Abstract
Atomic force microscopy, infrared spectroscopy and NMR studied morphological and physicochemical properties of calcium hydroxyapatite powders produced by changing the temperature parameters of synthesis. Features of morphology formation of calcium hydroxyapatite nanoparticles with an annealing temperature within 200°C to 1,100°C were determined. It is shown that the particle size of the apatite obtained that annealed 700°C is 40 nm corresponding to the particle size of apatite in native bone. The effect of dimension factor on structural parameters of calcium hydroxyapatite is manifested in a more local symmetry of the PO43− tetrahedra at nanodispersed calcium hydroxyapatite.
Keywords: Atomic force microscopy, Hydroxyapatite, Morphology, Electronic structure
Background
Bioactive materials based on calcium hydroxyapatite (Ca10(PO4)3(OH)2, HAP), the chemical composition and structure of which approaches the mineral component of bones, are widely used in medical practice over the past 20 years. All the time, the researches continue to work for improvement of these materials [1-4]. However, the prospect of their application may not be quite complete without clarifying the relationship of morphological, physical and chemical properties, and atomic architecture surfaces of these materials because biocompatibility is dependent not only on their chemical composition but also to a large extent on the morphology of the particles, which affect the interaction of mineral constituent of bones with the organic matrix. In view of the fact that such material should be included in the metabolism of the body and be replaced with a full bone tissue, it remains relevant to obtain nanodispersed calcium hydroxyapatite with parameters that match the parameters of particle maximum mineral component of bones [5-8]. This article presents the results of a study of morphological and physicochemical properties of calcium hydroxyapatite powders obtained at various temperature parameters of synthesis.
Methods
Preparation of calcium hydroxyapatite
For preparation of hydroxyapatite powder, calcium nitrate tetrahydrate and diammonium hydrogen phosphate (reagent grade) were used as calcium and phosphorus precursors, respectively. Both reagents were purchased from Merck, Darmstadt, Germany. Urea (R&M Chemicals, Edmonton, UK) was used as gelling and ammonium donor agent. EDTA (Merck) was used as a chelating agent to prevent an immediate precipitate formation calcium ion in the course of gel formation. The reaction was conducted in a basic solution using ammonium solution (R&M Chemicals) as a solvent. Ammonium solution was heated at 60°C, and EDTA (182 g) was added while stirring until it dissolved. Into this, 200 mL of aqueous solution of 128 g calcium nitrate tetrahydrate was poured. Diammonium hydrogen phosphate (39.83 g) and urea (45.20 g) were subsequently added. The mixture was heated at 100°C for 3 to 4 h. The obtained gel was dried at 150°C under ambient static air and subsequently subjected to an 200°С, 500°С, 700°С, 900°С, 1,000°С, and 1,100°С calcination under flowing air for 1 h. The powder was examined by X-ray diffraction techniques to determine the phases formed. It was observed that Ca/P molar ratio is 1.667.
Morphology investigation
Morphology of hydroxyapatite powder has been investigated by scanning probe microscope JSPM-4500/4610 (JEOL, Tokyo, Japan) using atomic force microscopy. Imaging parameters were as follows: image size 90.0 × 90.0 nm, image height 12.2 nm, and exposure time at 83.33 μs. As the scanning probe made of cantilever with a diamond tip of the NSG-10-DLC. Operational vacuum was no worse than 10−7 Pa.
Infrared spectroscopy investigation
IR spectra were obtained on double-beam spectrophotometer SPECORD M80 (Carl Zeiss, Jena, Germany). To provide the study, the samples were prepared by compressing into the mixed tablets of the compound and powdered KBr. Tablets of pure KBr powder were also prepared to measure of the sample phonon transmission. Transmission spectra were recorded in the absorption range of the tetrahedral sublattice PO43− with vibrational modes lying in the 4,000 to 400 cm−1 range.
In the region of slow electromagnetic waves, sharp KBr powder absorption begins; therefore, the measurement in the range <400 cm−1 is not correct. The measurements were made at a constant level of signal/noise ratio in the whole measurement range. To optimize the recording of the spectra, an electromagnetic radiation attenuator was also installed in comparison channel, so that the absorption background level of KBr pellet approaches to 80%. During the measurements, the sample chamber was additionally blown through with dry air for thorough drying of water vapor.
NMR investigation
NMR spectra were recorded on a spectrometer Avance 400 (Bruker, Ettlingen, Germany) at room temperature. NMR spectra were obtained from rotation of the sample under the magic angle (MAS NMR) at frequencies of 10 and 15 kHz, which eliminates the effects of anisotropy and dipole-dipole interaction, but does not exclude the quadrupole interaction of the second order.
Results and discussion
Morphology of nanoscale powders
It should be noted that the powders obtained by sol-gel method formation of apatite are similar with the formation of apatite in bone native mechanism: colloidal solution-amorphous condensate-crystal phase. Kinetic chain synthesis involves the transformation of sol in the gel, condensation precipitate, rinsed with water, and drying. Since the composition of the amorphous shell in particles cannot be determined, we can conclude only a disordered agglomeration of the molecules. Transition of amorphous condensate-crystal phase is observed in samples obtained at 200°C. Simultaneous formation of amorphous particles begins with the process of crystallization, i.e., begins with the formation of the long-range order within the amorphous phase. There are clusters of crystal phase size 10 to 15 nm in an amorphous matrix (Figure 1).
Figure 1.

AFM image of HAP samples obtained at the temperature of 200°C. Transition of amorphous condensate-crystal phase is observed in samples obtained at 200°C. There are clusters of crystal phase size 10 to 15 nm in an amorphous matrix. Image size is 90.0 × 90.0 nm.
When the temperature reaches up to 500°C, it shows an increase in the size of the crystal phase clusters. Aspiration systems reduce the surface energy leads to the activation of aggregation processes: coagulation and coalescence of clusters, resulting in an increase in grain size crystal phase. In Figure 2 shows a picture of nanoparticles obtained at 500°C temperature conditions in which the collective process of recrystallization completed.
Figure 2.
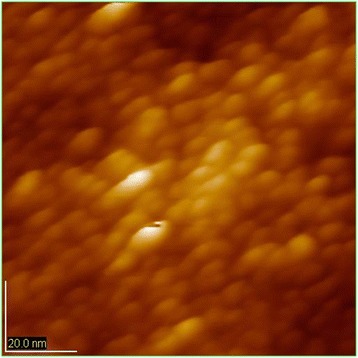
AFM image of HAP samples obtained at the temperature of 500°C. There has been an increase in the size of the crystal phase clusters. The particle size is 15 to 20 nm. Image size is 90.0 × 90.0 nm.
This process continues up to a temperature of 700°C. It should be noted that the most rapid growth of crystals occurs in the temperature range of 600°C to 700°C [1-3]. The particle size of the apatite obtained by annealing at 700°C is 40 nm (Figure 3), corresponding to a particle size of apatite in native bone. There is a horseshoe shape of the particles. However, the bone apatite particles are needle shaped. The horseshoe shape is due to the application in the synthesis of drying powder in which the ends of the particles are not committed and bent under the influence of surface tension, creating a shape that resembles a horseshoe.
Figure 3.
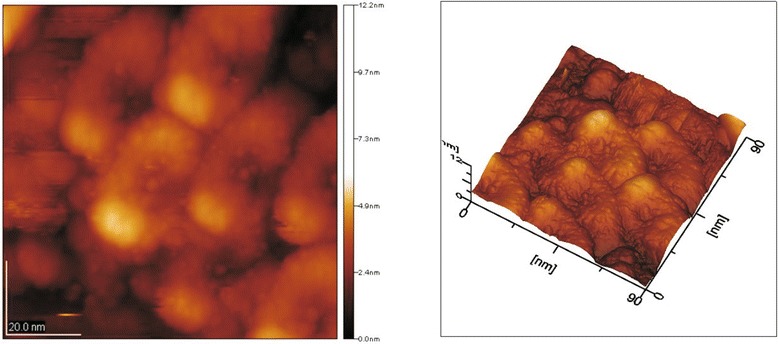
AFM image of HAP samples obtained at the temperature of 700°C. The particle size of the apatite is 40 nm. There is a horseshoe shape of the particles. Image size is 90.0 × 90.0 nm; image height is 12.2 nm.
Annealing at temperatures above 900°C leads to complex processes of mass transfer and formation of crystals of different morphology and particle size range from 200 to 500 nm.
The crystallization process takes place before the full absorption of the crystalline phase of amorphous matrix. Structural changes in the crystal lattice during the transition amorphous condensate-crystal phase can mostly be traced back to change the width of the vibrational bands in the spectra of crystalline lattice.
IR and NMR spectroscopy of nanodispersed apatites
It is known [1] that spectrum of initial phosphate hydroxyapatite is characterized by two intense groups of bands around 1,040 and 570 cm−1. Nine possible variations of XO4 group in the case of equivalence of all X-O bonds, i.e., tetrahedral symmetry Td, give only two bands of IR spectrum: one band of v3 vibrations and another of v4 ones, the vibrations v2 and v1 being active only in Raman spectra [9].
If only three of four bonds are equal (symmetry C3v), then total symmetric vibration v1 becomes active, and the splitting of three degenerate vibrations v3 and v4 is removed partially. When there is a non-equivalence of the two X-O bonds with respect to the other two (point symmetry C2v) degenerations of vibrations, v3 and v4 are removed completely. Finally, in the case when all four bonds are different (symmetry Cs), there is another change in the spectrum - the removal of doubly degenerated vibration v2 [9,10]. Thus, widening the bands that correspond to vibrations of the PO4 groups indicates a decreasing of surrounding symmetry of the (РО4)3− anion in apatite sublattice.
Study on IR spectra of samples (Table 1) revealed a lower bandwidth, characterizing PO43− tetrahedra for nanodispersed samples one to three, indicating a higher local symmetry of the PO43− tetrahedra in nanodispersed apatite in comparison with macrosamples four to five.
Table 1.
Half width of the absorption band ν 3 of studied compounds
| Temperature of the HAP samples annealing, T ,°C | Half width of the absorption band at 1,040 cm −1 , characterizing PO 4 3− tetrahedra |
|---|---|
| 200 | 130 |
| 500 | 116 |
| 700 | 120 |
| 900 | 193 |
| 1,000 | >272 |
It should be noted that the presence of impurity atoms and other defects significantly affects vibrational infrared spectra. In the investigated samples are the presence of CO2 from the atmosphere and a small amount of residual organic reaction (Figure 4). Thermal annealing of HAP leads to changes in the hydroxyl sublattice: the number of OH− ion decreases with increasing annealing temperature, as evidenced by the decrease in the intensity of the bands at approximately 3,400 and 2,900 cm−1, which correspond to vibration stretching of O-H and C-H bonds (Figure 4).
Figure 4.
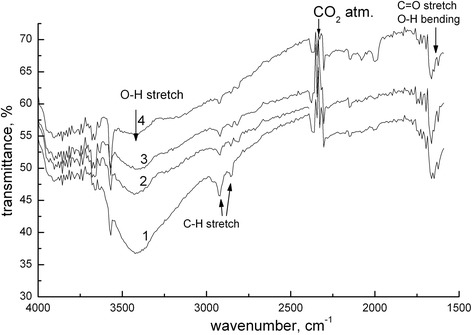
The presence of organic impurities in the samples according to IR spectroscopy. Annealing temperature samples: 1 - 200°C; 2 - 500°C; 3 - 900°C; 4 - 1,000°C. Transmission spectra were recorded in the absorption range in the 4,000 to 400 cm−1.
To determine the effects of annealing modes on HAP samples has been used in NMR method (Figure 5). It is known that 31P-NMR line quite narrows for the reference compound (phosphoric acid), whereas in the HAP, its intensity decreases, increases its half width, and maximum shifted to higher frequencies (approximately 850 Hz).
Figure 5.
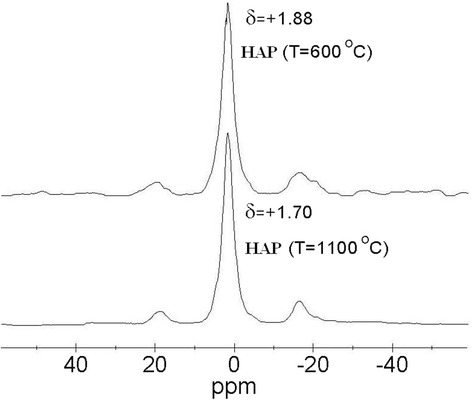
31 P-NMR spectra of compounds.
31Р-NMR spectra at annealing temperature increase are offset of the dominant signal in the direction of a strong field of δ = 1.88 to δ = 1.70 ppm (Figure 5). A significant offset of the 31Р spectrum to lower frequency range (Figure 5) with annealing temperature increases points to an increase in magnetic shielding of the phosphorus nuclei, which in this case means an increase in electron density on the phosphorus atoms.
Conclusions
This is a study of crystal growth mechanism of calcium hydroxyapatite in the synthesis of sol-gel method with variation of temperature parameters of synthesis within 200°C to 1,100°C. The origin of crystal phase occurs in the amorphous matrix by clustering, growth, and subsequent coagulation grains with the formation of crystals in a wide range of dispersion.
Temperature determines the kinetics of structural transformation of amorphous phase in the crystal phase, which in turn affects the characteristics of the particle substructure. It is shown that the particle size of the apatite obtained that annealed 700°C is 40 nm corresponding to the particle size of apatite in native bone.
The effect of dimension factor on structural parameters of calcium hydroxyapatite is manifested in a more local symmetry of the PO43− tetrahedra at nanosized calcium hydroxyapatite. Thermal annealing of HAP leads to changes in the hydroxyl sublattice: of number OH− ions decreases with increasing annealing temperature. A significant offset of the 31P spectrum to a low frequency range with increasing annealing temperature indicates an increase of electron density on the phosphorus atoms.
Acknowledgements
The authors gratefully acknowledge the financial support from the National Academy of Sciences of Ukraine, Ukraine (contract no. 5.16.1.25).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KN and KasV conceived and carried out the experiments, analyzed the data, and wrote the paper. KV designed the study, analyzed the data, and wrote the paper. All authors read and approved the final manuscript.
Contributor Information
Nataly Kurgan, Email: nataly_kurgan@mail.ru.
Volodymyr Karbivskyy, Email: karb@imp.kiev.ua.
Vasyl Kasyanenko, Email: kasiyanenko59@mail.ru.
References
- 1.Karbivskyy VL, Shpak AP. Apatite and apatite compounds. Electronic structure and properties. Kyiv: Naukova Dumka; 2010. [Google Scholar]
- 2.Dorozhkin SV. Nanodimensional and nanocrystalline apatites and other calcium orthophosphates in biomedical engineering, biology and medicine. Materials. 2009;4:1975–2045. doi: 10.3390/ma2041975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorozhkin SV. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomaterialia. 2010;3:715–49. doi: 10.1016/j.actbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Olszta MJ. Bone structure and formation: a new perspective. Mater Sci Eng. 2007;58:77–116. doi: 10.1016/j.mser.2007.05.001. [DOI] [Google Scholar]
- 5.Abidi SSA, Murtaza Q. Synthesis and characterization of nanohydroxyapatite powder using wet chemical precipitation reaction. UPB Sci Bull B. 2013;75:3–12. [Google Scholar]
- 6.Monmaturapoj N. Nano-size hydroxyapatite powders preparation by wet-chemical precipitation route. J Met Mater Miner. 2008;18:15–20. [Google Scholar]
- 7.Jothinathan E, Vanmeensel K, Vleugels J. Synthesis of nano-crystalline apatite type electrolyte powders for solid oxide fuel cells. J Eur Ceram Soc. 2010;30:1699–706. doi: 10.1016/j.jeurceramsoc.2010.01.003. [DOI] [Google Scholar]
- 8.Cunniffe GM, O'Brien FJ, Partap S, Levingstone TJ, Stanton KT, Dickson GR. The synthesis and characterization of nanophase hydroxyapatite using a novel dispersant-aided precipitation method. J Biomed Mater Res A. 2010;95:1142–9. doi: 10.1002/jbm.a.32931. [DOI] [PubMed] [Google Scholar]
- 9.Nakamoto K. Infrared spectra of inorganic and coordination compounds. New Jersey: John Wiley & Sons; 1965. [Google Scholar]
- 10.Cheng ZH, Yasukawa A, Kandori K, Ishikawa T. FTIR study of adsorption of CO2 on nonstoichiometric calcium hydroxyapatite. Langmuir. 1998;14:6681–6. doi: 10.1021/la980339n. [DOI] [Google Scholar]


