Abstract
OBJECTIVES
We investigated the development of binding and neutralizing antibodies to GM-CSF in patients receiving prolonged therapy with GM-CSF as adjuvant therapy of melanoma and the impact of these antibodies on biologic effects.
METHODS
Fifty-three patients with high-risk melanoma which had been surgically excised were treated with GM-CSF, 125 µg/m2 daily for 14 days every 28 days for 1 year following surgical resection of disease. Serum samples for antibodies to GM-CSF were measured before treatment and on Study Days 155 and 351. Blood draws for testing biologic effects were keyed to GM-CSF administration: Days 0 (before), 15 (after 14 days on GM-CSF), 29 (after 14 days off GM-CSF), 155, and 351 (after 14 days on GM-CSF in the 6th and 13th cycle of treatment).
RESULTS
Of 53 patients enrolled, 43 were evaluable for the development of anti-GM-CSF antibodies. Of these, 93% developed binding antibodies and 42% developed both binding and neutralizing antibodies. The increase in the white blood cell (WBC) count, percent eosinophils, or neopterin levels engendered by GM-CSF administration, was abrogated or markedly decreased in patients with neutralizing antibodies but not in patients who developed only binding antibodies.
CONCLUSIONS
Ninety-three percent of patients with melanoma treated with GM-CSF as adjuvant therapy develop antibodies to GM-CSF. In those with neutralizing antibodies, a diminution of the biologic effects of GM-CSF was observed. The development of neutralizing antibodies might also abrogate the potential clinical benefit of this treatment and should be considered in the design of future clinical trials.
Keywords: GM-CSF, melanoma, adjuvant therapy, clinical trial, neutralizing antibodies
INTRODUCTION
Patients with a thick primary melanoma (≥ 4 mm) or melanoma metastatic to regional lymph nodes are at high risk for melanoma recurrence and death.1,2 Currently, in 2014, high-dose interferon alfa-2b (IFN-α) and peginterferon alfa-2b (pegIFN-α2b) are the only agents approved by the Federal Food and Drug Administration (FDA) for adjuvant therapy of this patient population. These treatments have marginal efficacy. While IFN does prolong progression-free survival (PFS), the overall survival (OS) benefit is minimal.3–5 Moreover, the regimens are associated with considerable toxicity. These considerations led the National Comprehensive Cancer Network (NCCN) in 2014 to recommend participation in a clinical trial or observation for these patients.6 The NCCN Panel relegated treatment with IFN-α to category 2B, meaning that there is a lower level of evidence and no uniform NCCN consensus. It is clear that more effective, less toxic treatments are needed for adjuvant therapy of melanoma patients who are at high risk for recurrence. Prospective Phase III randomized pivotal trials of other agents for adjuvant therapy of melanoma are ongoing7, including a trial of ipilimumab vs. observation, ipilimumab vs. IFN-α, and trials of two vaccines (MAGE-A3 and a polyvalent melanoma vaccine, Pol-103A), but final results have not yet been released. An interim analysis of the MAGE-A3 trial showed that the study did not meet its first co-primary endpoint as it did not significantly extend disease-free survival in the overall patient population. Analysis regarding the second co-primary endpoint of efficacy in patients who demonstrate a favorable gene signature will be reported in 2015. An interim analysis of the trial of ipilimumab vs observation showed that it met its primary end-point of improved progression-free survival.8 A prospective randomized multicenter Phase III trial intended to test the efficacy of GM-CSF (Leukine) vs. vaccine vs. placebo as adjuvant therapy of melanoma (E4697) is ongoing. Accrual to this trial has now been completed and preliminary results reported,9 but patients are still being followed, and the study is expected to be completed in 2014.
GM-CSF is a hematopoietic growth factor which stimulates proliferation and differentiation of hematopoietic progenitor cells and is approved for this purpose.10 In addition, GM-CSF has immunologic activities that play a vital role in various diverse functions of the immune system. These include its ability to activate macrophages, which distinguish tumor cells from normal cells and kill only the tumor cells,9 stimulation of peripheral blood monocytes in vitro to become cytotoxic for human melanoma cells,10,11 production of monocyte activation and tumoricidal activity following in vivo administration,11,12 and stimulation of production of an angiogenesis inhibitor by macrophages.13 GM-CSF also serves as the principal mediator of proliferation, maturation and migration of dendritic cells,14–16 antigen presenting cells that play a major role in the induction of primary and secondary T-cell immune responses.
These considerations led to the design and conduct of several small hypothesis-generating clinical trials which showed that administration of GM-CSF might offer clinical benefit as adjuvant therapy of melanoma.17–20 Moreover, a randomized Phase II trial of GM-CSF + ipilimumab vs. ipilimumab monotherapy for patients with metastatic melanoma suggested that patients treated with the combination enjoyed significantly longer overall survival and less toxicity than patients treated with ipilimumab monotherapy.21
GM-CSF is a recombinant human granulocyte-macrophage colony stimulating factor (rhu GM-CSF) produced by recombinant DNA technology in a yeast (S. cerevisiae) expression system. Like the native protein, it is a glycoprotein of 127 amino acids but differs from the native protein in molecular mass.22,23 Moreover, the amino acid sequence of GM-CSF differs from the natural human GM-CSF by a substitution of leucine at position 23, and glycosylation is different from that of the native protein. These differences from the native GM-CSF could lead to immunogenicity of this molecule. In this study, we evaluated the biologic effects of GM-CSF on the WBC and percent eosinophils because these are routine clinical laboratory tests available to all physicians who are treating patients with GM-CSF. In addition, we performed serial determinations of serum neopterin levels as a means to measure monocyte/macrophage activation since release of neopterin is a sign of macrophage activation24,25 and administration of GM-CSF results in increased production of neopterin.26,27
The approved use of GM-CSF is for short-term administration. Long-term (1 year) administration of GM-CSF does not appear to be associated with untoward clinical side effects. In this report, we present results of a systematic evaluation of the development of antibodies to GM-CSF in patients treated with prolonged GM-CSF therapy and the effect of such antibody development on the biologic effects of GM-CSF.
PATIENTS AND METHODS
Patients
Fifty-three adult patients with histologically proven melanoma who were at high risk for recurrence (AJCC Stage II (T4), III, and IV surgically excised) were enrolled in a clinical trial to determine the effect of adjuvant treatment with GM-CSF on immune and biologic responses. Eligible patients were those in whom all known melanoma had been excised and had no evidence of disease on metastatic workup. Patients were required to start treatment with the study drug within 90 days of the last surgical procedure in which melanoma was present. Prior treatment with other adjuvant therapies, including IFN, before disease recurrence that led to study eligibility was allowed and adjuvant radiation therapy was allowed. The research protocol was approved by the relevant Institutional Review Boards and all participants gave written informed consent. The trial was registered on ClinicalTrials.gov with Identifier NCT00350597.
Patients were treated with GM-GSF, 125 µg/m2 once daily (maximum dose 250 µg) subcutaneously for 14 days followed by 14 days rest (28-day cycle). Treatment was continued for 1 year (13 28-day cycles) or until disease progression that required systemic therapy. Blood samples for testing of the biologic effects of GM-CSF (WBC, differential cell count, and serum neopterin levels) were keyed to the timing of GM-CSF administration (Fig. 1). The samples were obtained on Study Day 0 (pretreatment), Day 15 (after 14 days of GM-CSF treatment in the first cycle), Day 29 (after 14 days of rest in the first cycle), Day 155 (after 14 days of treatment in the 6th cycle), and Day 351 (after 14 days of treatment in the 13th cycle). Serum samples for determination of binding and neutralizing antibodies to GM-CSF were obtained at the same time points. The serum was separated, stored frozen at −70°C, and shipped in batches from the clinical site to the test site.
Figure 1.
Timing of Biologic Assessments Relative to GM-CSF Administration
Assays for anti-GM-CSF antibodies
Assays to evaluate both binding antibodies assay and neutralizing antibodies were developed for use in human serum. Cut-point, selectivity, sensitivity, precision, freeze and thaw stability, short-term stability, long-term stability, linearity of dilutions, parallelism, recovery and robustness/ruggedness of the method have been studied. However, these assays would not allow us to distinguish whether the anti-GM-CSF antibodies are neutralizing or that the apparent neutralizing effect was due to high-titer binding antibodies.
Binding Antibodies
The measurement of binding antibodies is based on a qualitative bridging assay design where antibody in the sample is bound bi-valently (e.g. IgG) or multi-valently (e.g. IgM) to biotinylated GM-CSF captured to streptavidin coated wells in a 96-well microtiter plate and detected by using Europium radio-isotope (Eu) labeled GM-CSF.
Briefly, biotinylated GM-CSF (10 ng/well) is applied to streptavidin-coated wells. After incubation with slow shaking at room temperature, the wells are washed. Subsequently, calibration standards, quality control (QC) samples or study samples are applied to the wells, followed by the addition of assay buffer. After incubation and slow shaking at room temperature, the wells are washed. Subsequently, Eu-labeled GM-CSF (50 ng/well) is added and incubated. After washing, the Enhancement Solution is added to each well and incubated. Finally, the plates are submitted for Eu-measurement using the Victor multilabel reader (Perkin-Elmer Life Sciences, Turku Finland).
Neutralizing Antibodies
The bioassay is based on the TF-1 cell line the growth of which is highly dependent on GM-CSF concentration. The TF-1 cell based bioassay is calibrated with GM-CSF. QC and study samples are added to TF-1 cells and grown with a constant amount of GM-CSF (1.00 IU/mL). In the presence of neutralizing antibodies the proliferation of TF-1 cells is decreased. Proliferation is measured using commercially available DELFIA cell proliferation kit (Perkin-Elmer Life Sciences, Turku, Finland) based on the measurement of 5-bromo-2´-deoxyuridine (BrdU) incorporation during DNA synthesis in proliferating cells.
Briefly, GM-CSF calibration standards + assay media and QC or study samples + assay media + GM-CSF (1.00 IU/mL) are added to the wells of 96-well microtiter plates and incubated for 1–2 h at 37 °C. Subsequently, a TF-1 cell suspension (2000 cells/well) is added. Plates are then incubated at 37 °C in an atmosphere of 5% CO2. Then the anti-BrdU-Eu solution is added (40 µL/well). Plates are again incubated at 37 °C (5% CO2) followed by washing and incubation steps, and the measurement of time-resolved fluorescence (TR-FIA) according to manufacturer's instructions.
Sample Analysis Scheme
A four-step sample analysis scheme has been employed:
Initial immunoassay screening assay: All study samples were assessed to be either positive or negative for binding antibodies, based on an assay plate-specific cut point. The cut point has been determined by calculating the average of matrix blank values multiplied by a constant cut point factor of 5.16 (determined using 95th percentile, corresponding to 5% false positive rate, during the assay evaluation phase using sera from healthy individuals, n=27). This procedure using an assay plate-specific cut point has been applied to control inter-assay variability (day-to-day variability and change of materials or reagents).
Confirmatory assay: Samples determined as potentially positive for binding antibodies in the initial screening assay (signal above cut point) were further investigated by means of an immuno depletion experiment. An excess amount of GM-CSF was added to the wells containing study samples. A sample was considered as true positive for binding antibodies if the signal was reduced below <50% compared to the reference signal (no GM-CSF added).
Cell-based neutralizing antibody assay: Study samples previously confirmed positive for total binding antibodies were further tested for neutralizing antibodies against GM-CSF. Sample values below the specific cut point of 0.158 IU/mL (determined using mean – 3×SD, corresponding to 0.1% false positive rate, of the LN converted concentrations from sera of healthy individuals, n=21) are determined positive and values at or above cut point are determined negative for neutralizing antibodies.
Titration: Samples determined positive for antibodies after step II and III were further investigated quasi-quantitatively using assays I and III. Samples were diluted in buffer (e.g. 1:10 serial dilution steps) until the response in the assay was negative (below the assay cut point). The titer was determined as the last dilution factor with a positive response.
Assay for Neopterin
Serum neopterin levels (normal range: 0.3–3.0 ng/ml) were determined with a validated ELISA procedure using a test kit (MP Biochemicals, Orangeburg, N.Y.). All specimens were tested immediately after thawing in one assay.
In brief, each of neopterin standards, controls and patient samples is added to microtiter plates with neopterin coated wells. Diluted enzyme conjugate is added to all wells except for the non-specific binding wells. The plates are then incubated at room temperature on a rotator. After that, contents of all wells are aspirated and blotted on absorbent paper. Wells are further washed with diluted wash buffer and aspirated to dryness. Finally, color substrate is added to all wells, incubated for thirty minutes on a rotator and followed by stopping solution. Results are read from absorbance at 450 nm and calculated using the supplied formula from the manufacturer.
Statistical Analysis
Clinical data and the results of the WBC and differential counts were collected on Case Report Forms at the Clinical Study Sites. The Neopterin determinations were done under the supervision of Theresa Whiteside at the University of Pittsburgh. The determinations of binding and neutralizing anti-GM-CSF antibodies were done by Timo Piironen in Turku, Finland. The Case Report Forms and spreadsheets of laboratory test results were all sent to the statistician, Scott Cruickshank, who performed the analysis.
Descriptive statistics were prepared for demographics and baseline disease-related characteristics. Categorical variables were summarized by number and percent; continuous variables by mean and standard deviation, median, and range. WBC, eosinophil, and neopterin values from blood samples obtained at baseline (Day 0) and post-baseline (Days 15 and 29) were summarized in a descriptive manner. The Wilcoxon signed rank test was used to compare baseline versus post-baseline values. Given the exploratory nature of this study, p-values are not adjusted for multiple testing.
RESULTS
Characteristics of the Study Population
Of the 53 patients who enrolled in this study, there were 43 who were considered evaluable for determination of the incidence of binding and neutralizing antibodies to GM-CSF. Patients were considered evaluable for antibody formation if they had a serum sample available at baseline and on Study Day 155, 351, or both. There were 31 male (72.1%) and 12 female (27.9%) with a mean age of 56.4 ± 12.04 and a median of 55.0 (range 31–83). Eleven patients were over 65 (25.6%). The baseline melanoma diagnosis and staging characteristics of these patients are shown in Table 1. The biologic effects of the antibodies were reported only for patients who had samples for analysis available at baseline and on Study Days 155 and 351. There were 32 – 34 patients evaluable for assessment of biologic effects of the antibodies, depending on the particular assay involved.
Table 1.
Melanoma Diagnosis and Staging (Evaluable Patients, N=43)
| Characteristic | Patients (n=43) | |
|---|---|---|
| Current stage — no. (%) | ||
| Stage IIC | 3 | (7.0) |
| Stage IIIA | 5 | (11.6) |
| Stage IIIB | 26 | (60.5) |
| Stage IIIC | 3 | (7.0) |
| Stage IV: M1a | 3 | (7.0) |
| Stage IV: M1b | 1 | (2.3) |
| Stage IV: M1c | 2 | (4.6) |
| Location of primary — no. (%)† | ||
| Head/neck | 15 | (34.9) |
| Trunk | 12 | (27.9) |
| Leg | 6 | (13.9) |
| Arm | 5 | (11.6) |
| No known primary | 5 | (11.6) |
| Breslow thickness — mm‡ | ||
| N | 35* | |
| Mean (± SD) | 2.7 (± 3.06) | |
| Median (Minimum, Maximum) | 1.5 (0.3, 14.0) | |
| Breslow thickness category — mm‡ | ||
| ≤ 1.0 | 12 | (34.3) |
| 1.01 to 2.0 | 10 | (28.6) |
| 2.01 to 4.0 | 6 | (17.1) |
| > 4.0 | 7 | (20.0) |
No reported Breslow thickness for 8 patients: no known primary (n=6), not applicable (n=1), and unknown (n=1).
Anti-GM-CSF Antibody Determinations
Of the 43 evaluable patients, none had anti-GM-CSF antibodies at baseline. Forty (93%) of the patients developed anti-GM-CSF binding antibodies by the time of testing on Study Day 155 and/or 351; 3 patients remained antibody negative. Eighteen (42%) had anti-GM-CSF neutralizing antibodies (or high-titer binding antibodies), and 22 (51%) had binding antibodies only. All patients with neutralizing antibodies also had binding antibodies. No patient had neutralizing antibodies when tested on Study Day 15 or 29; 1 patient had binding antibodies on Study Day 15 and 9 patients had binding antibodies on Study Day 29.
Biologic Effects of GM-CSF during the First Cycle of Treatment
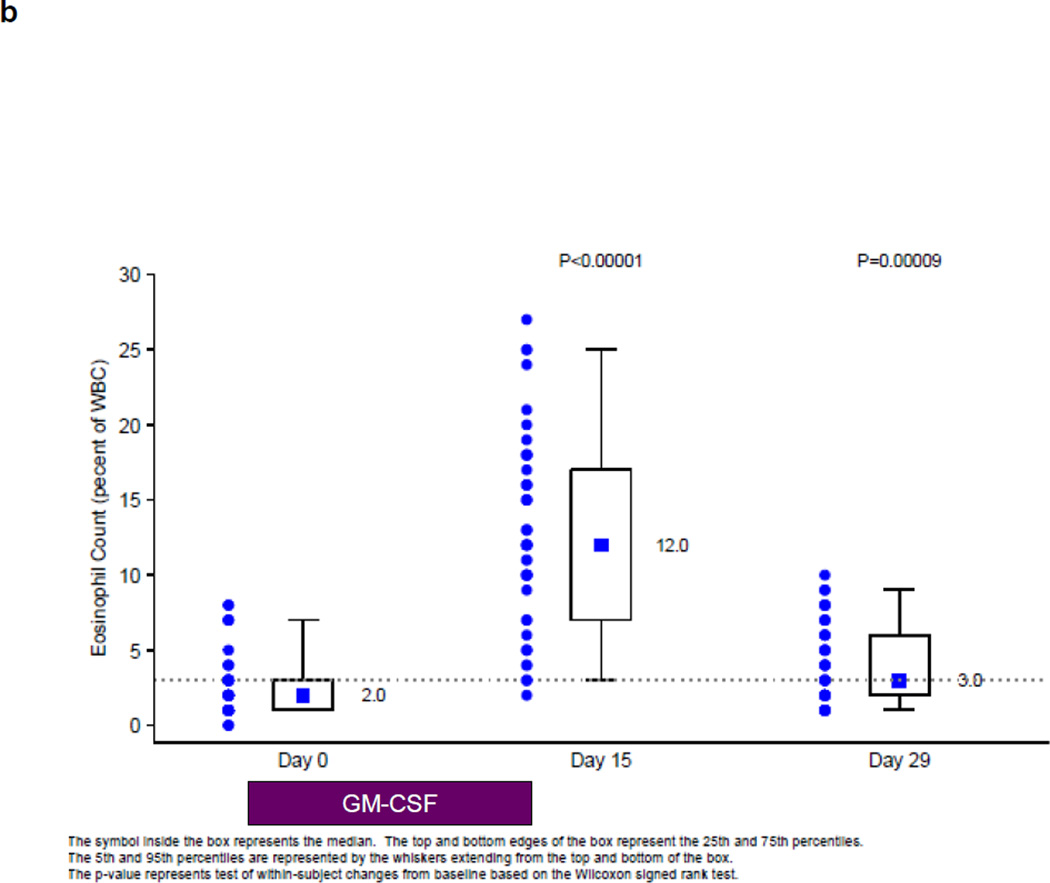
During the first cycle of treatment, before the development of neutralizing antibodies to GM-CSF, there was a significant increase in the WBC counts, eosinophil counts as a percentage of the WBC count and neopterin levels on Study Day 15, after 2 weeks on GM-CSF therapy (Fig. 2). The WBC and neopterin levels returned to baseline by Study Day 29, after 2 weeks rest. The eosinophil counts as a percentage of the WBC count also decreased after 2 weeks rest, but did not reach baseline levels. These analyses only included patients for whom values were available for all 3 study points.
Figure 2.
Biologic Assessments during the First Cycle of Therapy before Development of Neutralizing Antibodies
a: Summary of WBC counts at Baseline, Study Day 15, and Study Day 29 (n = 49)
b: Summary of Eosinophil Counts as a percentage of the WBC at Baseline, Study Day 15, and Study Day 29 (n = 46)
c: Summary of Neopterin Levels at Baseline, Study Day 15, and Study Day 29 (n = 49)
Biologic Effects of GM-CSF in Patients with and without Neutralizing Antibodies in the 6th and 13th Cycles of Treatment
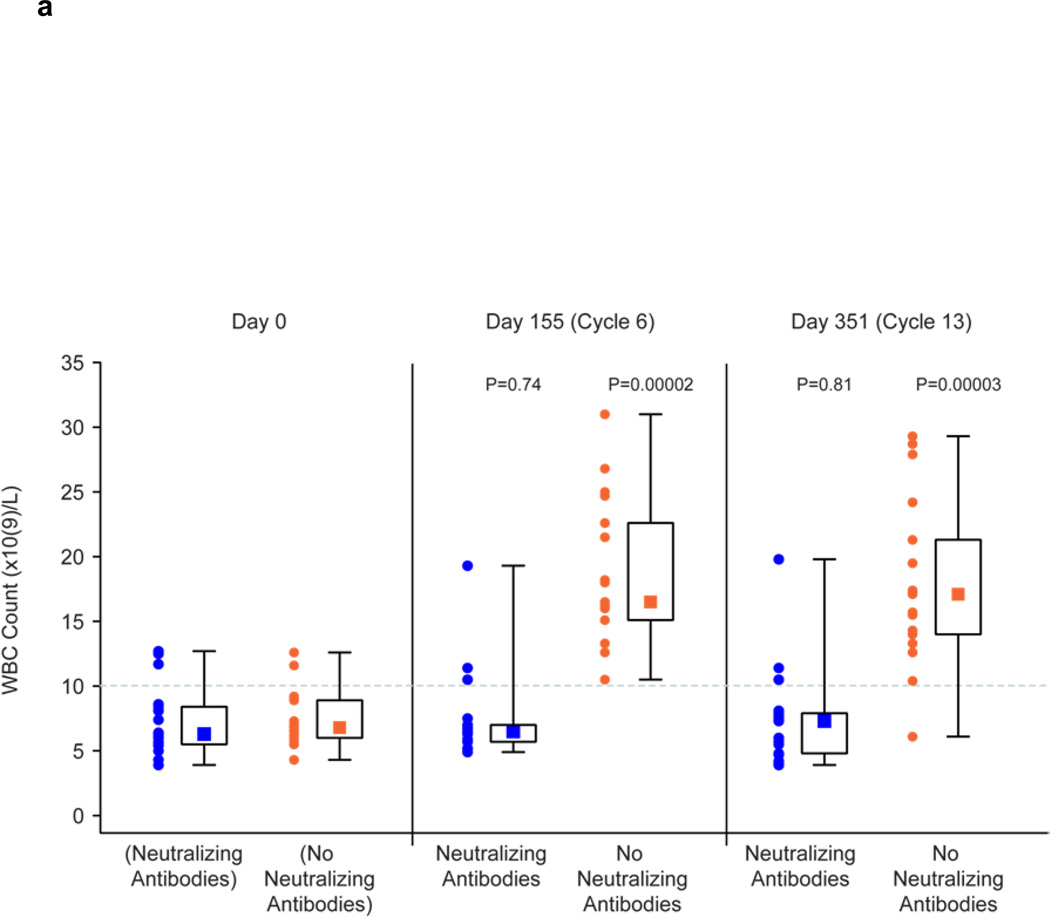
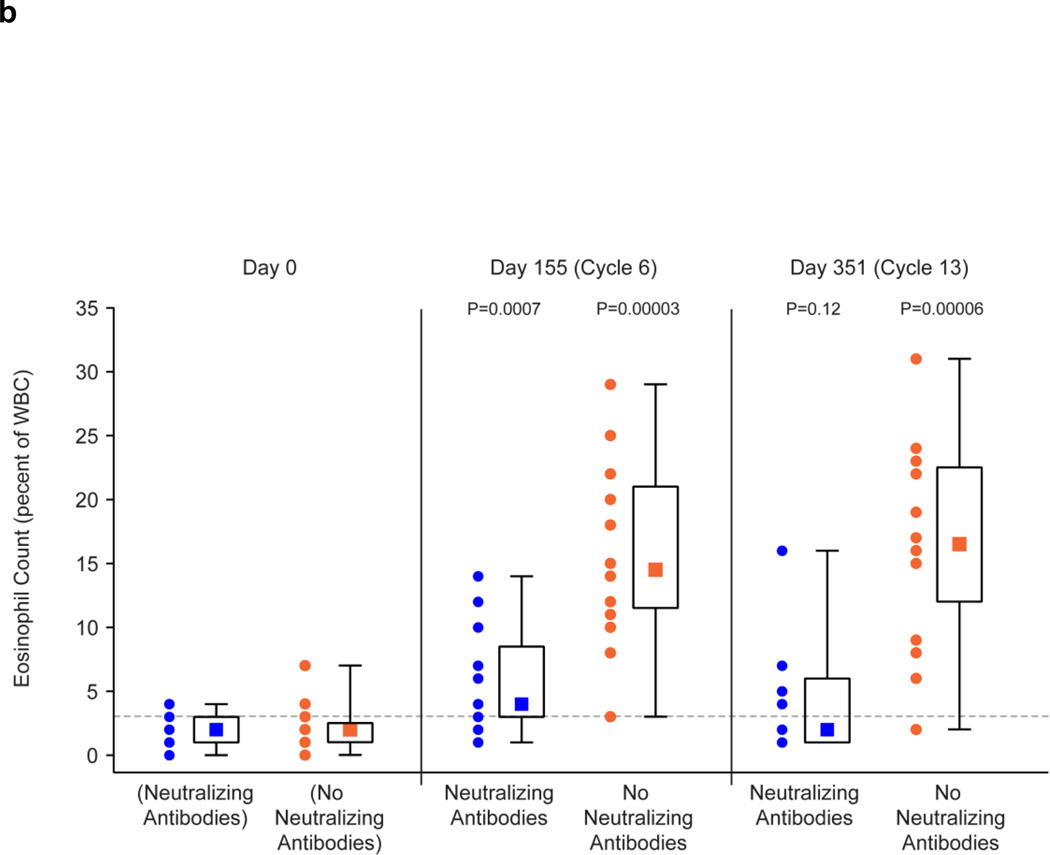
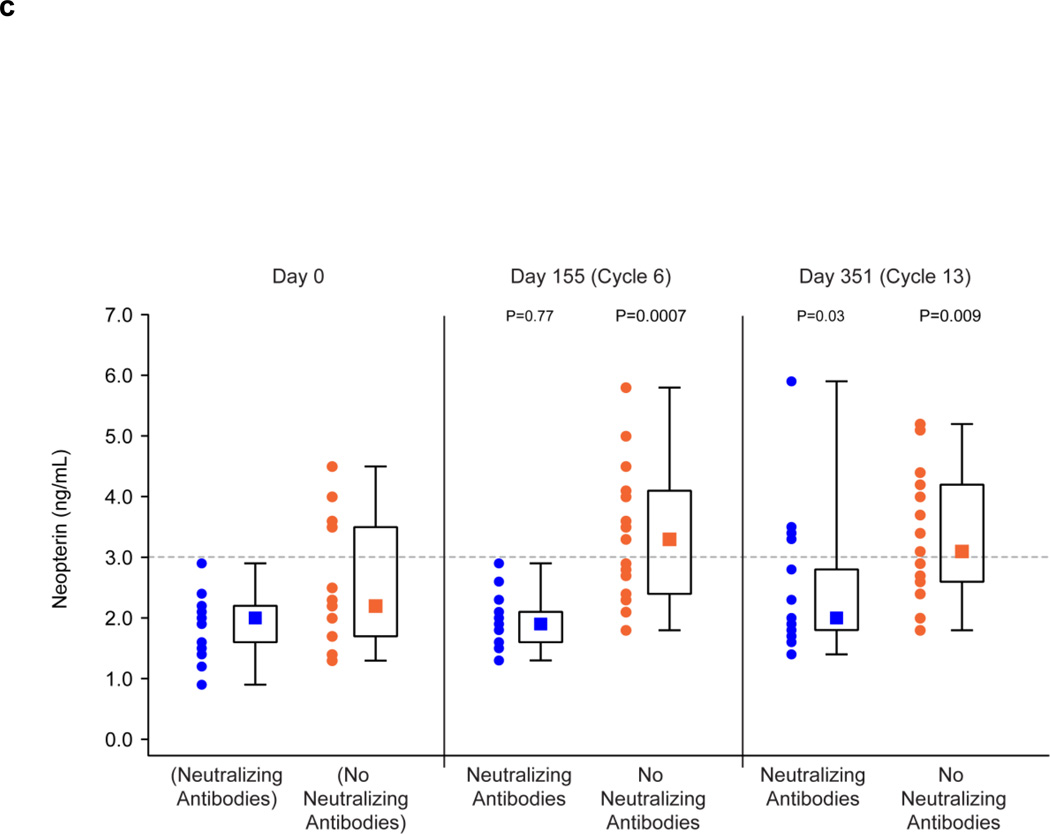
The presence of neutralizing antibodies abrogated or reduced the biologic effects of GM-CSF therapy in patients following 14 days of administration of GM-CSF in the 6th and 13th cycles of treatment (Fig. 3). The WBC counts on Study Days 155 (cycle 6) and 351 (cycle 13) were no different from the baseline counts in patients with neutralizing antibodies, whereas there was a significant increase from baseline levels following 14 days of GM-CSF therapy in patients without neutralizing antibodies (Fig. 3a). Results were less clear-cut for the impact of neutralizing antibodies on the eosinophil counts as a percentage of the WBC count and also the neopterin levels. In the absence of neutralizing antibodies, these values were significantly increased as compared to baseline following 14 days of GM-CSF therapy. In patients with neutralizing antibodies, the increase was abrogated on Study Day 351 for the eosinophil count as a percentage of the WBC count (Fig. 3b) and was abrogated on Study Day 155 for neopterin levels (Fig. 3c). For the other Study Days, the values of these tests were less in the presence of neutralizing antibodies than in their absence, but were still significantly above baseline values.
Figure 3.
Effect of Neutralizing and No Neutralizing Antibodies Present on Biologic Effects of GM-CSF Administration for 14 Days in Cycles 6 and 13.
a: Summary of WBC Counts on before Study and on Study Day 155 (Cycle 6) and 351 (Cycle 13) in Patients with Neutralizing Antibodies (n = 17) and No Neutralizing Antibodies (n = 17) to GM-CSF. WBC counts before Study are shown for patients who subsequently developed Neutralizing Antibodies and did not subsequently develop Neutralizing Antibodies.
b: Summary of Eosinophil Counts as a percentage of the WBC Count on before Study and on Study Day 155 (Cycle 6) and 351 (Cycle 13) in Patients with Neutralizing Antibodies (n = 16) and No Neutralizing Antibodies (n = 16) to GM-CSF. Eosinophil counts before Study are shown for patients who subsequently developed Neutralizing Antibodies and did not subsequently develop Neutralizing Antibodies.
c: Summary of Neopterin Levels before Study and on Study Day 155 (Cycle 6) and 351 (Cycle 13) in Patients with Neutralizing Antibodies (n = 17) and No Neutralizing Antibodies (n = 15) to GM-CSF. Neopterin Levels before Study are shown for patients who subsequently developed Neutralizing Antibodies and did not subsequently develop Neutralizing Antibodies.
DISCUSSION
GM-CSF is approved for short-term use for acceleration of myeloid recovery and/or mobilization of hematopoietic progenitor cells in certain circumstances. For that use, consideration of the development of anti-GM-CSF antibodies is not an issue because therapy is short-term and would generally be completed before development of such antibodies would occur. The drug prescribing information states that neutralizing antibodies were detected in 5 of 214 patients (2.3%) after receiving GM-CSF by continuous IV infusion (three patients) or subcutaneous injection (SC) (two patients) for 28 to 84 days in multiple courses It further states that “1 of 75 patients (1.3%) receiving GM-CSF for Crohn’s disease developed neutralizing antibodies.” It also states that “The clinical relevance of the presence of these antibodies are (sic) unknown”. “Drug-induced neutropenia, neutralization of endogenous GM-CSF activity and diminution of the therapeutic effect of Leukine secondary to formation of neutralizing antibody remain a theoretical possibility.”10 No references are given regarding the incidence of neutralizing or binding anti-GM-CSF antibodies nor are the methods used to detect them described.
In clinical trials, GM-CSF monotherapy is being used as adjuvant therapy of cancer, as a vaccine adjuvant in patients with cancer, and in combination with other agents as therapy of metastatic disease.7 When GM-CSF is used in cancer therapy, it is administered repeatedly on a long-term basis (months to years). In this circumstance, the development of anti-GM-CSF antibodies is a relevant consideration because in these studies the treatment is given long enough to allow time for the development of antibodies including those which might neutralize the effects of the GM-CSF. Moreover, the administration of intermittent courses is a method known in vaccinations for infectious diseases to induce strong immune responses.
We report herein that 93% of 43 evaluable patients with melanoma treated with GM-CSF had binding antibodies to GM-CSF when determinations were made after 6 or 12 months of therapy, or both. Moreover, 42% of the patients had neutralizing anti-GM-CSF antibodies and these resulted in abrogation or diminution of the biological effects of ongoing GM-CSF administration. We do not know the reason for the apparent discordance of Day 155 and Day 351 test results in Figure 4b (Eosinophil Count) and Figure 4c (Neopterin). Taken as a whole, however, the data strongly support that the development of neutralizing antibodies abrogates the biologic responses to GM-CSF. It seems reasonable that the development of such antibodies which diminish the biological effects of GM-CSF administration might also diminish the therapeutic efficacy of GM-CSF administration. Moreover, we do not know which of the immunologic effects of GM-CSF are relevant to the efficacy and whether this is impacted by the development of neutralizing antibodies. Our study was not designed to evaluate the effect of the development of anti-GM-CSF antibodies on clinical outcome; the number of patients included and the timing of the sampling for antibody analysis was not sufficient to make any statement regarding this.
There have been previous reports of the development of binding and neutralizing antibodies following treatment of patients with GM-CSF, but in most of these studies, the GM-CSF utilized was produced in an Escherichia coli (E. coli) expression system.28–34 The E. coli derived GM-CSF is unlike the yeast-derived GM-CSF utilized in the studies reported herein because it is not glycosylated. One report indicated that the antibodies react with unprotected glycosylation sites on the native protein backbone which are exposed in the non-glycosylated GM-CSF.28 Therefore the development of antibodies described in these studies may not correlate with the potential for the development of antibodies following treatment with GM-CSF produced in yeast. The E. coli-derived product is no longer manufactured. Nonetheless, it is noteworthy that in one study, in which the E. coli-derived product was administered to patients with metastatic colorectal carcinoma, results were similar to our findings; a high percentage of patients developed anti-GM-CSF binding antibodies and 40% developed neutralizing antibodies.33 Moreover, these neutralizing antibodies, but not binding antibodies, were associated with a significant reduction on the GM-CSF-induced expansion of leucocytes, neutrophils, and eosinophils.
There have been several reports of small studies showing the development of anti-GM-CSF antibodies in patients treated with yeast-derived GM-CSF. All 15 patients with prostate cancer treated with yeast-derived GM-CSF developed binding antibodies to GM-CSF and 60% developed antibodies that neutralized the biological activity of GM-CSF in vitro.35 In 32 patients given GM-CSF by embolization of the hepatic artery, 69% developed binding antibodies and 47% developed neutralizing antibodies to GM-CSF.36 The authors noted that in patients with neutralizing antibodies, some of the systemic effects of intrahepatic GM-CSF (leukocytosis and transient thrombocytopenia) seem to have diminished. In one report in which GM-CSF was given as a vaccine adjuvant along with cancer-associated peptides in patients with cancer, 13 of the 18 patients (72%) developed antibodies to GM-CSF; none of the analyzed sera expressed neutralizing activity. 37
Our data indicates that 93% of patients with melanoma treated with GM-CSF as adjuvant therapy develop antibodies to GM-CSF. In those with neutralizing antibodies the biologic effects of GM-CSF was diminished or abrogated. The development of such antibodies might also diminish the potential clinical benefit of this treatment and should be considered in the design of future clinical trials.
Acknowledgments
Source of Funding: This work was supported by Bayer Healthcare Pharmaceuticals, Wayne, N.J. and the Melanoma Research Institute, Joyce N. Furman Memorial Trust. No NIH Grants supported this work.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Melanoma of the Skin . In: AJCC Cancer Staging Manual. Seventh Edition. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti IA, editors. Springer; 2010. pp. 325–344. [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of Eastern Cooperative Oncology Group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 4.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20:1818–1825. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 5.Suciu S, Ives N, Eggermont AM, Kirkwood JM, et al. Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): An individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7500 melanoma patients. J Clin Oncol. 2014;32:5s. (suppl; abstrc 9064). [Google Scholar]
- 6.NCCN Guidelines Melanoma V 22014. www.nccn.org. [Google Scholar]
- 7.Clinical Trials Registry. 2014 at www.clinicaltrials.gov.)
- 8.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, et al. Ipilimumab versus placebo after complete resection of stage III melanoma: Initial efficacy and safety results from the EORTC 18071 phase III trial. J Clin Oncol. 2014 Abstrc No: LBA9008. [Google Scholar]
- 9.Lawson DH, Lee SJ, Tarhini AA, Margolin KA, Ernstoff MS. J.M. K. E4697: Phase III cooperative group study of yeast-derived granulocyte macrophage colony-stimulating factor (GM-CSF) versus placebo as adjuvant treatment of patients with completely resected stage III-IV melanoma. J Clin Oncol. 2010;28 doi: 10.1200/JCO.2015.62.0500. (suppl; abstr 8504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leukine (Sanofi), A Recombinant GM CSF - Yeast Expressed, Prescribing Information. 2013 [Google Scholar]
- 11.Chachoua A, Oratz R, Hoogmoed R, et al. Monocyte activation following systemic administration of granulocyte- macrophage colony-stimulating factor. J Immunother Emphasis Tumor Immunol. 1994;15:217–224. doi: 10.1097/00002371-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Demir G, Klein HO, Tuzuner N. Low dose daily rhGM-CSF application activates monocytes and dendritic cells in vivo. Leuk Res. 2003;27:1105–1108. doi: 10.1016/s0145-2126(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 14.Young JW, Szabolcs P, Moore MA. Identification of dendritic cell colony-forming units among normal human CD34+ bone marrow progenitors that are expanded by c-kit-ligand and yield pure dendritic cell colonies in the presence of granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha [published erratum appears in J Exp Med 1996 Mar 1;183(3):1283] J Exp Med. 1995;182:1111–1119. doi: 10.1084/jem.182.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995;154:5851–5861. [PubMed] [Google Scholar]
- 16.Szabolcs P, Avigan D, Gezelter S, et al. Dendritic cells and macrophages can mature independently from a human bone marrow-derived, post-colony-forming unit intermediate. Blood. 1996;87:4520–4530. [PubMed] [Google Scholar]
- 17.Daud AI, Mirza N, Lenox B, et al. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2008;26:3235–3241. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- 18.Grotz TE, Kottschade L, Pavey ES, Markovic SN, Jakub JW. Adjuvant GM-CSF Improves Survival in High-risk Stage IIIC Melanoma: A Single-center Study. Am J Clin Oncol. 2013 doi: 10.1097/COC.0b013e31827def82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor [see comments] J Clin Oncol. 2000;18:1614–1621. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 20.Spitler LE, Weber RW, Allen RE, et al. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim) administered for 3 years as adjuvant therapy of stages II(T4), III, and IV melanoma. Journal of immunotherapy. 2009;32:632–637. doi: 10.1097/CJI.0b013e3181a7d60d. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, Lee SJ, McDermott DF, et al. Multicenter, randomized phase II trial of GM-CSF (GM) plus ipilimumab (Ipi) versus Ipi alone in metastatic melanoma: E1608. ASCO. J Clin Oncol. 2013;31 (suppl; abstr CRA9007); 2013; Chicago. [Google Scholar]
- 22.Cantrell MA, Anderson D, Cerretti DP, et al. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1985;82:6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorr RT. Clinical properties of yeast-derived versus Escherichia coli-derived granulocyte-macrophage colony-stimulating factor. Clin Ther. 1993;15:19–29. discussion 18. [PubMed] [Google Scholar]
- 24.Huber C, Batchelor JR, Fuchs D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reibnegger G, Fuchs D, Fuith LC, et al. Neopterin as a marker for activated cell-mediated immunity: application in malignant disease. Cancer Detect Prev. 1991;15:483–490. [PubMed] [Google Scholar]
- 26.Crispino S, Lissoni P, Ardizzoia A, et al. Effects of granulocyte-macrophage colony stimulating factor on soluble interleukin-2 receptor serum levels and their relation to neopterin and tumor necrosis factor-alpha in cancer patients. J Biol Regul Homeost Agents. 1993;7:92–94. [PubMed] [Google Scholar]
- 27.Marth C, Weiss G, Koza A, et al. Increased production of immune activation marker neopterin by colony-stimulating factors in gynecological cancer patients. Int J Cancer. 1994;58:20–23. doi: 10.1002/ijc.2910580105. [DOI] [PubMed] [Google Scholar]
- 28.Gribben JG, Devereux S, Thomas NS, et al. Development of antibodies to unprotected glycosylation sites on recombinant human GM-CSF. Lancet. 1990;335:434–437. doi: 10.1016/0140-6736(90)90665-r. [DOI] [PubMed] [Google Scholar]
- 29.Ragnhammar P, Friesen HJ, Frodin JE, et al. Induction of anti-recombinant human granulocyte-macrophage colony-stimulating factor (Escherichia coli-derived) antibodies and clinical effects in nonimmunocompromised patients. Blood. 1994;84:4078–4087. [PubMed] [Google Scholar]
- 30.Sredni B, Caspi RR, Klein A, et al. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature. 1987;330:173–176. doi: 10.1038/330173a0. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JA, Lee DJ, Kidd P, et al. Subcutaneous granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndrome: toxicity, pharmacokinetics, and hematological effects. J Clin Oncol. 1989;7:629–637. doi: 10.1200/JCO.1989.7.5.629. [DOI] [PubMed] [Google Scholar]
- 32.Ullenhag G, Bird C, Ragnhammar P, et al. Incidence of GM-CSF antibodies in cancer patients receiving GM-CSF for immunostimulation. Clin Immunol. 2001;99:65–74. doi: 10.1006/clim.2000.4999. [DOI] [PubMed] [Google Scholar]
- 33.Wadhwa M, Bird C, Fagerberg J, et al. Production of neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies in carcinoma patients following GM-CSF combination therapy. Clin Exp Immunol. 1996;104:351–358. doi: 10.1046/j.1365-2249.1996.11704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadhwa M, Skog AL, Bird C, et al. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clin Cancer Res. 1999;5:1353–1361. [PubMed] [Google Scholar]
- 35.Rini B, Wadhwa M, Bird C, Small E, Gaines-Das R, Thorpe R. Kinetics of development and characteristics of antibodies induced in cancer patients against yeast expressed rDNA derived granulocyte macrophage colony stimulating factor (GM-CSF) Cytokine. 2005;29:56–66. doi: 10.1016/j.cyto.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Terai M, Sakashita H, Mastrangelo MJ, et al. Development of antibodies to granulocyte-macrophage colony stimulating factor (GM-CSF) after embolization of the hepatic artery with GM-CSF. Proceedings of AACR; Anaheim, CA: 2005. p. 5994a. [Google Scholar]
- 37.McNeel DG, Schiffman K, Disis ML. Immunization with recombinant human granulocyte-macrophage colony-stimulating factor as a vaccine adjuvant elicits both a cellular and humoral response to recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1999;93:2653–2659. [PubMed] [Google Scholar]