Abstract
Disruption of intestinal epithelial tight junctions is an important event in the pathogenesis of ulcerative colitis. Dextran sodium sulfate (DSS) induces colitis in mice with the symptoms similar to ulcerative colitis. However, the mechanism of DSS-induced colitis is unknown. We investigated the mechanism of DSS-induced disruption of intestinal epithelial tight junctions and barrier dysfunction in Caco-2 cell monolayers in vitro and mouse colon in vivo. DSS treatment resulted in disruption of tight junctions, adherens junctions and actin cytoskeleton leading to barrier dysfunction in Caco-2 cell monolayers. DSS induced a rapid activation of c-jun N-terminal kinase (JNK), and the inhibition or knockdown of JNK2 attenuated DSS-induced tight junction disruption and barrier dysfunction. In mice, DSS administration for 4 days caused redistribution of tight junction and adherens junction proteins from the epithelial junctions, which was blocked by JNK inhibitor. In Caco-2 cell monolayers, DSS increased intracellular Ca2+ concentration, and depletion of intracellular Ca2+ by BAPTA or thapsigargin attenuated DSS-induced JNK activation, tight junction disruption and barrier dysfunction. Knockdown of Ask1 or MKK7 blocked DSS-induced tight junction disruption and barrier dysfunction. DSS activated c-Src by a Ca2+ and JNK-dependent mechanism. Inhibition of Src kinase activity or knockdown of c-Src blocked DSS-induced tight junction disruption and barrier dysfunction. DSS increased Tyr-phosphorylation of occludin, ZO-1, E-cadherin and β-catenin. SP600125 abrogated DSS-induced Tyr-phosphorylation of junctional proteins. Recombinant JNK2 induced threonine phosphorylation and auto phosphorylation of c-Src. This study demonstrates that Ca2+-Ask1-MKK7-JNK2-cSrc signaling cascade mediates DSS-induced tight junction disruption and barrier dysfunction.
Keywords: barrier function, epithelium, occludin, ZO-1, actin, stress, phosphorylation
INTRODUCTION
A major function of the gastrointestinal epithelium is to form a physical barrier between the hostile environment of the gastrointestinal lumen and the sub-epithelial tissue. The epithelial tight junctions provide this barrier against the paracellular penetration of noxious substances from the gut lumen. Immediately basal to tight junction is the adherens junction, which is known to indirectly regulate the integrity of tight junctions [1]. These intercellular junctions associate with the perijunctional actin cytoskeleton and numerous signaling molecules to form an integrated functional unit called the apical junctional complex (AJC)[2]. The integrity of AJC is compromised in numerous gastrointestinal diseases, including inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis [3, 4]. Colitis induced by dextran sodium sulfate (DSS) is an extensively applied animal model of the human ulcerative colitis [5, 6]. This model exhibits clinical features very similar to those in ulcerative colitis patients. However, the mechanism involved in DSS-induced colitis is poorly understood. Similar to ulcerative colitis patients, DSS-induced colitis in rodents is associated with increased intestinal mucosal permeability [7–10]. Elevated mucosal permeability appears to precede the onset of inflammatory process [8, 9]. Although epithelial barrier dysfunction in DSS-induced colitis is well documented, the precise mechanism involved in DSS-induced epithelial barrier dysfunction is poorly understood. DSS-induced colitis was found to be associated with a depletion of zona occludens-1 (ZO-1), a tight junction protein, in the intestinal epithelium, suggesting that tight junctions may be altered [11].
Tight junction assembly involves at least four types of transmembrane proteins: occludin, tricellulin, claudins, and junctional adhesion molecules [12]. Transmembrane proteins interact with cytoplasmic adapter proteins such as ZO-1, which anchors the transmembrane proteins to the actin cytoskeleton [13]. A significant body of evidence indicates that a variety of intracellular signaling molecules are associated with AJC and that activities of such signaling molecules regulate the integrity of epithelial tight junctions [14]. The major groups of signaling elements that regulate tight junctions include protein kinases and protein phosphatases, such as Src kinases [15, 16], protein kinase C [17–19], MAP kinases [20–22], protein tyrosine phosphatase [14, 23, 24] and protein serine/threonine phosphatases [25, 26].
c-Jun N-terminal kinases (JNKs), a subgroup of MAP kinases, are activated by various types of stress, such as UV irradiation, osmotic stress, heat shock, as well as by direct activation of cytokine and T toll-like receptors [27]. JNK activation causes tissue injury by induction of apoptosis, production of pro-inflammatory cytokines and delayed differentiation[28]. Three JNK isoforms are JNK1, JNK2, and JNK3. While JNK3 expression is limited to neural cells, JNK1 and JNK2 are ubiquitously distributed in different cell types [28]. Although JNK activation was detected in inflamed colonic mucosa of patients with inflammatory bowel disease [29], the role of JNK in the disease activity is unclear. JNK inhibitor SP600125 ameliorated trinitrobenzoyl sulfonic acid-induced colitis in mice, predominantly by inhibiting JNK activity in the infiltrating inflammatory cells [30].
In the present study, we investigated the direct effect of DSS on the epithelial integrity and determined the role of JNK in DSS-induced tight junction disruption and barrier dysfunction using Caco-2 cell monolayers and mouse intestine. We further investigated the importance of intracellular calcium ([Ca2+]i) in DSS-induced JNK2 activation, the JNK-mediated c-Src activation and c-Src-mediated Tyr phosphorylation of tight junction and adherens junction proteins.
METHODS AND MATERIALS
Chemicals
Cell culture supplies, transfection reagents, Fura-2AM, pluronic acid and phalloidin (Cy3 or AlexaFlour-488 conjugated) were procured from Cellgrow® (Manassas, VA) or Invitrogen (Carlsbad, CA). Transwells were purchased from Costar (Cambridge, MA). SP600125, thapsigargin and PP2 were from EMD Chemicals (San Diego, CA). Other chemicals were purchased from either Sigma Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Tustin, CA). JNK2 and c-Src recombinant proteins were purchased from MyBioSource (San Diego, CA).
Antibodies
Anti-JNK(pTpY183/185), anti-c-Src(pY418), anti-phospho-threonine, anti-ZO-1, anti-occludin, and anti-claudin-4 antibodies were purchased from Invitrogen (Carlsbad, CA). Anti-JNK, anti-Ask1, anti-MKK7 and anti-c-Src antibodies were purchased from Millipore (Billerica, MA). HRP-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG and anti-β-actin antibodies were obtained from Sigma Aldrich. AlexaFlour-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR). Anti-E-cadherin, anti-β-catenin, and biotin-conjugated anti-p-Tyr antibodies were purchased from BD Biosciences (Billerica, MA). FITC-conjugated anti-mouse Gr-1 antibodies were from MyBioSource (San Diego, CA).
Antisense oligonucleotides and siRNA
Antisense oligonucleotides for JNK1 (AS-Jnk1) and JNK2 (AS-Jnk2) and the scrambled sequence for AS-Jnk1 (MS-Oligo) were prepared as described previously [31]. Human c-Src-specific siRNA and corresponding control RNA were purchased from Dharmacon (Lafayette, CO) and OriGene (Rockville, MD). Sets of siRNA against Ask1 and MKK7 were purchased also from OriGene.
Cell culture and transfection
Caco-2 cells (ATCC, Rockville, MD) were grown under standard cell culture conditions as described before [32]. Experiments were conducted using cells grown in transwell inserts of varying diameters (6.5–24 mm) for 10–15 days.
Cells grown in 6-well costar plates for 24 h showing ≅75% confluence were transfected using serum-free Opti-MEM®, 150 nM oligonucleotides (MS-oligo, AS-Jnk1, AS-Jnk2, c-Src siRNA, siAsk1, siMKK7 and nonspecific RNA) and 3.15 µl of Oligofectamine® as described previously [31]. Transfected cells were seeded onto transwell inserts. Experiments were performed on day 3 or 4 after seeding.
Cell treatments
Cell monolayers in transwell inserts were incubated with 2.5–3.0% DSS in DMEM (apically). SP600125 (1 µM) was administered 50 min prior to DSS treatment and PP2 (10 µM) administered 30 min prior to DSS. For Ca2+ depletion, DSS was prepared in DMEM lacking Ca2+. Depletion of [Ca2+]i was achieved by incubation of cells with 10 µM BAPTA-AM or 1 µM thapsigargin for 30 min.
Epithelial barrier function
Transepithelial electrical resistance (TER) was measured using a Millicell-ERS Electrical Resistance System and macromolecular permeability evaluated by measuring unidirectional flux of FITC-inulin as described previously [31]. The basal TER values for Caco-2 cell monolayers in these experiments were 400–500 Ohms/cm2.
Cell viability assay
Lactate dehydrogenase release from cells into medium and the mitochondrial dehydrogenase activity (WST assay) in cells were measured using commercial kits as described before to evaluate cell viability [31].
DSS administration in mice
All animal experiments were performed according to the protocols approved by the institutional animal care and use committee at the University of Tennessee Health Science Center, Memphis. Adult female mice (C57BL/6; 12–14 weeks) were used for these studies. Colitis was induced by administering 2.5% (w/v) DSS in drinking water for 4 or 6 days. Body weights, spleen and colon length and weights were measured. Stool consistency and rectal bleeding were graded on 0–3 scales. Stool was graded as: 0 - well-formed pellets, 1 - semisolid stools that do not adhere to anus, 2 - semisolid stool that adhere to anus, and 3 - liquid stool that adhere to anus. Bleeding was graded as: 0 – no blood in stool as tested by hemoccult analysis (ColoScreen-ES, Heleno), 1 – positive hemoccult, 2 – positive hemoccult with trace of blood in stool, and 3 – visible rectal bleeding.
Fluorescence microscopy
Caco-2 cell monolayers and Cryosections (10 µm thickness) of colon were fixed in 3% paraformaldehyde for 15 min at room temperature. Following permeabilization with 0.2% Triton X-100™, sections were blocked and stained for different proteins as described before [31]. The fluorescence was examined by using a Zeiss LSM 510/710 laser scanning confocal microscope and 20× objective lens. Images from x-y (1 µm) sections were collected using LSM Pascal or Zen software. Images from sections were stacked using Image J (NIH) and processed by Adobe Photoshop (Adobe Systems, San Jose, CA).
Measurement of [Ca2+]i
[Ca2+]i was measured as previously described[33]. Briefly, serum-starved Caco-2 cell monolayers on glass-bottom microwell dishes (MatTek; Ashland, MA) were incubated with Fura-2AM (10 µM) in 0.5% pluronic acid for 30 min. Fura-2-loaded cells were alternately excited at 340 or 380 nm using a PC-driven hyper-switch (Ionoptix; MA, USA). Ratios were collected every second at 510 nm using a Dage MTI iCCD camera and Ionwizard software (Ionoptix). [Ca2+]i was calculated using the following equation: [Ca2+]i = Kd (R − Rmin) (Sf2) / (Rmax − R) (Sb2), where R is the 340/380 nm ratio, Rmin and Rmax are the minimum and maximum ratios determined in Ca2+-free and saturated Ca2+ solutions, respectively, Sf2/Sb2 is the Ca2+ free/Ca2+-replete ratio of emissions at 380 nm excitation, and Kd is the dissociation constant for Fura-2. Rmin, Rmax, Sf2, and Sb2 were determined by increasing the Ca2+ permeability of Caco-2 cells with ionomycin (10 µM), and perfusing cells with a high-Ca2+ (10 mM) or Ca2+-free (10 mM EGTA) solution. The in situ apparent dissociation constant (Kd) for Fura-2 used in this study was 224 nM. Eight to ten cells in each monolayer were analyzed simultaneously, and the experiments were repeated in 3–5 monolayers.
c-Src activation by JNK2 in vitro
Recombinant c-Src (500 ng) was incubated with or without recombinant JNK2 (200 ng) in the presence or absence of ATP (0.5 mM) and in the presence or absence of 1 µM SP600125 or 10 µM PP2 in Tris kinase buffer (50 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 0.5 mM ethylene diaminotetraacetic acid, 0.02% Triton X-100, and 2 mM DTT) for one hour at 30°C. Samples were then immunoblotted for p-Thr, c-Src(pY418), c-Src or JNK.
Immunoprecipitation
Caco-2 cell monolayers were washed with phosphate buffered saline and proteins extracted in heated lysis buffer-D (0.3% sodium dodecyl sulfate in 10 mM Tris buffer, pH 7.4, containing 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM phenyl methyl sulfonylfluoride, and 10 µl/ml protease inhibitors cocktail). Phospho-Tyr was immunoprecipitated as described before using biotin-conjugated anti-phospho-Tyr antibody [32]. Immunoprecipitates were immunoblotted for AJC proteins. Similarly, extracts from colonic mucosa was used to immunoprecipitate phosphor-Tyr and immunoblotted for ZO-1.
Immunoblot analysis
Proteins in cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel (7%) electrophoresis and transferred to polyvinylidene fluoride membranes. Membranes were immunoblotted for different proteins as described before [31].
Statistical analyses
Comparison between two groups was made by the Student’s t tests (unpaired) for grouped data. The significance in all tests was derived at the 95% or greater confidence level.
RESULTS
DSS disrupts AJC and induces barrier dysfunction in Caco-2 cell monolayers
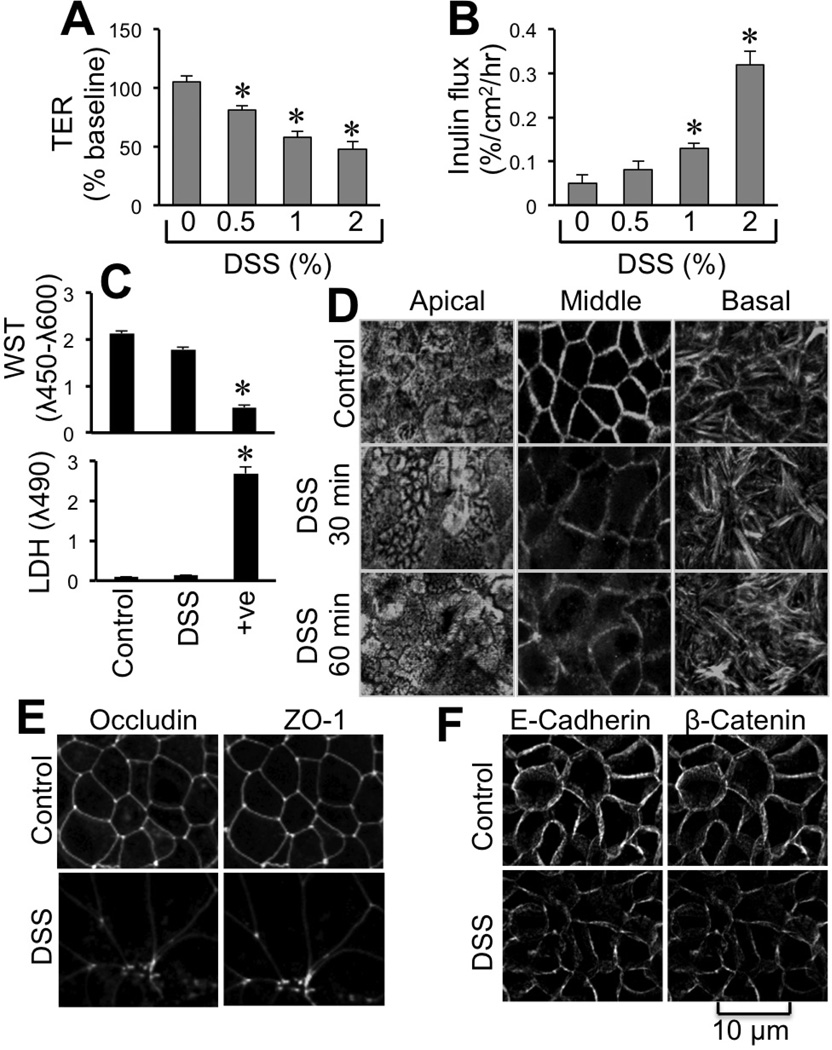
Evidence suggests that DSS-induced colitis in mice may involve elevated mucosal permeability [5, 7, 8, 11]. But, the role of tight junction disruption in DSS-induced mucosal permeability and whether DSS directly affects the epithelial cells are not well understood. Therefore, studies were conducted to evaluate the effect of DSS on epithelial junctional complexes and barrier function in Caco-2 cell monolayers. Treatment of cell monolayers with DSS for 3 hours dose-dependently reduced TER (Fig. 1A) and increased transepithelial inulin permeability (Fig. 1B). DSS treatment did not affect cell viability as assessed by lactate dehydrogenase release into medium and cellular mitochondrial activity (Fig. 1C). Stain for actin cytoskeleton was reorganized by DSS treatment (Fig. 1D). DSS reduced F-actin stain at the upper mid region of the epithelial cell by 30 min, whereas actin organization at the apical and basal regions was unaffected until 60 min. DSS treatment for 2 hours caused a redistribution of tight junction proteins, occludin and ZO-1 from the intercellular junctions into the intracellular compartment (Fig. 1E). Junctional distribution of E-cadherin and β-catenin was also reduced by DSS treatment (Fig. 1F).
Figure 1. DSS disrupts apical junctional complexes in Caco-2 cell monolayers.
A–C: Caco-2 cell monolayers were incubated with DSS at varying concentrations for 3 hours. TER (A) and inulin permeability (B) were measured. Cell viability evaluated after 4 hour DSS treatment by measuring LDH activity in incubation medium and cellular mitochondrial activity by WST assay (C). Values are mean ± SEM (n = 6). Asterisks indicate the values that are significantly (P < 0.05) different from corresponding control (0% DSS) values. D–F: Cell monolayers incubated with or without DSS for 30–90 min were fixed and stained for F-actin (D), tight junction proteins (E) and adherens junction proteins (F) by immunofluorescence method. For actin cytoskeleton, 2 µm stacked optical sections at the apical, upper middle and distal regions of cell monolayer are presented.
JNK activity mediates DSS-induced tight junction disruption and barrier dysfunction
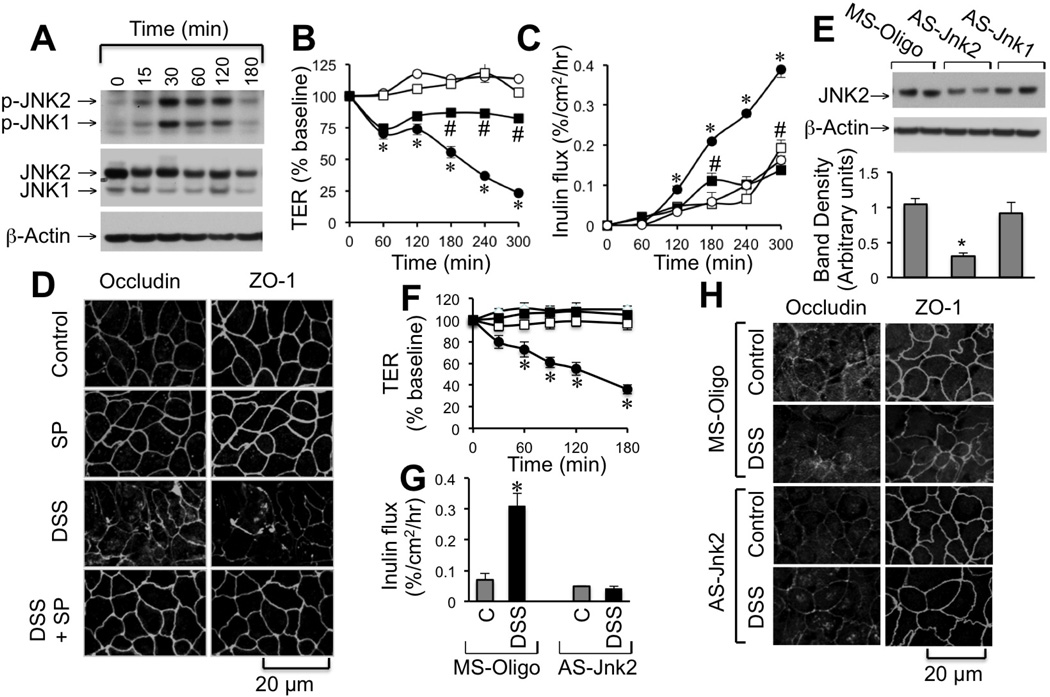
DSS treatment caused a rapid increase in the levels of p-JNK1/2 (JNK1/2pT183/pY185) in Caco-2 cells without considerable effect on the total JNK1/2 levels (Fig. 2A). Pretreatment of cell monolayers with SP600125 (a JNK inhibitor) significantly attenuated DSS-induced decrease in TER (Fig. 2B), increase in inulin permeability (Fig. 2C). SP600125, by itself had no significant effect on TER or inulin permeability. SP600125 also attenuated DSS-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 2D). Transfection of Caco-2 cells with AS-Jnk2, the JNK2-specific antisense oligonucleotide [31], reduced JNK2 levels (Fig. 2E) and significantly attenuated DSS-induced decrease in TER (Fig. 2F), increase in inulin permeability (Fig. 2G) and redistribution of occludin and ZO-1 (Fig. 2H).
Figure 2. JNK activity mediates DSS-induced tight junction disruption in Caco-2 cell monolayers.
A: Caco-2 cell monolayers were incubated with DSS (2.5%) for varying times and cell extracts were immunoblotted for p-JNK, JNK and actin. B–D: Cell monolayers were incubated with (■, □) or without (●, ○) SP600125 (1 µM) 30 min prior to incubation with (●, ■) or without (○, □) 2% DSS for varying times. TER (B) and inulin permeability (C) were measured at varying times. Values are mean ± SEM (n = 6). Asterisks indicate the values that are significantly (P < 0.05) different from corresponding control values (○), and # symbol indicates values that are significantly different from corresponding values for DSS group (●). Cell monolayers were fixed and stained for tight junction proteins. Fluorescence images were collected by confocal microscopy (D). E–H: Caco-2 cell monolayers were transfected with missense (MS-Oligo; ●, ○) or antisense (As-Jnk1 or As-Jnk2; ■, □) oligonucleotides. Protein extracts were immunoblotted for JNK2 and band densities quantitated (E). Transfected cell monolayers were incubated with (●, ■) or without (○, □) 2% DSS for varying times. TER (F) and inulin permeability (G) were measured at varying times. Values are mean ± SEM (n = 6). Asterisks indicate values that are significantly (P < 0.05) different from corresponding control values. After 3 hours of DSS treatment, cell monolayers were fixed and stained for tight junction proteins (H).
DSS administration disrupts AJC in mouse colon in vivo by a JNK-dependent mechanism
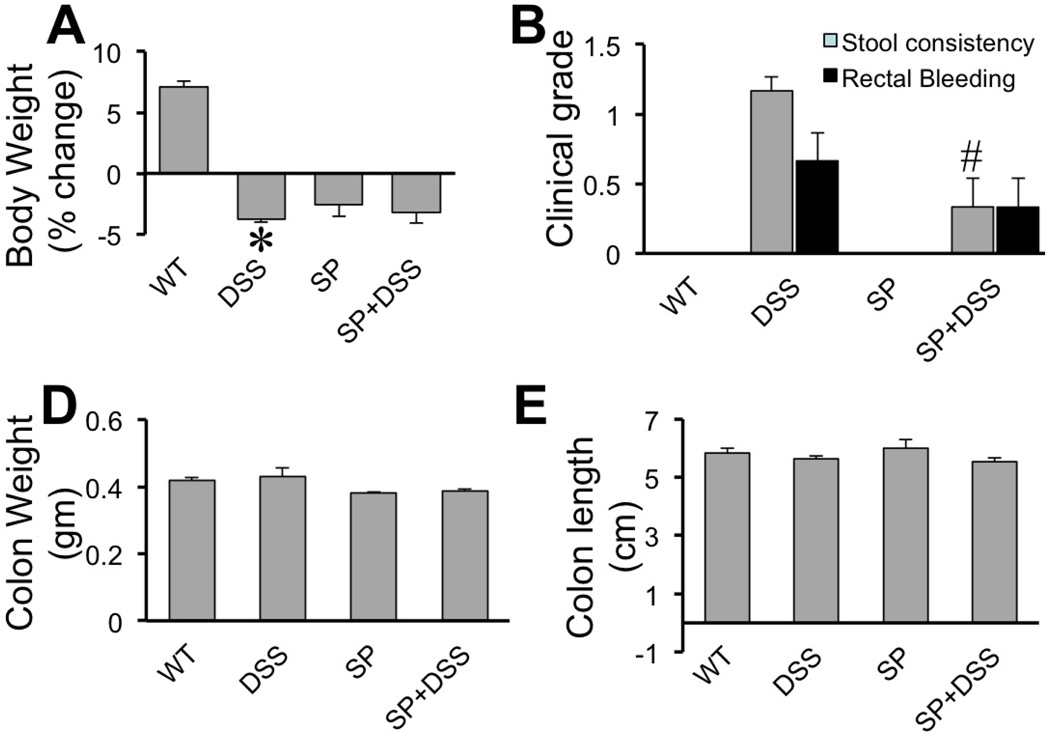
To determine the influence of DSS on colonic mucosal barrier function in vivo mice were treated with 2.5% DSS (w/v) in drinking water for 4 days and evaluated its effect on the integrity of epithelial tight junction and adherens junction in colon. DSS administration resulted in significant loss of body weight (Fig. 3A). Administration of SP600125 by itself reduced the body weight, but DSS administration did not further alter the body weight in the presence of SP600125. Stool consistency was only slightly affected, which was significantly attenuated by SP600125 administration (Fig. 3B). DSS did not cause rectal bleeding at this stage, but slight hemoccult was detected in DSS-treated mice (Fig. 3B). Length and weight of colon were unaffected by DSS or SP600125 (Fig. 3C–D). Similarly, spleen weight and length were also unaffected by DSS or SP600125 (data not shown).
Figure 3. Effect of SP600125 on DSS-induced effects on mouse colon.
Adult female mice were fed 2.5% DSS in drinking water with or without intra peritoneal injection of SP600125 (5 mg/kg body weight). Control groups received no treatment or received only SP600125. Body weights (A), stool consistency & rectal bleeding (B) and colon length & weights (C & D) were evaluated. Values are mean ± SEM (n = 5–7). Asterisk indicates the value that is significantly (p<0.05) different from corresponding control value, and # symbol represents the value that is significantly (p<0.05) different from corresponding DSS without SP600125 value.
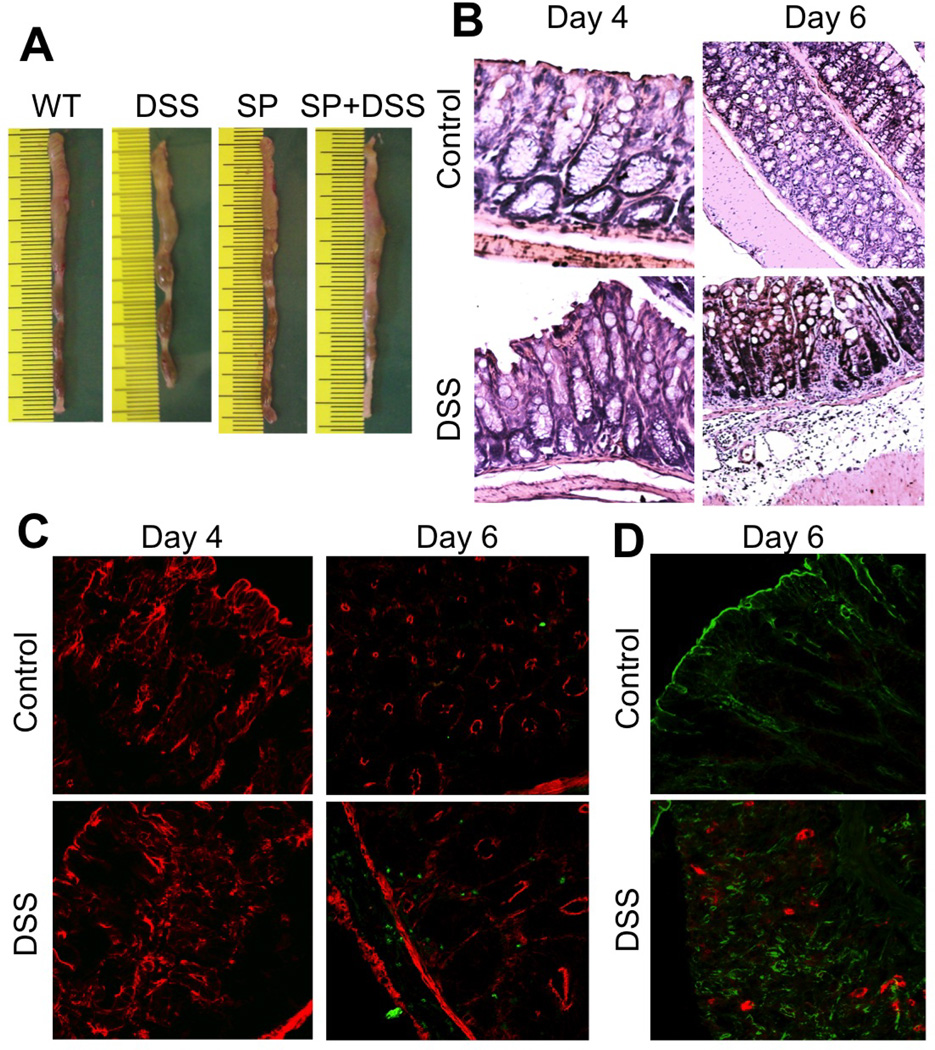
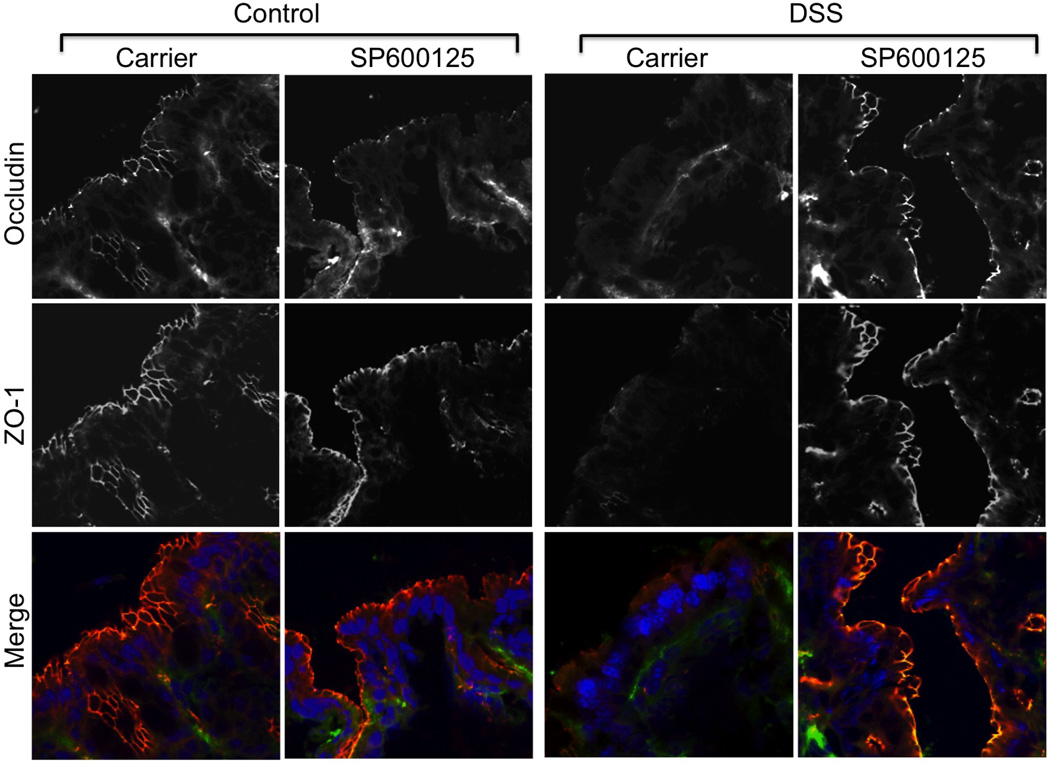
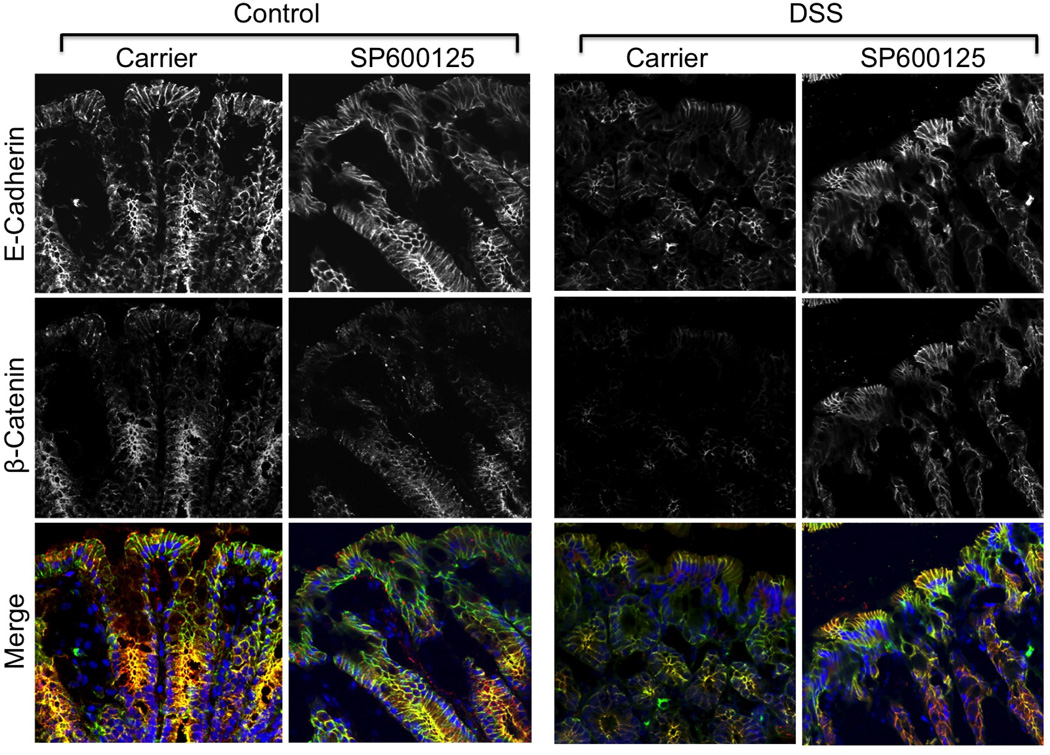
DSS administration for 4 days did not show any sign of colitis (Fig. 4A) nor altered the gross morphology of mucosa and sign of inflammation (Fig. 4B). On the other hand, DSS treatment for 6 days did result in colitis. Inflammatory process was further assessed by co-staining cryosections of colon for Gr1, a marker of polymorphonuclear neutrophil (PMN) and F-actin. DSS administration for 4 days did not induce neutrophil infiltration, but 6 days of DSS administration resulted in significant infiltration of PMN in colonic mucosa (Fig. 4C). DSS treatment for 4 days increased p-JNK levels in colonic mucosa (Fig. 4D) and caused depletion of occludin and ZO-1 at the epithelial junctions (Fig. 5). SP600125 administration attenuated this DSS-induced depletion of tight junction proteins at the intercellular junctions. DSS treatment also reduced junctional organization of E-cadherin and β-catenin in distal colon, which was blocked by SP600125 administration (Fig. 6).
Figure 4. DSS-induced neutrophil infiltration in mouse colon is time-dependent.
Adult female mice were treated with or without 2.5% DSS in drinking water for 4 or 6 days. A: Photographs of representative colon on day 4 after DSS treatment. B: Formalin-fixed paraffin sections were stained with hematoxylin and eosin and bright field images collected at 10× magnification. C: Cryosections of colon were stained for Gr1 (green) and F-actin (Red). D: Cryosections of colon were stained for JNK(pT183pY185) (red) and F-actin (green). Staining and imaging are repeated with two more mice for each group with similar results.
Figure 5. DSS disrupts tight junctions by a JNK-dependent mechanism.
Adult female mice were fed 2.5% DSS in drinking water with or without intra peritoneal injection of SP600125 (5 mg/kg body weight). Control groups received no treatment or received only SP600126. Cryosections of colon were stained for occludin (green), ZO-1 (red) and nucleus (blue). Staining and imaging are repeated with similar results in two more mice for each group.
Figure 6. DSS disrupts adherens junctions by a JNK-dependent mechanism.
Adult female mice were fed 2.5% DSS in drinking water with or without intra peritoneal injection of SP600125 (5 mg/kg body weight). Control groups received no treatment or received only SP600126. Cryosections of colon were stained for E-cadherin (green), β-catenin (red) and nucleus (blue). Staining and imaging are repeated with similar results in two more mice for each group.
Calcium signaling mediates DSS-induced JNK activation and tight junction disruption in Caco-2 cell monolayers
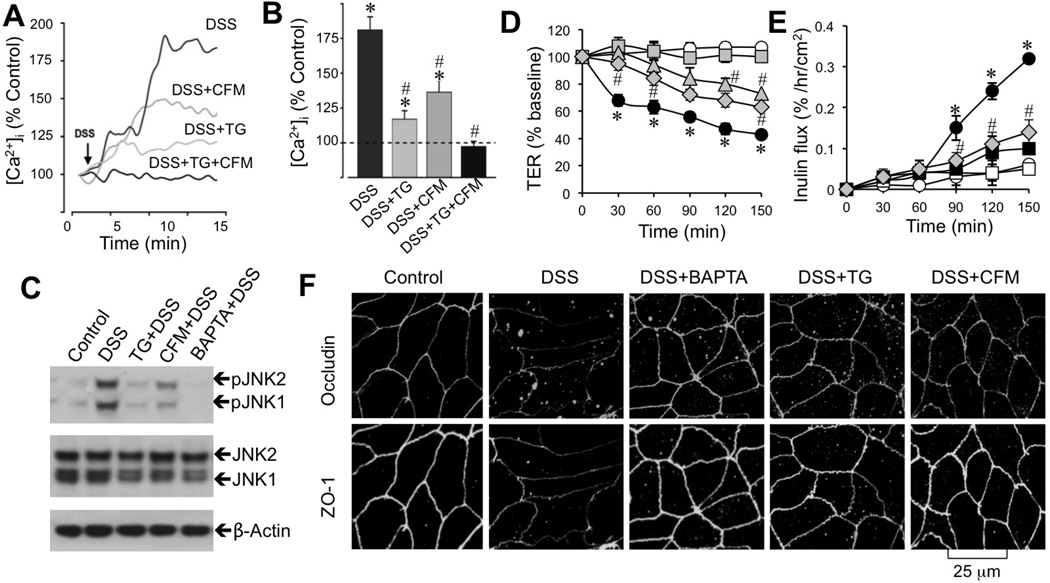
Studies were conducted to investigate the upstream mechanism involved in DSS-induced JNK activation. A recent study indicated a potential role of [Ca2+]i in JNK activation and regulation of epithelial tight junctions during osmotic stress [33]. Therefore, we examined the effect of DSS on [Ca2+]i in Caco-2 cells. DSS rapidly increased [Ca2+]i, and this effect was partially attenuated by either removal of extracellular Ca2+ or depletion of endoplasmic reticulum (ER) Ca2+ using thapsigargin, an ER Ca2+-ATPase inhibitor (Fig. 7A & B). Simultaneous treatment with thapsigargin and extracellular Ca2+ depletion completely blocked DSS-induced rise in [Ca2+]i (Fig. 7B). Consistent with this observation, extracellular Ca2+ depletion or thapsigargin partially reduced DSS-induced JNK activation (Fig. 7C). Treatment of cells with 1,2-bis(o-aminophenoxy)ethane-N,N,N'N'-tetra acetic acid tetra(acetoxymethyl) ester (BAPTA-AM) abolished DSS-induced JNK activation (Fig. 7C). Pretreatment of cell monolayers with BAPTA-AM, thapsigargin or extracellular Ca2+ depletion attenuated DSS-induced decrease in TER (Fig. 7D), increase in inulin permeability (Fig. 7E), and redistribution of occludin and ZO-1 from intercellular junctions (Fig. 7F). BAPTA-AM, thapsigargin or extracellular calcium depletion by itself had no significant effect on JNK activation, TER or inulin permeability.
Figure 7. Calcium signaling mediates DSS-induced JNK activation and tight junction disruption.
A–B: Fura-2 loaded Caco-2 cell monolayers were incubated with 3% DSS in the absence or presence of thapsigargin (TG) or Ca2+-free medium (CFM). Real-time change in [Ca2+]i was measured and quantitated. Values are mean ± SEM (n = 10). Asterisks indicate the values that are significantly (p<0.05) different from corresponding basal values; symbol # indicates values that are significantly (p<0.05) different from value for DSS group. C: Protein extracts from cells treated with 3% DSS in the absence or presence of TG, CFM, or BAPTA for 30 min were immunoblotted for JNK and JNK(pT183pY185) (pJNK). D–F: Caco-2 cell monolayers were incubated with 3% DSS in the absence (●) or presence of BAPTA-AM (□), TG (△), or CFM (◇). Control cell monolayers (○) received no treatments. TER (D) and inulin permeability (E) were measured at varying times. Values are mean ± SEM (n = 8). Asterisks indicate the values that are significantly different (p<0.05) from corresponding control values (○); symbol # indicates values that are significantly (p<0.05) different from value for DSS group (●). Cell monolayers at 2 h after DSS under varying conditions were fixed and stained for tight junction proteins (F).
Ask1 and MKK7 mediates DSS-induced tight junction disruption in Caco-2 cell monolayers
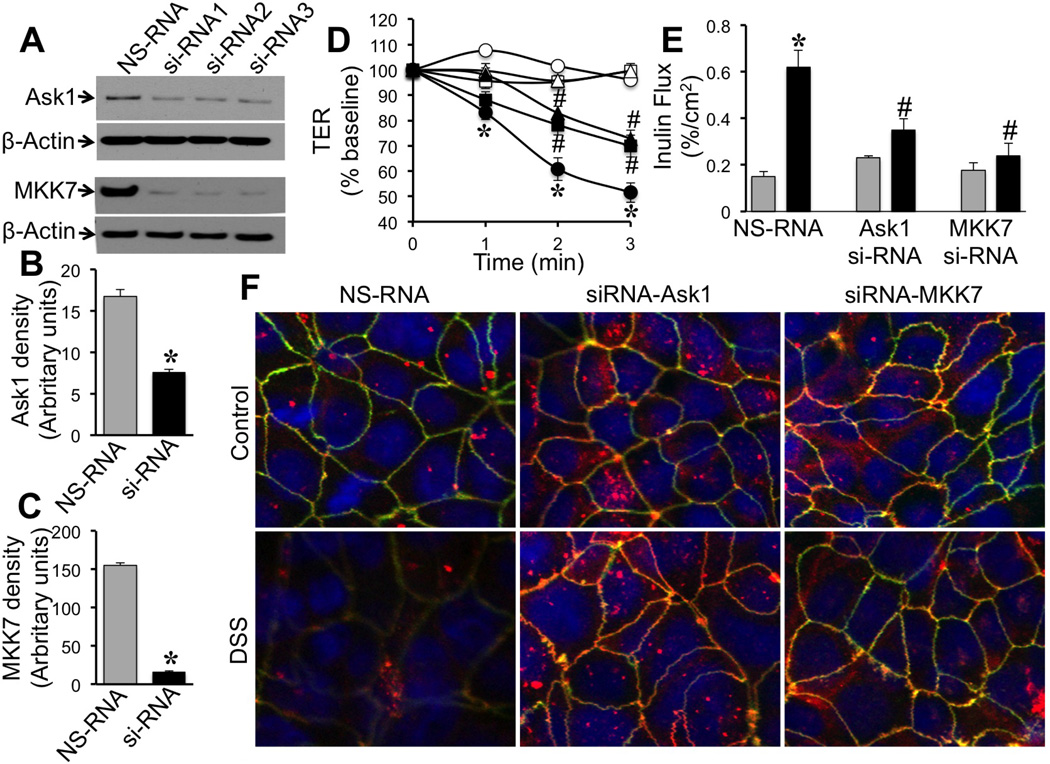
To establish the link between [Ca2+]i and JNK2 activation we evaluated the effects of specific knockdown of Ask1 or MKK7, the signaling elements known to play role in activation of JNK2. Specific siRNAs targeting 3 different sequences of Ask1 and MKK7 genes were transfected to Caco-2 cells, which significantly reduced the levels of corresponding protein (Fig. 8A–C). in NS-RNA-transfected cell monolayers, DSS treatment significantly reduced TER in a time-dependent manner (Fig. 8D). Knockdown of Ask1 or MKK7 significantly attenuated DSS-induced decline in TERAks1 or MKK7 knockdown also significantly blocked DSS-induced inulin permeability (Fig. 8E). DSS induced redistribution of occludin and ZO-1 from the intercellular junctions of NS-RNA-transfected cell monolayers (Fig. 8F). Knockdown of either Ask1 or MKK7 preserved the junctional organization of occludin and ZO-1 during DSS treatment.
Figure 8. Ask1 and MKK7 are involved in the mechanism of DSS-induced barrier dysfunction and tight junction disruption.
A–C: Caco-2 cells were transfected with sets of siRNA for 3 different sites in the gene for human Ask1 (Ask1-siRNA) and MKK7 (MKK7 si-RNA) or non-specific RNA (NS-RNA). Effect of siRNA on Ask1 and MKK7 protein levels was determined by immunoblot analysis (A) and quantitated by densitometric analysis (B & C). Values in panels B and C are mean SE (n = 3). Asterisks indicate the values that are significantly different (p<0.05) from corresponding NS-RNA values. D & E: Cell monolayers transfected with NS-RNA (○, ●), Ask1-siRNA (□, ■) or MKK7-siRNA (△, ▲) were incubated with (●, ■, ▲ or black bars) or without (○, □, △ or gray bars) DSS and TER (D) was measured at varying times and inulin permeability (E) measured at 3 hours after DSS. Values are mean ± SE (n = 4–6). Asterisks indicate the values that are significantly different (p<0.05) from corresponding control values; symbol # indicates values that are significantly (p<0.05) different from value for NS-RNA DSS group. F: At 3 hours after DSS treatment cell monolayers were fixed and stained for occludin (green), ZO-1 (red) and nucleus (blue) by immunofluorescence method and images collected by confocal microscopy.
Calcium and JNK-dependent activation of c-Src mediates DSS-induced tight junction disruption
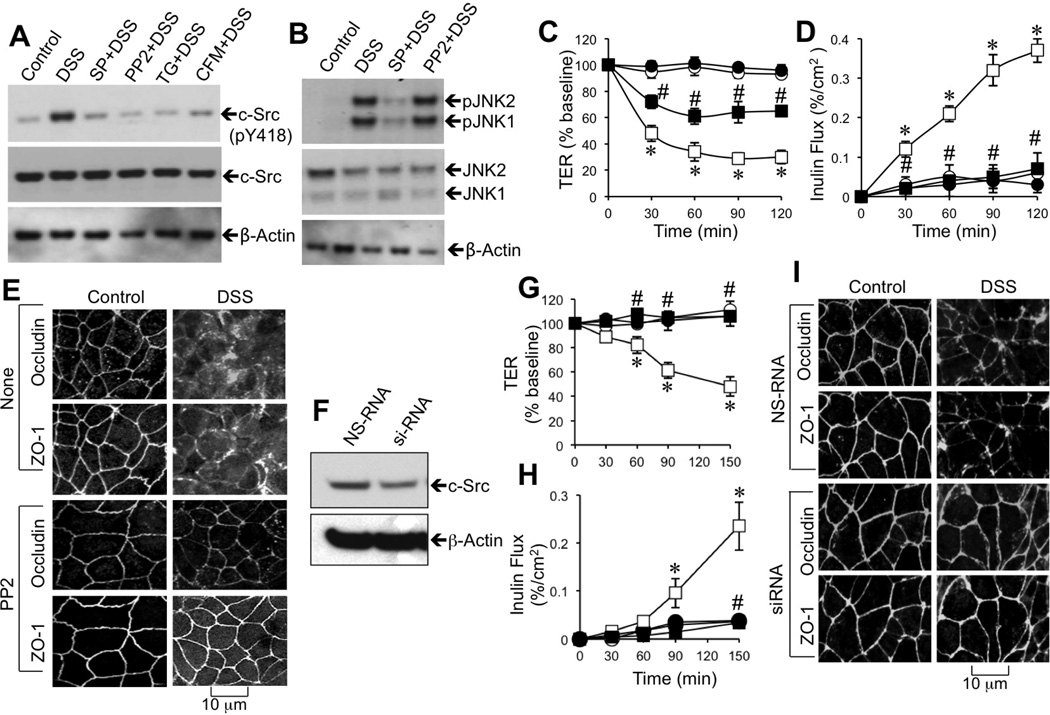
Studies were targeted to investigate the potential signaling down stream to JNK activation during DSS-induced tight junction disruption. Previous studies have demonstrated that c-Src activation leads to epithelial tight junction disruption [15]. Therefore, in the present study, we investigated the role of c-Src in DSS-induced tight junction disruption. Incubation of Caco-2 cell monolayers with DSS for 30 min increased the level of c-Src(pY418), which was blocked by SP600125, PP2 (Src kinase inhibitor), thapsigargin, and extracellular Ca2+ depletion (Fig. 9A). The DSS-induced increase in pJNK1/2, on the other hand, was unaffected by PP2 (Fig. 9B). PP2 treatment significantly blunted DSS-induced decrease in TER (Fig. 9C) and increase in inulin permeability (Fig. 9D). PP2 also attenuated DSS-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 9E). Transfection of Caco-2 cells with c-Src-specific siRNA significantly reduced the level of c-Src (Fig. 9F) and attenuated DSS-induced decrease in TER (Fig. 9G) and increase in inulin permeability (Fig. 9H). Knockdown of c-Src also attenuated DSS-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 9I). The role of c-Src in DSS-induced barrier dysfunction and tight junction disruption was further confirmed by specific knockdown by using another set of siRNA. The data, similar to the effect of above siRNA, are presented in the supplemental figure 1.
Figure 9. Calcium and JNK2-mediated c-Src activation is involved in DSS-induced tight junction disruption.
A–D: Caco-2 cell monolayers were incubated with 3% DSS for 30 min in the presence or absence of SP600125 (SP), PP2 (Src kinase inhibitor), thapsigargin (TG), or Ca2+-free medium (CFM). Protein extracts were immunoblotted for c-Src(pY418) and total c-Src (A) or for JNK and JNK(pT183pY185) (pJNK) (B). TER (C) and inulin permeability (D) were measured in cell monolayers treated with (■, □) or without (●, ○) 3% DSS in the presence (●, ■) or absence (○, □) of PP2. Values are mean ± SEM (n = 6); Asterisks indicate the values that are significantly (p<0.05) different from corresponding control values (○), and the symbol # indicates the values that are significantly (p<0.05) different from corresponding value for DSS group (□). Fixed cell monolayers were stained for occludin and ZO-1 (E). F–I: Caco-2 cell monolayers were transfected with nonspecific RNA (NS-RNA; ○, □) or c-Src-specific siRNA (●, ■), and incubated with (■, □) or without (●, ○) 3% DSS for varying times. Proteins were immunoblotted for c-Src (E). TER (F) and inulin permeability (G) were measured at varying times. Values are mean ± SEM (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding control values (■), and the symbol # indicates the values (p<0.05) that are significantly (p<0.05) different from corresponding values for DSS-treated NS-RNA cells (□). Cell monolayers fixed after 2 h of DSS treatment and stained for tight junction proteins (H).
DSS-induced barrier dysfunction is associated with JNK-dependent Tyr-phosphorylation of AJC proteins
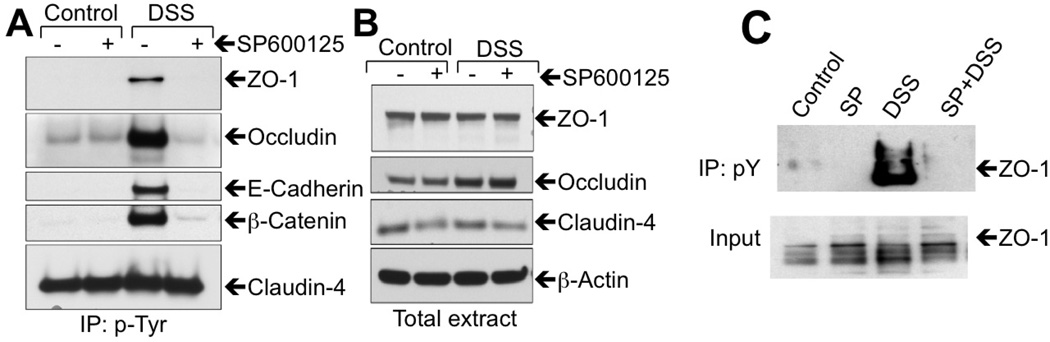
Previous studies demonstrated that hydrogen peroxide-induced disruption of epithelial tight junctions is associated with c-Src-mediated phosphorylation of occludin [15], and that occludin phosphorylation on specific Tyr residues results in a loss of its interaction with ZO-1, leading to disruption of tight junctions [16]. The involvement of c-Src activity in the present study raised the question whether Tyr-phosphorylation of junctional proteins is associated with DSS-induced tight junction disruption. Therefore, we investigated the influence of DSS on Tyr-phosphorylation of tight junction and adherens junction proteins. Incubation of Caco-2 cell monolayers with DSS for 1 h induced a robust increase in Tyr-phosphorylation of occludin, ZO-1, E-cadherin and β-catenin (Fig. 10A). Pretreatment of cell monolayers with SP600125 abrogated DSS-induced Tyr-phosphorylation of these proteins. Claudin-4 was found to be Tyr-phosphorylated in control cell monolayers, which was unaffected by DSS or SP600125 (Fig. 10A). The total amounts of AJC proteins were unaffected by DSS or SP600125 (Fig. 10B). Immunoprecipitation of phospho-Tyr and immunoblot analysis showed that DSS administration for 4 days induced a dramatic increase and Tyr-phosphorylation of ZO-1 in mouse colonic mucosa, which was blocked by SP600125 administration (Fig. 10C).
Figure 10. DSS induces Tyr-phosphorylation of tight junction and adherens junction proteins by a JNK-dependent mechanism.
A–B: Anti-phospho-Tyr immunoprecipitates (IP:p-Tyr) (A) and total cell extracts (B) from Caco-2 cell monolayers treated with DSS for 2 h with or without SP600125 were immunoblotted for tight junction and adherens junction proteins. C: Anti-p-Tyr immunoprecipitates (IP:pY) and total extracts (input) from mucosal extracts of distal colon from mice treated with DSS for 4 days with or without SP600125 administration were immunoblotted for ZO-1.
JNK2 directly phosphorylates c-Src and activates its auto phosphorylation
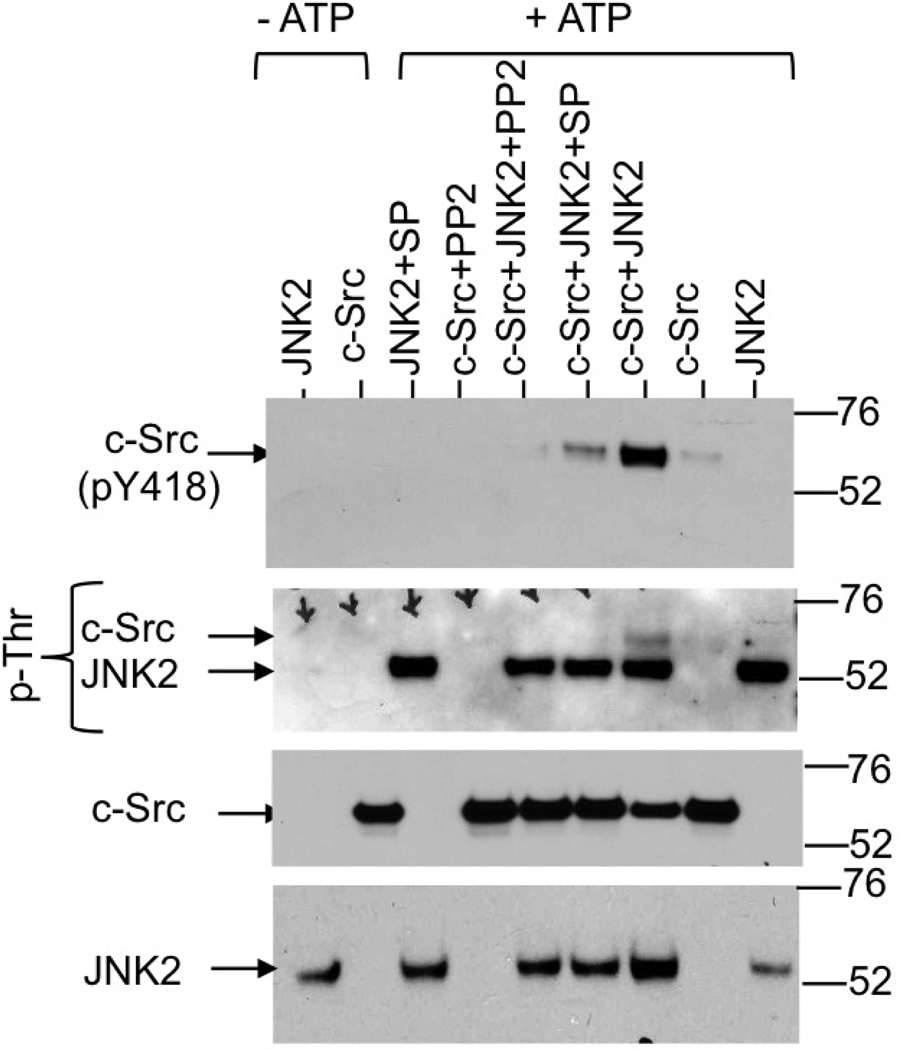
Above studies show that DSS activates c-Src by a JNK-dependent mechanism. The mechanism involved in this JNK2 activity is unclear. This effect of JNK2 may be caused by a direct interaction with c-Src or an indirect mechanism involving other proteins that are known to regulate c-Src activity. To determine whether or not JNK2 directly activates c-Src we incubated recombinant c-Src with recombinant JNK2 in the presence or absence of ATP and with or without selective kinase inhibitors. Results show that incubation with JNK2 and ATP increased the auto phosphorylation of c-Src on Y418, an indicator of activation of c-Src (Fig. 11). In the absence of JNK2, incubation with ATP slightly elevated auto phosphorylation of c-Src. JNK2-medaited activation of c-Src was blocked by SP600125 (JNK inhibitor) as well as PP2 (Src inhibitor). Activation of c-Src by JNK2 was accompanied by the phosphorylation of c-Src on threonine residue(s).
Figure 11. JNK2 directly phosphorylates c-Src on Thr residues and increases its activity.
Recombinant c-Src (500 ng) was incubated with or without recombinant JNK2 (200 ng) in the presence or absence of ATP (0.5 mM) and in the presence or absence of SP (SP600125) or PP2 in Tris kinase buffer for one hour at 30°C. Samples were then immunoblotted for p-Thr, c-Src(pY418), c-Src or JNK.
DISCUSSION
Ulcerative colitis is a chronic inflammatory disease of the colon and rectum of multifactorial etiology [3]. DSS-induced colitis in mice is a well-established model of ulcerative colitis [6]. DSS administration induces erosion and ulceration in the colonic mucosa that is associated with infiltration of inflammatory cells. These symptoms of DSS-induced colitis are similar to those seen in ulcerative colitis patients. However, the mechanisms associated with DSS-induced colitis are poorly understood. Disruption of epithelial barrier and increased mucosal permeability are associated with DSS-induced colitis, and few reports suggest that barrier dysfunction may occur prior to the onset of inflammatory process. Here we provide evidence that a rapid activation of JNK2 is involved in DSS-induced disruption of the AJC in Caco-2 cell monolayers in vitro and in mouse colonic epithelium in vivo at an initial stage prior to inflammation. Furthermore, this study shows that Ca2+-Ask1-MKK7-JNK2-cSrc signaling cascade mediates DSS-induced tight junction disruption and barrier dysfunction.
Evidence indicates that DSS-induced colitis in mice is associated with colonic mucosal permeability [5, 7, 8, 11]. However, it is not clear whether the increased permeability was caused by a direct effect on the epithelial junctions. A previous study showed that DSS decreases TER in Caco-2 cell monolayers [34], but it was unclear whether the decline in TER was caused by changes in the transcellular or paracellular transports. The observation in the present study that DSS decreases TER and increases inulin permeability in Caco-2 cell monolayers without altering cell viability indicates that DSS can directly affect the epithelial barrier function. Redistribution of tight junction and adherens junction proteins from the intercellular junctions demonstrates that DSS disrupts both tight junctions and adherens junctions in the intestinal epithelium. DSS-induced disruption of tight junction and adherens junction was associated with the reorganization of actin cytoskeleton. Actin reorganization occurred predominantly in the upper mid-region of epithelial cells, indicating that DSS impacts the adherens belt region of the actin cytoskeleton that interacts with the tight junction and adherens junction protein complexes.
A rapid increase in pJNK in DSS-treated cells indicates that DSS activates JNK1 and JNK2 in Caco-2 cell monolayers. A significant attenuation of DSS-induced barrier dysfunction and redistribution of occludin and ZO-1 from the junctions by SP600125 demonstrate that JNK activity mediates DSS-induced tight junction disruption in Caco-2 cell monolayers. Although SP600125 is a well-established JNK inhibitor and extensively used to probe the cellular of JNK activity, it inhibits both JNK1 and JNK2 activities. Attenuation of DSS-induced barrier dysfunction and redistribution of occludin and ZO-1 by specific knockdown of JNK2 indicates the importance of JNK2 isoform in the DSS-induced tight junction disruption. The antisense oligo used in this study was previously shown to specifically knockdown JNK2, without altering the levels of JNK1. Selective activation of JNK1 by EGF failed to disrupt tight junctions in Caco-2 cell monolayers, JNK1 activation rather reduced inulin permeability.
The role of JNK activity in DSS-induced barrier dysfunction was further confirmed in mouse colon in vivo. DSS administration for 4 days resulted in redistribution of occludin, ZO-1, E-cadherin and β-catenin from the junctions in colonic epithelium, indicating the DSS-induced loss of tight junction and adherens junction integrity. Attenuation of DSS-induced redistribution of tight junction and adherens junction proteins by SP600125 administration demonstrates the role of JNK activity in DSS-induced tight junction and adherens junction disruption in mouse colon in vivo. DSS administration for 4 days resulted in low-grade diarrhea, which was prevented by SP600125 administration. Histopathology and staining colonic cryosections for PMN marker, Gr1, indicated that inflammatory process was absent in colon at 4 days of DSS treatment. On the other hand, colon from mice at 6 days of DSS treatment showed significant PMN infiltration. Disruption of tight junctions and adherens junctions at 4 days of DSS treatment indicates that tight junction disruption and barrier dysfunction occur prior to the onset of inflammatory process, which is in agreement with the previous suggestions that mucosal permeability may precede the inflammatory process [8, 9]. These in vivo studies establish the physiologic relevance of the observations made in Caco-2 cell monolayers.
Previous study showed that the JNK inhibitor, SP600125, partially reduced DSS-induced wasting and colonic mucosal ulceration in mice [30], whereas another study indicated the lack of a role for JNK in IBD pathogenesis [35]. Therefore, the precise role of JNK activation in the pathogenesis of colitis is unknown. Interestingly, our study shows that DSS-induced diarrhea and disruption of tight junctions and adherens junctions were attenuated by SP600125 administration. Therefore, JNK-mediated disruption of AJC may play an important role in DSS-induced colitis. Specific inhibition of JNK or its up stream or down stream signals may serve as therapeutic target in the treatment of ulcerative colitis.
To determine the upstream signal involved in DSS-induced JNK activation we investigated the potential role of [Ca2+]i in DSS-induced JNK activation and barrier dysfunction. Ca2+ signaling is known to play roles in both assembly and disruption of tight junctions [33]. In the present study, we found that DSS rapidly increases [Ca2+]i, which can be partially reduced by either extracellular Ca2+ depletion or blocking intracellular ER Ca2+ release by thapsigargin. A combined absence of extracellular Ca2+ and ER Ca2+ release abrogated DSS-induced rise in [Ca2+]i, indicating that DSS increases [Ca2+]i by stimulating both extracellular Ca2+ influx and ER Ca2+ release. Blocking the [Ca2+]i elevation with BAPTA-AM, thapsigargin or extracellular Ca2+ depletion attenuated DSS-induced increase in pJNK levels, indicating that DSS-induced JNK activation is mediated by [Ca2+]i. Blocking [Ca2+]i significantly dampened DSS-induced barrier dysfunction and redistribution of tight junction proteins from the intercellular junctions. These results demonstrate that rise in [Ca2+]i is essential for DSS-induced tight junction disruption.
JNK activation by a calcium-dependent mechanism raised the question, what is the mechanism involved in calcium-induced JNK activation. There is no evidence in the literature supporting a direct activation of JNK by calcium. However, the known upstream regulator of JNK2 activation is MKK7 [36]. Therefore, we investigated the role of MKK7 in DSS-induced tight junction disruption. Knockdown of MKK7 in Caco-2 cell monolayers significantly blocked DSS-induced decline in TER and increase in inulin permeability, indicating the requirement of MKK7 in the mechanism of DSS-induced barrier dysfunction. MKK7 depletion also attenuated DSS-induced loss of junctional organization of tight junction proteins, occludin and ZO-1. These data demonstrate the role of MKK7. Upstream to MKK7 is Ask1, a signaling molecule know to be regulated by calcium via calmodulin kinase [36]. Therefore, we investigated the potential role of Ask1 in DSS-induced tight junction disruption. Knockdown of Ask1 significantly blocked DSS-induced drop in TER, rise in inulin permeability and loss of junctional organization of occluding and ZO-1, indicating that Ask1 is an important component of mechanism involved in DSS-induced tight junction disruption. These data demonstrate that Ask1 and MKK7 mediate calcium-induced JNK2 activation. This is the first report of tight junction regulation by Ask1 and MKK7.
We further investigated the signal down stream to JNK activation in DSS-induced tight junction disruption and barrier dysfunction. Previous studies demonstrated that c-Src activation leads to disruption of epithelial tight junctions and barrier dysfunction, and such a mechanism is involved in hydrogen peroxide-induced tight junction disruption in Caco-2 cell monolayers [15]. The mechanism of c-Src-mediated tight junction disruption involves Tyr-phosphorylation of tight junction and adherens junction proteins [16, 37]. Tyr-phosphorylation of tight junction and adherens junction proteins are known to weaken the integrity of AJC [38, 39]. Therefore, we investigated the potential role of c-Src activation in DSS-induced tight junction disruption. DSS rapidly increased the level of c-Src(pY418), indicating that DSS activates c-Src. DSS-induced c-Src activation was prevented by BAPTA-AM, thapsigargin and extracellular Ca2+ depletion as well as by SP600125, indicating that DSS activates c-Src by a Ca2+ and JNK-dependent mechanism. Inhibition of Src kinase activity or knockdown of c-Src attenuated DSS-induced tight junction disruption and barrier dysfunction. Therefore, c-Src activation is an important event downstream to JNK activation during DSS-induced tight junction disruption.
DSS-induced c-Src activation and tight junction disruption was associated with Tyr phosphorylation of occludin, ZO-1, E-cadherin, and β-catenin in Caco-2 cell monolayers. Prevention of DSS-induced Tyr-phosphorylation of tight junction and adherens junction proteins by SP600125 indicated that JNK activity mediates DSS-induced Tyr-phosphorylation, likely via c-Src activation. DSS administration in mice also caused Tyr-phosphorylation of ZO-1 in colonic mucosa, which was prevented by SP600125. Therefore, the mechanism involving JNK-mediated Tyr-phosphorylation of tight junction proteins, likely via c-Src activation, also exists in the mouse colonic epithelium in vivo. Phosphorylation of occludin on Y398 and Y402 was previously shown to attenuate its interaction with ZO-1 and weaken tight junction integrity [38, 39]. It is likely that DSS induces Tyr-phosphorylation of occludin on Y398 and Y402 residues.
The role of JNK activity in c-Src activation in DSS-treated cells raised question whether the effect is caused by a direct interaction of JNK2 with c-Src or it is indirect mediated by other proteins. At this time, there is no report to support a direct interaction between JNK2 and c-Src. To determine a potential direct interaction we conducted in vitro experiments using recombinant JNK2 and c-Src proteins. Incubation of c-Src with JNK2 and ATP induced a robust increase in the level of c-Src(pY418). Auto phosphorylation of c-Src on Y418 is an indicator of c-Src activation [40]. It is unclear at this time how JNK2 activates c-Src. Our data show that increase in c-Src auto phosphorylation by JNK2 is associated with threonine phosphorylation of c-Src, and therefore, needs further investigations to determine the precise mechanism of JNK2-induced activation of c-Src.
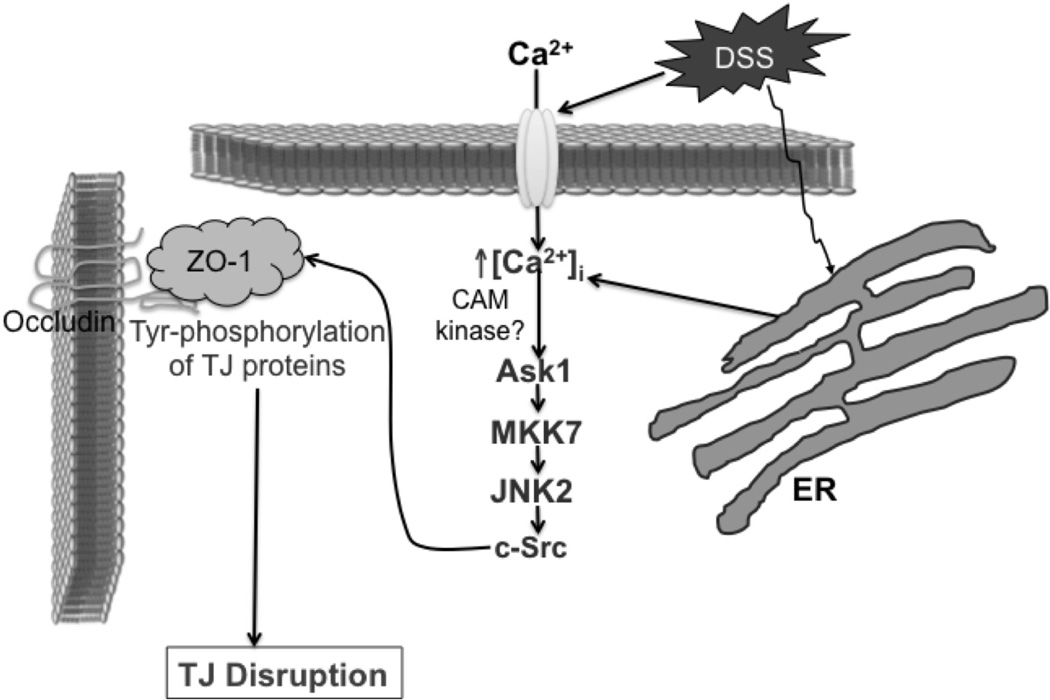
These studies in Caco-2 cell monolayers in vitro and mouse colon in vivo therefore demonstrate that DSS stimulates extracellular Ca2+ influx and ER Ca2+ release to elevate the [Ca2+]i (Fig. 12). [Ca2+]i activates JNK2 likely by Ask1 and MKK7-mediated mechanism, and JNK2 activates c-Src likely by direct phosphorylation of c-Src on threonine residues. Src activation leads to Tyr-phosphorylation of occludin, likely on Y398 and Y402 residues, resulting in weakening of tight junctions and causing barrier dysfunction. Tyrosine phosphorylation of ZO-1 and adherens junction proteins may also contribute to disruption of tight junction disruption. Disruption of tight junctions and barrier dysfunction may lead to mucosal translocation of luminal toxins and pathogens to induce inflammatory process and colitis.
Figure 12. Proposed mechanism of DSS-induced tight junction disruption.
DSS appears to mobilize calcium from both extracellular medium and endoplasmic reticulum (ER) that triggers Ask1-MKK7-JNK2-cSrc signaling and Tyr-phosphorylation of tight junction and adherens junction proteins leading to disruption of tight junctions.
Supplementary Material
A & B: Caco-2 cells were transfected with sets of siRNA for 3 different sites in the gene for human c-Src (Src-siRNA) or non-specific RNA (NS-RNA). Effect of siRNA on c-Src protein levels was determined by immunoblot analysis (A) and quantitated by densitometric analysis (B). Values in panels B are mean SE (n = 3). Asterisks indicate the values that are significantly different (p<0.05) from corresponding NS-RNA values. C & D: Cell monolayers transfected with NS-RNA (○, ●), or Src-siRNA (□, ■) were incubated with (●, ■ or black bars) or without (○, □ or gray bars) DSS and TER (C) was measured at varying times and inulin permeability (D) measured at 3 hours after DSS. Values are mean ± SE (n = 4–6). Asterisks indicate the values that are significantly different (p<0.05) from corresponding control values; symbol # indicates values that are significantly (p<0.05) different from value for NS-RNA DSS group. E: At 3 hours after DSS treatment cell monolayers were fixed and stained for occludin (green), ZO-1 (red) and nucleus (blue) by immunofluorescence method and images collected by confocal microscopy.
Acknowledgements
This study was supported by National Institute of Health grants R01-DK55532 and R01-AA12307.
Abbreviations
- AJC
apical junctional complex
- As-Jnk1/2
antisense oligonucleotides for JNK1 and JNK2
- Ask1
apoptosis BAPTA-AM, 1,2-bis(o-aminophenoxy)ethane-N,N,N’N’-tetra acetic acid tetra(acetoxymethyl) ester
- CFM
calcium free medium
- DSS
dextran sodium sulfate
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- MKK7
MAP kinase kinase
- MS-oligo
missense oligonucleotide
- NS-RNA
non specific RNA
- pJNK1/2
JNK1/2(pT183pY185)
- PMN
polymorphonuclear neutrophil
- p-cSrc
c-Src(pY418)
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- SP600125
(anthra[1,9-cd]pyrazol-6(2H)-one)
- TER
transepithelial electrical resistance
- TG
thapsigargin
- ZO-1
zona occludens-1
Footnotes
Statement of author contribution
Drs. Samak, Chaudhry and Gangwar were responsible for performing experiments, data analysis and organization, preparation of figures and editing manuscript. Dr. Narayan’s contribution include conducting experiments and editing manuscript. Dr. Jaggar has contributed to this study by design of experiments and data interpretation. Dr. Rao is responsible for the design of experiments, interpretation of data and manuscript writing.
References
- 1.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell. Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Turner JR. 'Putting the squeeze' on the tight junction: understanding cytoskeletal regulation. Semin. Cell Dev. Biol. 2000;11:301–308. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- 3.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr. Opin. Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 5.Bjorck S, Jennische E, Dahlstrom A, Ahlman H. Influence of topical rectal application of drugs on dextran sulfate-induced colitis in rats. Dig. Dis. Sci. 1997;42:824–832. doi: 10.1023/a:1018880501437. [DOI] [PubMed] [Google Scholar]
- 6.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J. Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 7.van Meeteren ME, van Bergeijk JD, van Dijk AP, Tak CJ, Meijssen MA, Zijlstra FJ. Intestinal permeability and contractility in murine colitis. Mediators Inflamm. 1998;7:163–168. doi: 10.1080/09629359891090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp. Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 9.Venkatraman A, Ramakrishna BS, Pulimood AB, Patra S, Murthy S. Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand. J. Gastroenterol. 2000;35:1053–1059. doi: 10.1080/003655200451171. [DOI] [PubMed] [Google Scholar]
- 10.Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JM, Fanning AS, Lapierre L, Van Itallie CM. Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem. Soc. Trans. 1995;23:470–475. doi: 10.1042/bst0230470. [DOI] [PubMed] [Google Scholar]
- 14.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008;13:7210–7226. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 16.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase Czeta phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem. J. 2011;437:289–299. doi: 10.1042/BJ20110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc. Natl. Acad. Sci. U S A. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J. Biol. Chem. 2008;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Suzuki T, Taylor WL, Bhargava A, Rao RK. Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem. J. 2011;433:51–63. doi: 10.1042/BJ20100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. 2006;393:69–77. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samak G, Aggarwal S, Rao RK. ERK is involved in EGF-mediated protection of tight junctions, but not adherens junctions, in acetaldehyde-treated Caco-2 cell monolayers. Am. J. Physiol. Gastrointest Liver Physiol. 2011;301:G50–G59. doi: 10.1152/ajpgi.00494.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N. Y. Acad. Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem. J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am. J. Physiol. Gastrointest Liver Physiol. 2012;303:G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- 27.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim. Biophys. Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J. Immunol. 2002;168:5342–5351. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 30.Assi K, Pillai R, Gomez-Munoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112–121. doi: 10.1111/j.1365-2567.2006.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samak G, Suzuki T, Bhargava A, Rao RK. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am. J. Physiol. Gastrointest Liver Physiol. 2010;299:G572–G584. doi: 10.1152/ajpgi.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samak G, Narayanan D, Jaggar JH, Rao R. CaV1.3 channels and intracellular calcium mediate osmotic stress-induced N-terminal c-Jun kinase activation and disruption of tight junctions in Caco-2 CELL MONOLAYERS. J. Biol. Chem. 2011;286:30232–30243. doi: 10.1074/jbc.M111.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh-Reitz MM, Huang EF, Musch MW, Chang EB, Martin TE, Kartha S, Toback FG. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am. J. Physiol. Gastrointest Liver Physiol. 2005;289:G163–G171. doi: 10.1152/ajpgi.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malamut G, Cabane C, Dubuquoy L, Malapel M, Derijard B, Gay J, Tamboli C, Colombel JF, Desreumaux P. No evidence for an involvement of the p38 and JNK mitogen-activated protein in inflammatory bowel diseases. Dig. Dis. Sci. 2006;51:1443–1453. doi: 10.1007/s10620-006-9116-2. [DOI] [PubMed] [Google Scholar]
- 36.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J. Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 37.Basuroy S, Dunagan M, Sheth P, Seth A, Rao RK. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. Am. J. Physiol. Gastrointest Liver Physiol. 2010;299:G186–G195. doi: 10.1152/ajpgi.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias BC, Bhattacharya S, Ray RM, Johnson LR. Polyamine-dependent activation of Rac1 is stimulated by focal adhesion-mediated Tiam1 activation. Cell Adh. Migr. 2010;4:419–430. doi: 10.4161/cam.4.3.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem. Biophys. Res. Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 40.Cole PA, Shen K, Qiao Y, Wang D. Protein tyrosine kinases Src and Csk: a tail's tale. Curr. Opin. Chem. Biol. 2003;7:580–585. doi: 10.1016/j.cbpa.2003.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A & B: Caco-2 cells were transfected with sets of siRNA for 3 different sites in the gene for human c-Src (Src-siRNA) or non-specific RNA (NS-RNA). Effect of siRNA on c-Src protein levels was determined by immunoblot analysis (A) and quantitated by densitometric analysis (B). Values in panels B are mean SE (n = 3). Asterisks indicate the values that are significantly different (p<0.05) from corresponding NS-RNA values. C & D: Cell monolayers transfected with NS-RNA (○, ●), or Src-siRNA (□, ■) were incubated with (●, ■ or black bars) or without (○, □ or gray bars) DSS and TER (C) was measured at varying times and inulin permeability (D) measured at 3 hours after DSS. Values are mean ± SE (n = 4–6). Asterisks indicate the values that are significantly different (p<0.05) from corresponding control values; symbol # indicates values that are significantly (p<0.05) different from value for NS-RNA DSS group. E: At 3 hours after DSS treatment cell monolayers were fixed and stained for occludin (green), ZO-1 (red) and nucleus (blue) by immunofluorescence method and images collected by confocal microscopy.