelletty D. Microbiology of bacterial respiratory infections. Pediatr Infect Dis J. 1998;17(8):55–61. doi: 10.1097/00006454-199808001-00002. [DOI] [PubMed] [Google Scholar]
- 114.Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis. 2012;206(6):902–6. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chuaybamroong P, Chotigawin R, Supothina S, Sribenjalux P, Larpkiattaworn S, Wu CY. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air. 2010;20(3):246–54. doi: 10.1111/j.1600-0668.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 116.Goswami DY, Vijayaraghavan S, Lu S, Tamm G. New and emerging developments in solar energy. Sol Energy. 2004;76(1–3):33–43. doi: 10.1016/S0038-092X(03)00103-8. [DOI] [Google Scholar]
- 117.Lee SH, Pumprueg S, Moudgil B, Sigmund W. Inactivation of bacterial endospores by photocatalytic nanocomposites. Colloids Surf B: Biointerfaces. 2005;40(2):93–8. doi: 10.1016/j.colsurfb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Venieri D, Markogiannaki E, Chatzisymeon E, Diamadopoulos E, Mantzavinos D. Inactivation of Bacillus anthracis in water by photocatalytic, photolytic and sonochemical treatment. Photochem Photobiol Sci. 2013;12(4):645–52. doi: 10.1039/C2PP25198A. [DOI] [PubMed] [Google Scholar]
- 119.Craun MF, Craun GF, Calderon RL, Beach MJ. Waterborne outbreaks reported in the United States. J Water Health. 2006;4(2):9–13. doi: 10.2166/wh.2006.016. [DOI] [PubMed] [Google Scholar]
- 120.Cheng YW, Chan RCY, Wong PK. Disinfection of Legionella pneumophila by photocatalytic oxidation. Water Res. 2007;41(4):842–52. doi: 10.1016/j.watres.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 121.Sadiq R, Rodriguez MJ. Disinfection by-products (DBPs) in drinking water and predictive models for their occurrence: a review. Sci Total Environ. 2004;321(1–3):21–46. doi: 10.1016/j.scitotenv.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 122.Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM. Occurrence, genotoxicity and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res. 2007;636(1–3):178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Belapurkar AD, Sherkhane P, Kale SP. Disinfection of drinking water using photocatalytic technique. Curr Sci. 2006;91(1):73–6. [Google Scholar]
- 124.Bonetta S, Bonetta S, Motta F, Strini A, Carraro E. Photocatalytic bacterial inactivation by TiO2-coated surfaces. AMB Express. 2013;3(1):59. doi: 10.1186/2191-0855-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Evans P, Sheel DW. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf Coat Technol. 2007;201(22–23):9319–24. doi: 10.1016/j.surfcoat.2007.04.013. [DOI] [Google Scholar]
- 126.Frank SN, Bard AJ. Heterogenous photocatalytical oxidation of cyanide and sulfite in aqueous solutions at semiconductor powders. J Phys Chem. 1977;81(15):1484–8. doi: 10.1021/j100530a011. [DOI] [Google Scholar]
- 127.Dadjour MF, Ogino C, Matsumura S, Nakamura S, Shimizu N. Disinfection of Legionella pneumophila by ultrasonic treatment with TiO2. Water Res. 2006;40(6):1137–42. doi: 10.1016/j.watres.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 128.Matsuyama M, Usami T, Masuda K, Niimi N, Ohta M, Ueda M. Prevention of infection in dental procedures. J Hosp Infect. 1997;35(1):17–25. doi: 10.1016/S0195-6701(97)90164-X. [DOI] [PubMed] [Google Scholar]
- 129.Danshvar D, Niaei A, Akbari S, Aber S, Kazemian N. Photocatalytic disinfection of water polluted by Pseudomonas aeruginosa. Global Nest J. 2007;9(3):1–5. [Google Scholar]
- 130.Sichel C, Tello J, de Cara M, Fernãndez-Ibáñez P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal Today. 2007;129(1–2):152–60. doi: 10.1016/j.cattod.2007.06.061. [DOI] [Google Scholar]
- 131.Gupta K, Singh RP, Pandey A, Pandey A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus, P. aeruginosa and E. coli. Beilstein J Nanotechnol. 2013;4:345–51. doi: 10.3762/bjnano.4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pathakoti K, Morrow S, Han C, Pelaez M, He X, Dionysiou DD, et al. Photoinactivation of Escherichia coli by sulfur-doped and nitrogen-fluorine-codoped TiO2 nanoparticles under solar simulated light and visible light irradiation. Environ Sci Technol. 2013;47(17):9988–96. doi: 10.1021/es401010g. [DOI] [PubMed] [Google Scholar]
- 133.Shiraishi K, Koseki H, Tsurumoto T. Antibacterial metal implant with a TiO2 conferred photocatalytic bactericidal effect against Staphylococcus aureus. Surf Interface Anal. 2009;41(1):17–22. doi: 10.1002/sia.2965. [DOI] [Google Scholar]
- 134.Upritchard HG, Yang J, Bremer PJ, Lamont IL, McQuillan AJ. Adsorption to metal oxides of the Pseudomonas aeruginosa siderophore pyoverdine and implications for bacterial biofilm formation on metals. Langmuir. 2007;23(13):7189–95. doi: 10.1021/la7004024. [DOI] [PubMed] [Google Scholar]
- 135.Chawengkijwanich C, Hayata Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int J Food Microbiol. 2008;123(3):288–92. doi: 10.1016/j.ijfoodmicro.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 136.Chorianopoulos NG, Tsoukleris DS, Panagou EZ, Falaras P, Nychas GJ. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiol. 2011;28(1):164–70. doi: 10.1016/j.fm.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 137.Oza G, Pandey S, Gupta A, Shinde S, Mewada A, Jagadale P, et al. Photocatalysis-assisted water filtration: using TiO2-coated vertically aligned multi-walled carbon nanotube array for removal of Escherichia coli O157:H7. Mater Sci Eng C Mater Biol Appl. 2013;33(7):4392–400. doi: 10.1016/j.msec.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 138.Matsunaga T, Tomoda R, Nakajima T, Nakamura N, Komine T. Continuous-sterilization system that uses photosemiconductor powders. Appl Environ Microbiol. 1988;54(6):1330–3. doi: 10.1128/aem.54.6.1330-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnol. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sekiguchi Y, Yao Y, Ohko Y, Tanaka K, Ishido T, Fujishima A, et al. Self-sterilizing catheters with titanium dioxide photocatalyst thin films for clean intermittent catheterization: basis and study of clinical use. Int J Urol. 2007;14(5):426–30. doi: 10.1111/j.1442-2042.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- 141.Nakamura H, Tanaka M, Shinohara S, Gotoh M, Karube I. Development of a self-sterilizing lancet coated with a titanium dioxide photocatalyticnano-layer for self-monitoring of blood glucose. Biosens Bioelectron. 2007;22(9–10):1920–5. doi: 10.1016/j.bios.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 142.Li Y, Leung P, Yao L, Song QW, Newton E. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62(1):58–63. doi: 10.1016/j.jhin.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 143.Marchi J, Amorim EM, Lazar DR, Ussui V, Bressiani AH, Cesar PF. Physico-chemical characterization of zirconia-titania composites coated with an apatite layer for dental implants. Dent Mater. 2013;29(9):954–62. doi: 10.1016/j.dental.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 144.Sawase T, Jimbo R, Wennerberg A, Suketa N, Tanaka Y, Atsuta M. A novel characteristic of porous titanium oxide implants. Clin Oral Implants Res. 2007;18(6):680–5. doi: 10.1111/j.1600-0501.2007.01404.x. [DOI] [PubMed] [Google Scholar]


