Abstract
Tau and its aggregates are linked to the pathology of Alzheimer’s disease (AD) and other tauopathies and, therefore, are explored as therapeutic targets for such disorders. Tau belongs to a family of microtubule-associated proteins (MAPs) that promote microtubule assembly. When hyperphosphorylated, tau becomes prone to forming aggregates. Increased brain levels of hyperphosphorylated tau correlate with dementia. Specificity protein 1 (Sp1), a transcription factor elevated in AD, is responsible for the transcription of AD-related proteins including the amyloid precursor protein (APP), tau, and its cyclin dependent kinase-5 (CDK5) activators. Tolfenamic acid promotes the degradation of Sp1, our previous studies demonstrated its ability to downregulate transcriptional targets of Sp1 like APP and reduce amyloid beta (Aβ), the main component of AD plaques. In this study, we administered tolfenamic acid daily to hemizygous R1.40 transgenic mice for 34 days, and examined tau and CDK5 gene and protein expression within the brain. Our results demonstrate that tolfenamic acid lowers tau mRNA and protein, as well as the levels of its phosphorylated form and CDK5. Thus, we present a drug candidate that inhibits the transcription of multiple major intermediates in AD pathology, thereby helping uncover a new mechanism-based approach for targeting AD.
Keywords: Alzheimer’s disease, CDK5, Sp1, Tau, Therapy, Tolfenamic acid
Introduction
Over 100 years have passed since the identification of Alzheimer's disease (AD) and no disease modifying drug has been found for this disorder. Current therapies try to recover the deteriorating mental functions by targeting symptoms, but fail to alter the debilitating course of the disease that ultimately leads to total memory loss and death. None of the few available medications targets the characteristic pathological aggregates in AD, the extracellular senile amyloid plaques and the intracellular neurofibrillary tau tangles.
The microtubule-associated protein tau was first isolated and recognized for its role in microtubule assembly in 1975 (Weingarten et al. 1975). In AD and other tauopathies, tau assembles forming pathological deposits. AD is the most common tauopathy where hyperphosphorylated tau aggregates as paired helical filaments (PHFs) and tangles (Lee et al. 2001, Brunden et al. 2009, Goedert 1997, Grundke-Iqbal et al. 1986, Lee et al. 1991). The normal function of tau is to stabilize microtubules, and the exact cause of its aggregation is unknown. Mutations in the tau gene have been coupled with frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17) where tau aggregates and not plaques are the characteristic deposits (Hong et al. 1998, Hutton et al. 1998, Gao et al. 2005). Tau mutations are responsible for 5% of frontotemporal dementia cases (Goedert & Spillantini 2011). Tau hyperphosphorylation reduces its binding to microtubules and plays a role in its aggregation (Brunden et al. 2009, Goedert 1997, Alonso et al. 1997, Drechsel et al. 1992, Iqbal et al. 1994). Hyperphosphorylated tau lacks its normal function of binding to microtubules and forms neurofibrillary aggregates (Beyreuther & Masters 1996). Moreover, hyperphosphorylated tau suppresses microtubules assembly and can sequester normal tau and high molecular weight microtubule binding proteins, restraining their normal functions (Iqbal et al. 2010, Drechsel et al. 1992, Alonso et al. 1997, Iqbal et al. 2009, Medina 2011). This suggests that phosphorylation regulates the functions of tau. The main enzymes responsible for tau phosphorylation are glycogen synthase kinase-3 beta (GSK3β) and cyclin-dependent kinase-5 (CDK5).
Specificity protein 1 (Sp1) is a transcription factor involved in AD pathology. Sp1 gene expression and protein levels are elevated within the frontal cortex of AD patients and animal models with AD-like pathology (Basha et al. 2005, Brock et al. 2008, Zawia & Basha 2005, Santpere et al. 2006, Citron et al. 2008). Sp1 binds to GC rich promoter regions within the amyloid precursor protein (APP), beta-site APP cleaving enzyme 1 (BACE1) and tau genes and promotes their transcription (Gao et al. 2005, Pollwein et al. 1992, Docagne et al. 2004, Hoffman & Chernak 1995, Salbaum et al. 1988, Citron et al. 2008, Heicklen-Klein & Ginzburg 2000, Christensen et al. 2004). Sp1 regulates the expression of tau and mutations on the Sp1 binding regions on the tau promoter decrease tau expression (Gao et al. 2005, Heicklen-Klein & Ginzburg 2000). Sp1 protein (SP1) is co-localized with hyperphosphorylated tau in AD tangles (Santpere et al. 2006). Sp1 also regulates the transcription of CDK5 activators p39 and p35 with Sp1 binding motifs found on CDK5, p39 and p35 promoter regions (Ohshima et al. 1995, Ohshima et al. 1996, Ross et al. 2002, Valin et al. 2009). CDK5 is responsible for the phosphorylation of tau on sites that are unusually hyperphosphorylated in AD (Ohshima et al. 1995, Paudel et al. 1993).
Tolfenamic acid, a drug used in Europe for migraine headaches, promotes SP1 degradation, and hence lowers the expression of genes regulated by Sp1 including APP and BACE1 and reduces their cleavage product amyloid β (Aβ) (Abdelrahim et al. 2006, Adwan et al. 2011, Adwan et al. 2014). Tolfenamic acid also improves cognition in mice (Subaiea et al. 2013), and is currently scheduled for a biomarker study in AD patients. Data obtained by our collaborators demonstrated that chronic administration of tolfenamic acid was not toxic and had no adverse effects on animals’ weight, hematocrit, stomach or intestinal lining integrity compared to control (Sankpal et al. 2013).
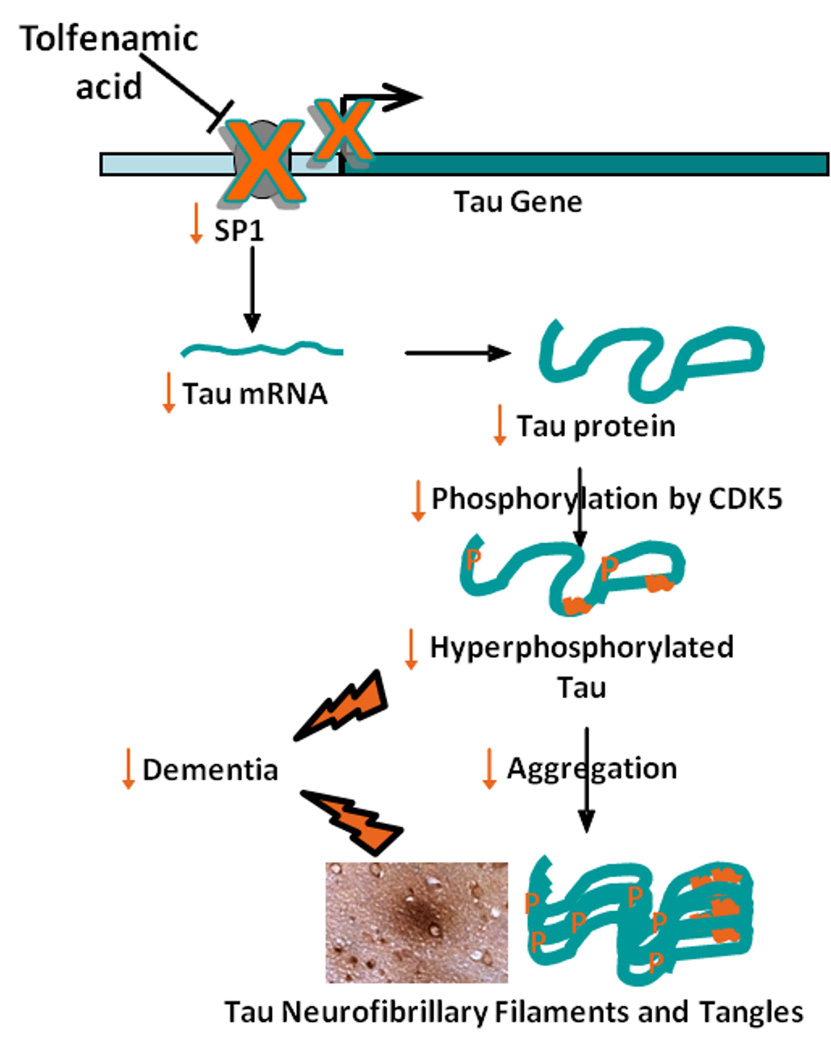
As lowering of hyperphosphorylated tau correlates with cognitive improvement (O'Leary et al. 2010, Medina 2011, Iqbal et al. 2009), this study was designed to test the ability of tolfenamic acid to downregulate the expression of tau and CDK5 via its unique capability to promote the degradation of SP1 (Fig. 1). This would provide more evidence for tolfenamic acid as a broad spectrum drug able to interrupt multiple pathways in the neurodegenerative process and offer more promise in its use in the upcoming clinical studies.
Fig. 1. Proposed transcription-based mechanism of tau and CDK5 downregulation by tolfenamic acid.
Tolfenamic acid induces the degradation of the transcription factor Sp1 (Abdelrahim et al. 2006, Adwan et al. 2011, Subaiea et al. 2013), which reduces the transcription of its target genes such as tau and CDK5 activator, resulting in a decrease in the total levels of tau as well as the pathogenic phosphorylated tau species.
Materials and methods
Chemicals and reagents
All materials used were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Animals
APP YAC transgenic mice, line R1.40, were used in this study (The Jackson Laboratory, Bar Harbor, ME, USA). These animals were particularly used to demonstrate in the same mouse model, the ability of tolfenamic acid to impact pathways associated with both amyloid and tau pathology. The ability of tolfenamic acid to decrease the levels of SP1, APP and Aβ and reduce BACE1 mRNA and activity in these same animals has already been published by us, along with behavioral tests demonstrating cognitive improvement following tolfenamic acid treatment in these mice (Subaiea et al. 2013, Adwan et al. 2014). Animals were housed in designated rooms within the animal facility at the University of Rhode Island. Female hemizygous mice bred in-house were assigned into 3 groups of similar age variations between 14 and 21 months of age, n= 6 in each group. Animals were administered 0, 5 or 50 mg/kg tolfenamic acid in corn oil everyday by oral gavage for 34 days. On day 35, mice were sacrificed and brain tissues were collected and stored at −80°C until further use. The detailed information on the breeding and exposure scenario have been already published along with the results from the biochemical and behavioral experiments conducted on these animals (Subaiea et al. 2013). All experiments were performed in accordance with the standard guidelines and the protocol approved by the Institutional Animal Care and Use Committee of the University of Rhode Island.
RNA isolation, cDNA synthesis and real time PCR
RNA was isolated from cerebral cortex tissue following the TRIzol® reagent method (Invitrogen, Carlsbad, CA, USA), checked for integrity by NanoDrop (Thermo Scientific, Wilmington, DE, USA), and reverse transcribed to cDNA using iScript™ Select cDNA Synthesis Kit following manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). About 1000 ng of RNA were diluted to 19.5 µL with nuclease free water, then 3 µL Oligo (dT) mix, 6 µL 5x iScript Select reaction mix, and 1.5 µL of iScript reverse transcriptase were added. Samples were incubated at 42°C for 90 minutes then at 85°C for 5 minutes to terminate the reaction. All incubations were conducted using MJ Research MiniCycler™ (Bio-Rad, Hercules, CA, USA). Primer pairs for mouse tau, CDK5, β-actin and GAPDH were obtained from Invitrogen (Carlsbad, CA, USA) as follows: tau sense: 5’- GTG GCC AGG TGG AAG TAA AA -3’ and antisense: 5’- TGG AAG ACA CAT TGC TGA GG -3’; CDK5 sense: 5’- GGC TAA AAA CCG GGA AAC TC -3’, and antisense: 5’- CCA TTG CAG CTG TCG AAA TA -3’; β-actin sense: 5’- TGT TAC CAA CTG GGA CGA CA -3’, and antisense: 5’- TCT CAG CTG TGG TGG TGA AG -3’; GAPDH sense: 5’- AGC TGA ACG GGA AGC TCA CT -3’, and antisense: 5’- AGG TCC ACC ACT GAC ACG TTG-3’. Each real time PCR reaction mix contained 2 µL of cDNA, 1 µL of each primer, 8.5 µL nuclease free water and 12.5 µL SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Real time PCR was conducted using the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA) following the standard protocol: 50°C for 2 minutes followed by 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Results were analyzed using the 7500 system software with relative quantification method and β-actin or GAPDH as endogenous control.
Protein extraction and Western blot analyses
Cerebral cortex tissue was homogenized with radio-immunoprecipitation assay lysis buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1 SDS, 1 mM ethylenediaminetetraacetic acid (Thermo Fisher Scientific, Waltham, MA, USA), and 0.1% protease inhibitor cocktail). The homogenates were centrifuged at 10,600 × g for 10 minutes at 4°C and supernatants were collected. Protein concentration was determined using the Micro BCA Protein Assay Kit (Thermo Scientific Pierce, Rockford, IL, USA). Protein extracts were stored at −80°C until further use. For Western blot analyses, approximately 40 µg of protein samples were separated onto 4–15% precast polyacrylamide gels (Bio-Rad, Hercules, CA, USA) at 150 V for 1-2 hours and then transferred to polyvinylidene fluoride membranes (GE-Healthcare, Piscataway, NJ, USA). Membranes were blocked and incubated with the appropriate dilution of the specific primary antibody for 1-2 hours. The antibodies used were as follows: 1:1000 dilution of T9450 for total tau levels (Sigma-Aldrich, St. Louis, MO, USA); 1:1000 of CDK5 #2506 (Cell Signaling, Beverly, MA, USA); 1:1000 of phosphorylated tau (P-tau) at Thr 181 #5383 (Cell Signaling, Beverly, MA, USA); 1:1000 of P-tau at Ser 235 ab30664 (Abcam, Cambridge, MA, USA); 1:5000 of β-actin A2013 (Sigma-Aldrich, St. Louis, MO, USA); or 1:2000 of GAPDH T9450 (Sigma-Aldrich, St. Louis, MO, USA), then the membranes were washed with TBST and incubated with the appropriate infrared dye-labeled secondary antibody (Li-Cor, Lincoln, NE, USA) for 1 hour at room temperature in the dark. Infrared signal of Western blot bands was detected and quantified using Odyssey® Infrared Imaging System (Li-Cor, Lincoln, NE, USA). Western blot protein levels for tau, CDK5, and p-tau were normalized against the levels of the house keeping proteins β-actin or GAPDH.
Statistical analysis
Data were represented as the mean ± SEM. Statistical analysis was performed using GraphPad Instat software (GraphPad software, San Diego, CA, USA) and statistical significance was determined by one-way ANOVA and Tukey-Kramer multiple comparisons post-test. Results with a p-value of <0.05 were considered statistically significant, and were marked with asterisks accordingly.
Results
Targeting neurofibrillary tau pathology of AD by influencing the transcription factor Sp1 is a new therapeutic approach that can be extended to other tauopathies. Studies from our lab have already provided evidence that tolfenamic acid crosses the blood brain barrier and is able to lower SP1 and subsequently reduce APP and BACE1 transcription and Aβ levels within mice brains as well as improve cognitive functions (Adwan et al. 2011, Subaiea et al. 2011, Subaiea et al. 2013, Adwan et al. 2014). The safety profile of tolfenamic acid has already been established. This drug has been approved and used in Europe for the management of migraine headaches and rheumatoid arthritis for decades. In our experiments, we did not observe any toxic effects on animals administered tolfenamic acid. In this study tolfenamic acid was given daily to mice for 34 days to study the effects on tau gene expression and protein levels. The data reported below also show the effects of tolfenamic acid treatment on various intermediates in tau pathology including CDK5 and P-tau at Ser 235 and Thr 181.
Tolfenamic acid lowers tau gene expression and total tau levels in vivo
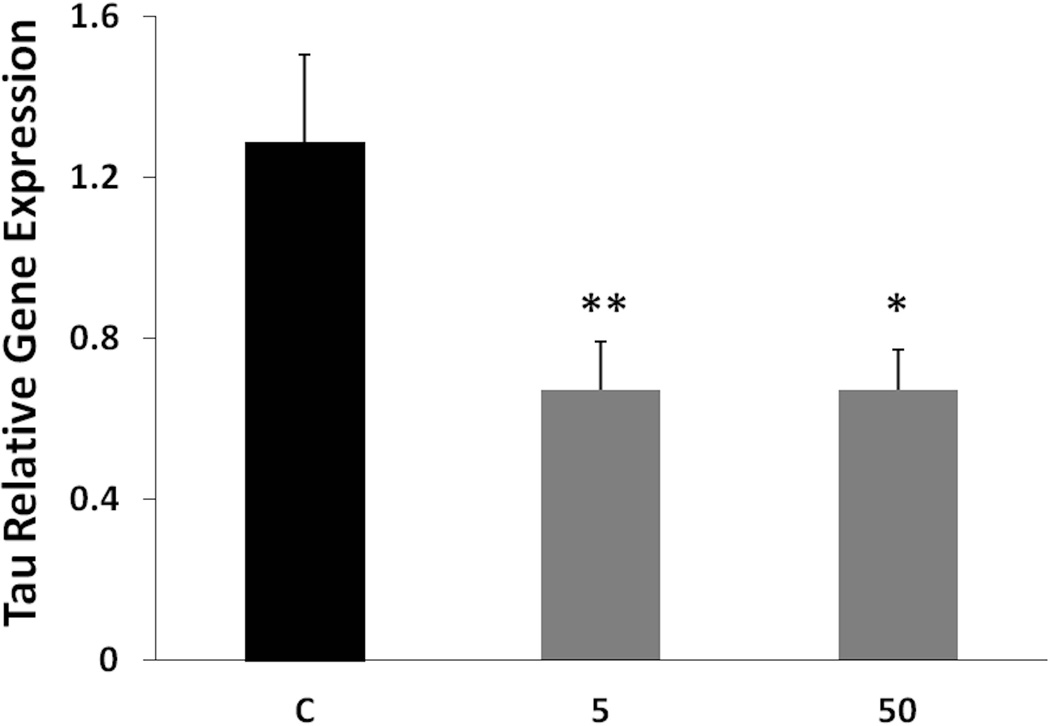
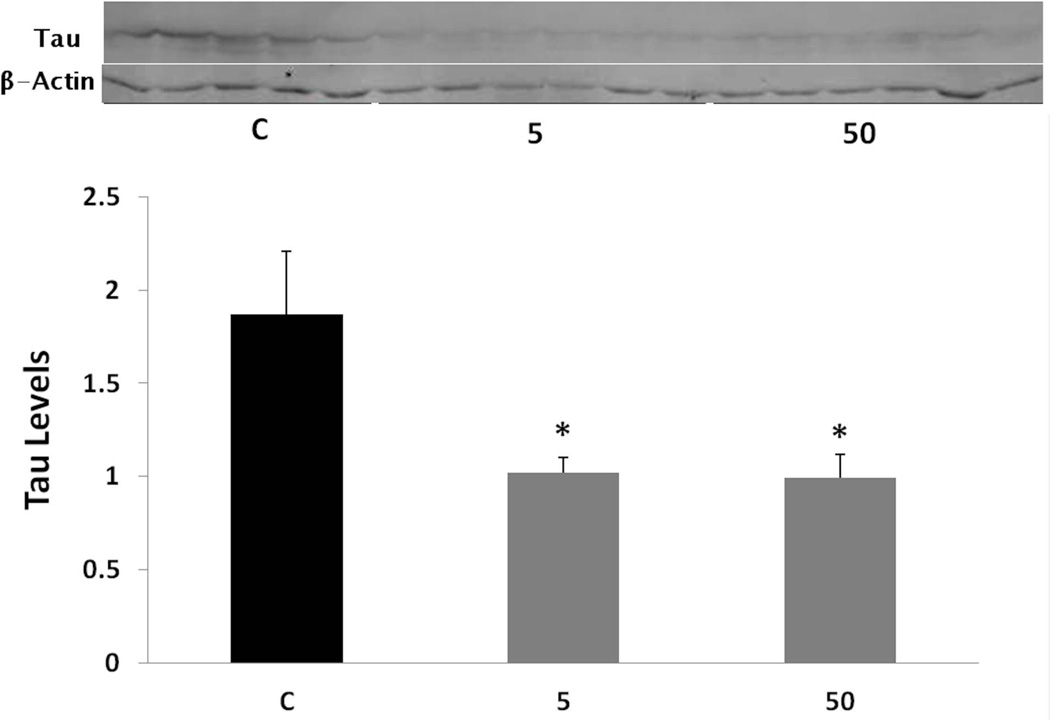
By inducing SP1 degradation and reducing its levels in these animals (Subaiea et al. 2013), we hypothesized that tolfenamic acid would also reduce the gene expression of its transcriptional targets like tau (Abdelrahim et al. 2006, Adwan et al. 2011). Following the administration of tolfenamic acid to mice daily for 34 days, tau gene expression was lowered by 48% with both the 5 and 50 mg/kg doses as demonstrated by real time PCR (Fig. 2). Statistical significance was determined by one-way ANOVA (F(2,14)=10.287, p=0.0018), followed by Tukey-Kramer multiple comparisons post-test p < 0.001 for the control (C) vs 5 mg/kg group, p < 0.05 for the C vs the 50 mg/kg group. Furthermore, tolfenamic acid decreased total tau protein levels by 46% with both doses as measured by Western blot analysis (Fig. 3). One-way ANOVA F(2,11)=6.446, p=0.014. Tukey-Kramer post-test p < 0.05 for the C vs the 5 mg/kg group and for the C vs the 50 mg/kg group.
Fig. 2. Tau relative gene expression in cerebral cortex tissues from mice treated with tolfenamic acid daily for 34 days.
Mice were administered 0, 5 or 50 mg/kg tolfenamic acid everyday for 34 days. Tau mRNA levels were measured in the cerebral cortex by real time PCR, with β-actin used as an endogenous control as mentioned in the methods section. Values shown are for the mean ± SEM, n=6 in each group, p=0.018 as determined by one-way ANOVA with Tukey-Kramer post-test *p < 0.05; **p < 0.01.
Fig. 3. Tau levels following tolfenamic acid administration.
Total tau protein levels were analyzed in the cerebral cortex following daily administration of tolfenamic acid to transgenic mice for 34 days by Western blot analysis. Values shown are for the mean ± SEM, n=5. Tau levels were normalized to the levels of the house keeping protein β-actin. One-way ANOVA p=0.014, with Tukey-Kramer post-test *p < 0.05. Insert shows representative control (C), 5 or 50 mg/kg treatment tau or β-actin Western blot bands.
Tolfenamic acid decreases the gene and protein expression of CDK5 in mice
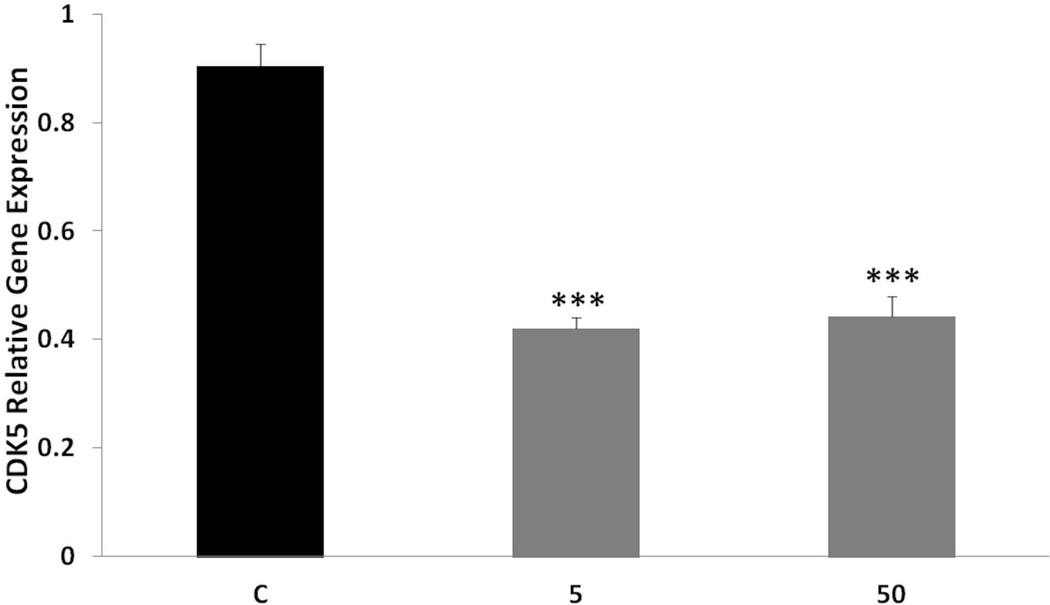
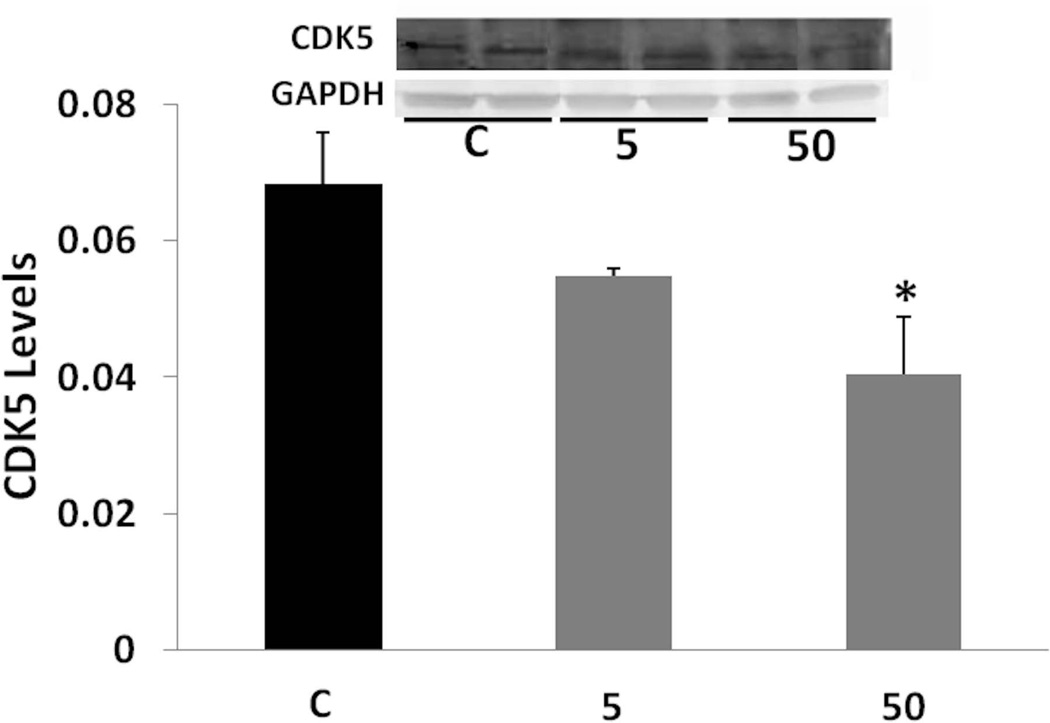
As Sp1 also regulates CDK5 activators (Valin et al. 2009), we tested the effects of tolfenamic acid on CDK5. We found that daily administration of tolfenamic acid to mice for a month lowered the gene expression of CDK5 in the cerebral cortex by about 50% (Fig. 4). One-way ANOVA F(2,13)=59.647, p=2.810−7. Tukey-Kramer post-test p < 0.05 for the C vs the 5 mg/kg group and for the C vs the 50 mg/kg group. There was a lowering trend in CDK5 levels (Fig. 5) that was not significant when analyzed with one-way ANOVA (F(2,8)=4.086, p=0.059). However, when comparing the 50 mg/kg dose group to the control group by Tukey-Kramer test, the 40% lowering in CDK5 from control was statistically significant (p < 0.05).
Fig. 4. CDK5 gene expression after tolfenamic acid treatment.
CDK5 mRNA levels in mice cortices were measured with real time PCR with GAPDH as an endogenous control as mentioned in the methods section. Values shown are for the mean ± SEM, n=5. One-way ANOVA ***p < 0.0001 as determined by Tukey-Kramer post-test.
Fig. 5. CDK5 levels following tolfenamic acid treatment.
CDK5 levels in cerebral cortices of mice administered tolfenamic acid or control for 34 days were obtained by Western blot analysis. CDK5 levels were normalized to GAPDH levels. Values shown are for the mean ± SEM, n=4. One-way ANOVA p=0.059. *p < 0.05 according to Tukey-Kramer post-test. Insert shows representative control (C), 5 or 50 mg/kg treatment CDK5 or GAPDH Western blot bands.
Tolfenamic acid reduces the expression of phosphorylated tau
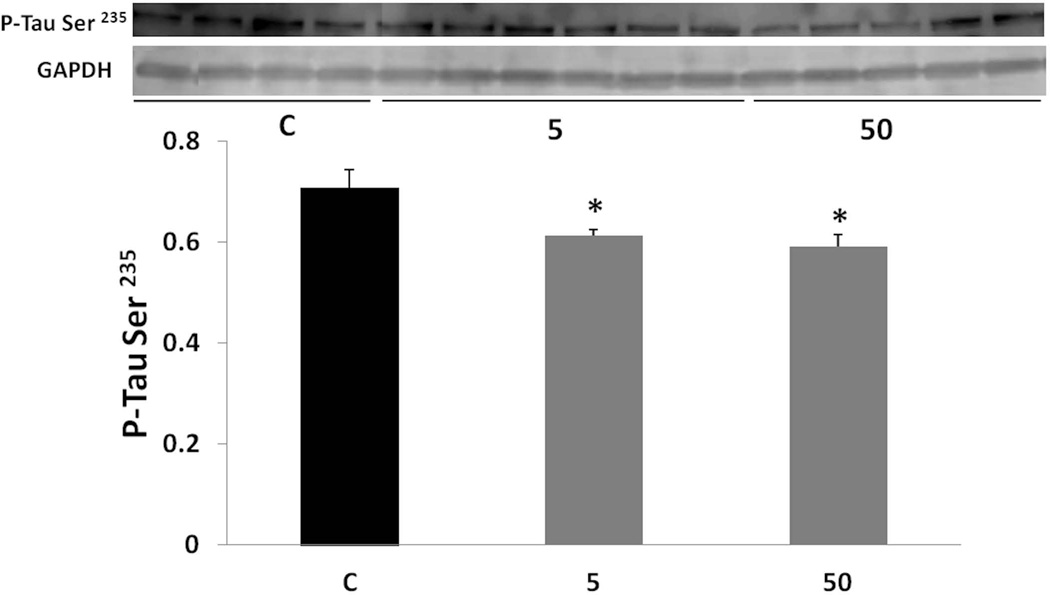
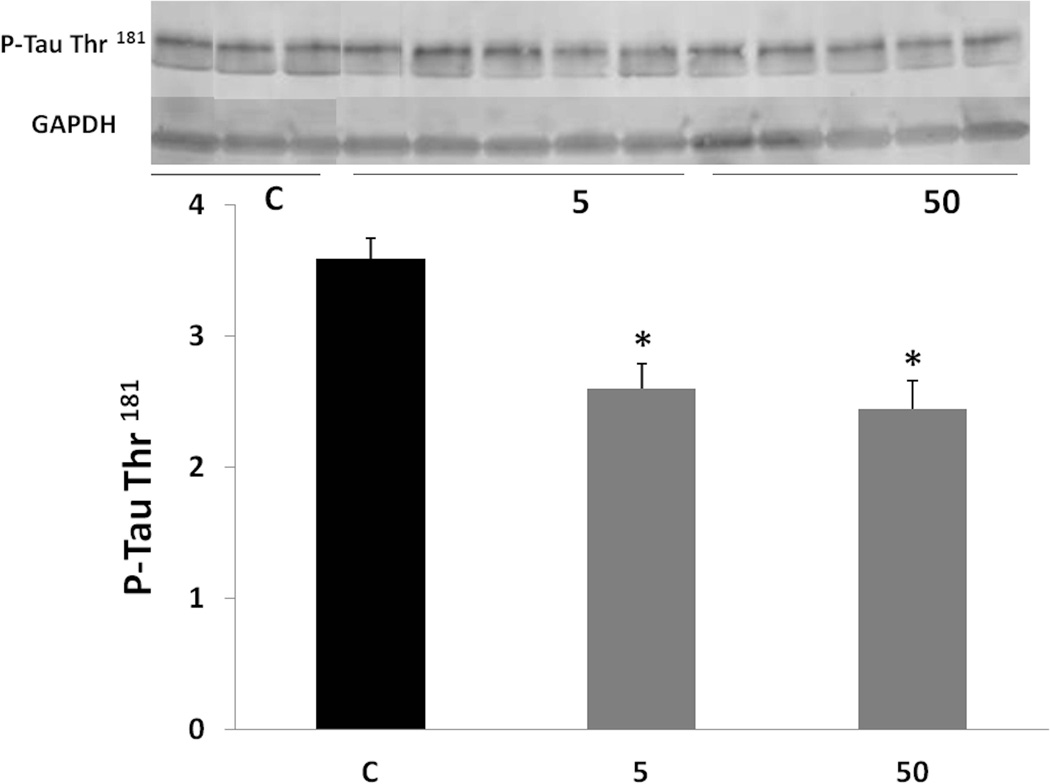
As phosphorylation of tau affects its function and its ability to bind to microtubules (Sengupta et al. 1998, Alonso et al. 1997, Alonso et al. 2008), it was important to test how phosphorylated tau is affected by the treatment. P-tau levels were analyzed by Western blotting using specific antibodies. P-tau at Ser 235 and P-tau at Thr 181 were lowered by both doses of tolfenamic acid (Fig. 6 and 7). Tau phosphorylated at Ser 235 was lowered by about 15% as indicated by one-way ANOVA (F(2,11)=6.105, p=0.0165), Tukey-Kramer post-test p < 0.05 for the C vs the 5 mg/kg group and for the C vs the 50 mg/kg group. P-tau at Thr 181 was lowered by about 30%, one-way ANOVA F(2,10)=7.272, p=0.0112, Tukey-Kramer post-test p < 0.05 for the C vs the 5 mg/kg group and for the C vs the 50 mg/kg group.
Fig. 6. Levels of tau phosphorylated at Ser 235 after tolfenamic acid treatment.
P-tau levels were measured by Western blot analysis and normalized to GAPDH as mentioned in the methods section. Values shown are for the mean ± SEM, n=5. One-way ANOVA p=0.0165. *p < 0.05 according to Tukey-Kramer post-test. Insert shows representative C, 5 or 50 mg/kg treatment P-tau at Ser 235 or GAPDH Western blot bands.
Fig. 7. Levels of tau phosphorylated at Thr 181 following tolfenamic acid exposure.
P-tau levels were measured by Western blot and normalized to GAPDH levels. Values shown are for the mean ± SEM, n=5. One-way ANOVA p=0.0112. *p < 0.05 according to Tukey-Kramer post-test. Insert shows representative C, 5 or 50 mg/kg treatment P-tau at Thr 181 or GAPDH Western blot bands.
Discussion
Tolfenamic acid, a drug already available in the European market for the management of migraine headaches, represents a novel class of drugs that could be repurposed for AD due to its unique ability to promote the degradation of SP1 (Abdelrahim et al. 2006, Adwan et al. 2011), a transcription factor that has been linked to AD tau and Aβ pathology (Brock et al. 2008, Citron et al. 2008, Docagne et al. 2004, Santpere et al. 2006). Previous studies from our lab demonstrate that by lowering SP1, tolfenamic acid was able to decrease the transcription of APP as well as Aβ levels in mice following two weeks of daily administration (Adwan et al. 2011). Our studies show that tolfenamic acid is readily available in the brain after dosing (Subaiea et al. 2011, Adwan et al. 2011). Behavioral and biochemical analyses have also revealed that tolfenamic acid lowers Sp1, APP, BACE1 mRNA and activity in addition to Aβ and improves cognition in the APP transgenic mouse model used in this study (Subaiea et al. 2013, Adwan et al. 2014).
Drug discovery for AD has focused on targeting intermediates mentioned in the amyloid hypothesis of AD including APP and Aβ, and so far no successful disease-modifying candidate has been found for this devastating disorder. Much less attention was paid to tau which is abnormally hyperphosphorylated and forms aggregates in AD. More recent studies have found a better correlation between tau and memory impairment in AD (Medina 2011). In a transgenic mouse model that expresses plaques and tangles, lowering both soluble tau and Aβ caused cognitive improvement, while lowering only soluble Aβ did not improve cognition (Oddo et al. 2006). Tangles are later manifestations of tau pathology and soluble phosphorylated tau is the species responsible for neurodegenerative damage (Iqbal et al. 2009, Medina 2011).
Since cognitive impairment is better correlated with the presence of tau and as Sp1 regulates tau expression (Medina 2011, Iqbal et al. 2009, Heicklen-Klein & Ginzburg 2000), we sought to study the effects of tolfenamic acid on the tau pathology in the same animals where we observed its cognitive benefits (Subaiea et al. 2013). Data presented within this manuscript demonstrate that tolfenamic acid lowers tau and CDK5 levels by inhibiting their transcription. However, the exact mechanism of action by which tolfenamic acid enhances SP1 degradation still remains to be established. Interestingly, we do not see much difference between the two doses used, suggesting that in order to get a dose response relationship we need to go lower beyond the 5 and 50 mg/kg doses used. Such low doses would resemble those approved for migraine headaches management in Europe.
Tau and its abnormal phosphorylation are becoming targets for AD therapeutics. Tau knockdown by siRNA in vitro does not alter cell viability or the availability of microtubules (Morris et al. 2011). Probably because other microtubule associated proteins (MAPs) like MAP1b carry out similar functions to tau (Morris et al. 2011). The ability of tolfenamic acid to lower total tau levels is of great importance (Fig. 3). It was found that lowering soluble hyperphosphorylated tau rather than the insoluble tangles correlates with cognitive improvement (O'Leary et al. 2010, Medina 2011, Iqbal et al. 2009). In fact, in a neurodegenerative mouse model, tau inhibition recovered memory function even though the buildup of tangles continued suggesting that tangles by themselves are not responsible for cognitive dysfunction (Santacruz et al. 2005).
It is important to note that tolfenamic acid has been used for years, and that its interference with Sp1 should not be alarming since it was found that Sp1 is vital during early embryonic development only but not necessary for the following later stages of cell growth and differentiation (Marin et al. 1997). CDK5 is also important during nervous system development but not crucial later in life and thus is considered a promising target for AD where aberrant hyperphosphorylation and aggregation of tau is a major pathological finding (Piedrahita et al. 2010, Lau et al. 2002, Lopez-Tobon et al. 2011).
Administration of tolfenamic acid reduced the levels of tau phosphorylated at two sites, Ser 235 and Thr 181 (Fig. 6 and 7). Both sites are phosphorylated by CDK5 and other kinases (Baumann et al. 1993, Liu et al. 2002). Tau phosphorylation occurs on multiple sites and is regulated by different kinases (Liu et al. 2006). Ser 235 was found to be one of 3 sites whose phosphorylation inhibits tau binding to microtubules (Sengupta et al. 1998). Moreover, it is one of the sites that are especially phosphorylated in PHF tau (Morishima-Kawashima et al. 1995, Hoffmann et al. 1997).
Decreasing the levels of the tangle forming tau protein by reducing its transcription is a novel approach for targeting AD and other tauopathies. Data from this study demonstrate that this can be achieved by promoting the degradation of the transcription factor Sp1. Tolfenamic acid is able to lower tau, CDK5, phosphorylated tau at Ser 235 and Thr 181. Hence tolfenamic acid represents a promising candidate that targets both the amyloid and tau neurofibrillary pathways of AD and improves cognition through a unique transcription driven mechanism.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) and by grants NIH- ES015867 and AG042695 awarded to NHZ. The RI-INBRE Research Core Facility was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 8 P20 GM103430-12.
Abbreviations used
- Aβ
amyloid β
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- BACE1
beta-site APP cleaving enzyme 1
- CDK5
cyclin-dependent kinase-5
- GSK3β
glycogen synthase kinase-3 beta
- MAPs
microtubule associated proteins
- P-tau
phosphorylated tau
- PHFs
paired helical filaments
- Sp1
Specificity protein 1
- SP1
Sp1 protein
Footnotes
Conflict of interest disclosure: Provisional patent application U.S. Serial No. 61/739930 (N.H.Z.) was filed related to the work in this manuscript. The authors declare no other conflict of interest.
References
- Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- Adwan L, Subaiea GM, Zawia NH. Tolfenamic acid downregulates BACE1 and protects against lead-induced upregulation of Alzheimer's disease related biomarkers. Neuropharmacology. 2014;79:596–602. doi: 10.1016/j.neuropharm.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adwan LI, Basha R, Abdelrahim M, Subaiea GM, Zawia NH. Tolfenamic acid interrupts the de novo synthesis of the beta-amyloid precursor protein and lowers amyloid beta via a transcriptional pathway. Curr Alzheimer Res. 2011;8:385–392. doi: 10.2174/156720511795745285. [DOI] [PubMed] [Google Scholar]
- Alonso AC, Li B, Grundke-Iqbal I, Iqbal K. Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2008;5:375–384. doi: 10.2174/156720508785132307. [DOI] [PubMed] [Google Scholar]
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U. S. A. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- Beyreuther K, Masters CL. Alzheimer's disease. Tangle disentanglement. Nature. 1996;383:476–477. doi: 10.1038/383476a0. [DOI] [PubMed] [Google Scholar]
- Brock B, Basha R, DiPalma K, Anderson A, Harry GJ, Rice DC, Maloney B, Lahiri DK, Zawia NH. Co-localization and distribution of cerebral APP and SP1 and its relationship to amyloidogenesis. J Alzheimers Dis. 2008;13:71–80. doi: 10.3233/jad-2008-13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat Rev Drug Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MA, Zhou W, Qing H, Lehman A, Philipsen S, Song W. Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by Sp1. Mol Cell Biol. 2004;24:865–874. doi: 10.1128/MCB.24.2.865-874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, Dennis JS, Zeitlin RS, Echeverria V. Transcription factor Sp1 dysregulation in Alzheimer's disease. J Neurosci Res. 2008;86:2499–2504. doi: 10.1002/jnr.21695. [DOI] [PubMed] [Google Scholar]
- Docagne F, Gabriel C, Lebeurrier N, Lesne S, Hommet Y, Plawinski L, Mackenzie ET, Vivien D. Sp1 and Smad transcription factors co-operate to mediate TGF-beta-dependent activation of amyloid-beta precursor protein gene transcription. Biochem J. 2004;383:393–399. doi: 10.1042/BJ20040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Tucker KL, Andreadis A. Transcriptional regulation of the mouse microtubule-associated protein tau. Biochim Biophys Acta. 2005;1681:175–181. doi: 10.1016/j.bbaexp.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Goedert M. The Neurofibrillary Pathology of Alzheimer's Disease. The Neuroscientist. 1997;3:131–141. [Google Scholar]
- Goedert M, Spillantini MG. Pathogenesis of the tauopathies. J Mol Neurosci. 2011;45:425–431. doi: 10.1007/s12031-011-9593-4. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heicklen-Klein A, Ginzburg I. Tau promoter confers neuronal specificity and binds Sp1 and AP-2. J Neurochem. 2000;75:1408–1418. doi: 10.1046/j.1471-4159.2000.0751408.x. [DOI] [PubMed] [Google Scholar]
- Hoffman PW, Chernak JM. DNA binding and regulatory effects of transcription factors SP1 and USF at the rat amyloid precursor protein gene promoter. Nucleic Acids Res. 1995;23:2229–2235. doi: 10.1093/nar/23.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann R, Lee VM, Leight S, Varga I, Otvos L., Jr Unique Alzheimer's disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry. 1997;36:8114–8124. doi: 10.1021/bi970380+. [DOI] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Zaidi T, Bancher C, Grundke-Iqbal I. Alzheimer paired helical filaments. Restoration of the biological activity by dephosphorylation. FEBS Lett. 1994;349:104–108. doi: 10.1016/0014-5793(94)00650-4. [DOI] [PubMed] [Google Scholar]
- Lau LF, Seymour PA, Sanner MA, Schachter JB. Cdk5 as a drug target for the treatment of Alzheimer's disease. J Mol Neurosci. 2002;19:267–273. doi: 10.1385/JMN:19:3:267. [DOI] [PubMed] [Google Scholar]
- Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Lett. 2002;530:209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- Liu F, Liang Z, Shi J, Yin D, El-Akkad E, Grundke-Iqbal I, Iqbal K, Gong CX. PKA modulates GSK-3beta- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett. 2006;580:6269–6274. doi: 10.1016/j.febslet.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Tobon A, Castro-Alvarez JF, Piedrahita D, Boudreau RL, Gallego-Gomez JC, Cardona-Gomez GP. Silencing of CDK5 as potential therapy for Alzheimer's disease. Rev Neurosci. 2011;22:143–152. doi: 10.1515/RNS.2011.015. [DOI] [PubMed] [Google Scholar]
- Marin M, Karis A, Visser P, Grosveld F, Philipsen S. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell. 1997;89:619–628. doi: 10.1016/s0092-8674(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Medina M. Recent developments in tau-based therapeutics for neurodegenerative diseases. Recent Pat CNS Drug Discov. 2011;6:20–30. doi: 10.2174/157488911794079091. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Watanabe A, Titani K, Ihara Y. Hyperphosphorylation of tau in PHF. Neurobiol Aging. 1995;16:365–371. doi: 10.1016/0197-4580(95)00027-c. [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JC, 3rd, Li Q, Marinec P, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Kozak CA, Nagle JW, Pant HC, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse gene encoding cyclin-dependent kinase 5 regulatory subunit p35. Genomics. 1996;35:372–375. doi: 10.1006/geno.1996.0370. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Nagle JW, Pant HC, Joshi JB, Kozak CA, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse cyclin-dependent kinase 5 gene. Genomics. 1995;28:585–588. doi: 10.1006/geno.1995.1194. [DOI] [PubMed] [Google Scholar]
- Paudel HK, Lew J, Ali Z, Wang JH. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J Biol Chem. 1993;268:23512–23518. [PubMed] [Google Scholar]
- Piedrahita D, Hernandez I, Lopez-Tobon A, et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic alzheimer's mice. J Neurosci. 2010;30:13966–13976. doi: 10.1523/JNEUROSCI.3637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollwein P, Masters CL, Beyreuther K. The expression of the amyloid precursor protein (APP) is regulated by two GC-elements in the promoter. Nucleic Acids Res. 1992;20:63–68. doi: 10.1093/nar/20.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Tienhaara A, Lee MS, Tsai LH, Gill G. GC box-binding transcription factors control the neuronal specific transcription of the cyclin-dependent kinase 5 regulator p35. J Biol Chem. 2002;277:4455–4464. doi: 10.1074/jbc.M110771200. [DOI] [PubMed] [Google Scholar]
- Salbaum JM, Weidemann A, Lemaire HG, Masters CL, Beyreuther K. The promoter of Alzheimer's disease amyloid A4 precursor gene. EMBO J. 1988;7:2807–2813. doi: 10.1002/j.1460-2075.1988.tb03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankpal UT, Lee CM, Connelly SF, et al. Cellular and Organismal Toxicity of the Anti-Cancer Small Molecule, Tolfenamic Acid: a Pre-Clinical Evaluation. Cell Physiol Biochem. 2013;32:675–686. doi: 10.1159/000354471. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santpere G, Nieto M, Puig B, Ferrer I. Abnormal Sp1 transcription factor expression in Alzheimer disease and tauopathies. Neurosci Lett. 2006;397:30–34. doi: 10.1016/j.neulet.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys. 1998;357:299–309. doi: 10.1006/abbi.1998.0813. [DOI] [PubMed] [Google Scholar]
- Subaiea GM, Adwan LI, Ahmed AH, Stevens KE, Zawia NH. Short-term treatment with tolfenamic acid improves cognitive functions in Alzheimer's disease mice. Neurobiol Aging. 2013;34:2421–2430. doi: 10.1016/j.neurobiolaging.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subaiea GM, Alansi BH, Serra DA, Alwan M, Zawia NH. The ability of tolfenamic acid to penetrate the brain: a model for testing the brain disposition of candidate Alzheimer's drugs using multiple platforms. Curr Alzheimer Res. 2011;8:860–867. doi: 10.2174/156720511798192691. [DOI] [PubMed] [Google Scholar]
- Valin A, Cook JD, Ross S, Saklad CL, Gill G. Sp1 and Sp3 regulate transcription of the cyclin-dependent kinase 5 regulatory subunit 2 (p39) promoter in neuronal cells. Biochim Biophys Acta. 2009;1789:204–211. doi: 10.1016/j.bbagrm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer's disease. Rev Neurosci. 2005;16:325–337. doi: 10.1515/revneuro.2005.16.4.325. [DOI] [PubMed] [Google Scholar]