Introduction
The establishment of systems to ensure a safe and sufficient supply of blood and blood products for all patients requiring transfusion is a core issue of every blood programme.
A spectrum of blood infectious agents is transmitted through transfusion of infected blood donated by apparently healthy and asymptomatic blood donors. Recent emerging-infectious-disease threats include West Nile virus1,2, chikungunya3, babesia4, dengue5, hepatitis E virus6, and variant of Creutzfeldt-Jakob disease7.
Parvovirus B19 (B19V), long known to be the causative agent of erythema infectiosum (fifth disease), is not a newly emerging agent. However, it deserves discussion because it may be present in blood and in plasma products, can circulate at extraordinarily high titres, can infect recipients, and, in some cases, can cause severe disease8. Its potentially severe pathological effects have become more apparent in the past decade with the widespread use of (pooled) plasma-derived medicinal products and are the main reason for the uneasy relationship between transfusion medicine specialists and B19V9.
The aim of this review is to analyse the role played by this virus in compromising safety in transfusion medicine and the progressive measures to reduce the risks associated with the virus.
The virus
B19V, a member of the Parvoviridae family, Parvovirinae subfamily, Erythroparvovirus genus, and Primate erythroparvovirus 1 species10, is a small non-enveloped DNA virus, discovered in 1975 by Yvonne Cossart in the blood of a healthy blood donor11. The name parvovirus originates from the Latin word parvum, which means small. In fact, parvoviruses are among the smallest known viruses with a virion diameter of 18–26 nm12. Their structure is simple: the icosahedral virion consists only of proteins and linear single-stranded-DNA genome (length 5–6 kb) with hairpin structures at both ends. The hairpins are palindromic and the 3′-end can fold and function as a primer during viral replication12 (Figure 1). Three B19V genotypes have been identified based on isolates having nucleotide divergence greater than 10%. Genotype 1 is the most prevalent type currently circulating worldwide and is the B19V prototype13. It largely replaced genotype 2 viruses, which were common in Europe half a century ago14 and, therefore, has been sporadically found in plasma donations from Europe and North America15. Genotype 3 has been found predominantly in West Africa (Ghana) thus implying a different and longer evolutionary history, probably rooted in Africa16. Interestingly, recent studies found B19V genotype 3 in samples from Europe, Asia, and Brazil thus raising the question that this genotype may be more widely distributed outside Africa than previously believed17.
Figure 1.

Schematic representation of Parvovirus B19 icosahedral virion and genome structure.
ORF: open reading Frame; kDa: kiloDaltons; X: Region X.
In its infection cycle, the virus links to a specific receptor on the surface of host cells18 (i.e. P blood group antigen globoside-4 [Gb4] in the case of human B19V) and is transported into the cell by endocytosis. Inside the host cell, the virion is transported to the nucleus where its replication takes place. Parvoviruses do not encode their own DNA-polymerase and are, therefore, all dependent on host cell polymerase and the S-phase of dividing cells12. Gb4 is also responsible for the phenomenon of B19V haemagglutination19. Individuals who lack the blood group P antigen on their erythroid cells are not, therefore, susceptible to B19V infection20. The B19V receptor is also present on platelets, cardiac, synovial, renal and thyroid cells as well as on hepatic progenitors8. Interestingly, between 1989 and 1992, B19V replication was shown in a primary culture of erythroid cells derived from foetal liver21, in haematopoietic progenitor cells from a blood donor22, and in human umbilical cord blood cells23.
The bone marrow cell tropism of B19V is now well recognised as an erythroid progenitor cell tropism24. In fact, erythroid progenitor cells are susceptible to infection and this susceptibility increases with differentiation. Therefore, the destruction of the source of mature red blood cells will result in dramatically low haematocrit levels and a temporary state of anaemia. Naturally, in patients with disorders that shorten red blood cell half-life the clinical picture can be more severe and there may also be a transient aplastic crisis20. On the other hand, the concentration of Gb4 on non-haematopoietic tissues does not appear sufficient for active infection, and other factors may be required for virus internalisation and replication19.
Markers of Parvovirus B19 infection
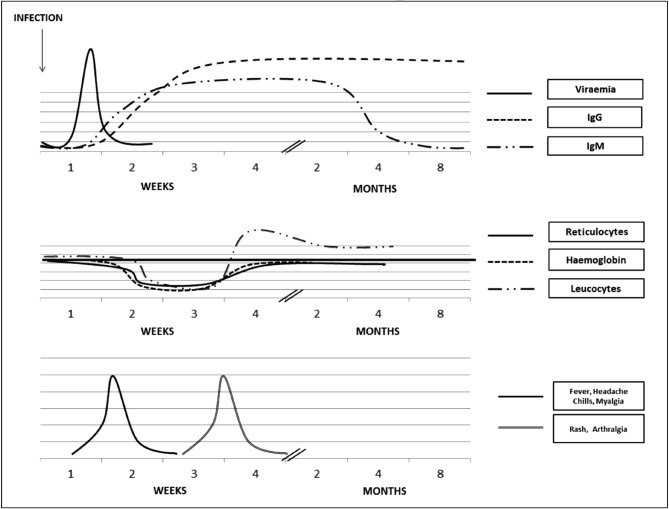
B19V viraemia occurs 1 week after exposure and usually lasts about 5 days, with virus titres peaking in the first 2 days (Figure 2). IgM antibodies against B19V are detected late in the viraemic stage. They appear 10 to 14 days after the infection and can persist for up to 5 months but, in some patients, they can last even longer (Figure 2)25. Specific IgG antibodies are detectable about 15 days after infection, remain high for several months and persist long-term25.
Figure 2.
Clinical, haematological and viral course after B19V infection in immunocompetent subjects.
B19V: Parvovirus B19; IgG: immunoglobulin G; IgM: immunoglobulin M.
Parvovirus serology (anti-B19V immunoglobulin [Ig] M and IgG antibodies) can be determined using enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, chemiluminescence or immunofluorescence25.
IgA antibodies are also detectable for a short period and may play a protective role in the respiratory tract26. In addition, long-term B19V-specific IgE antibodies have been found, but their biological functions are unclear27.
Given that there are different epidemiological patterns of infection, the seroprevalence of B19V-IgG varies widely from approximately 2% to 21% in children from 1 to 5 years of age, from 30% to 40% in adolescents, from 40% to 60% in adults, and more than 85% in elderly populations28,29. Neutralising IgG antibodies generally appear about 2 weeks after infection and persist lifelong (Figure 2). The development of the antibody response corresponds to virus clearance and also, in the vast majority of immunocompetent individuals, protection from disease25. The protective role of antibodies against B19V is demonstrated by the efficacy of commercial immunoglobulins used to treat B19V infection in immunocompromised subjects, in whom, due to the absence of antibodies, viral levels can be even higher than 1012 copies/mL30.
Results of IgM testing are particularly difficult to interpret31. Standardisation among laboratories is lacking. Even in a single laboratory, sensitivity and specificity partly depend on the operator’s skills. High-level viraemia in acutely infected subjects may cause virus-antibody complexes, which will result in a false-negative IgM test result31.
In this setting, polymerase chain reaction (PCR) analysis or alternative nucleic acid amplification technology (NAT) assay (e.g., transcription-mediated amplification) may be a better diagnostic tool as viral titres can reach more than 1012 genome equivalents (geq) per mL during acute B19V infection. On the other hand, in chronic B19V infection, viral DNA can persist in the host without the presence of B19V IgM or IgG. In the immunocompetent host, viral DNA is detectable for at least 1 month after infection30, but can persist even longer at low levels32. Therefore, B19V DNA, detected by qualitative PCR analysis, is not always indicative of recent infection25. This assay has been set up to include an internal control to eliminate false negative results caused by the presence of inhibitors in plasma. It is very useful for plasma pools for fractionation and could also be exploited for clinical diagnosis in primary red cell aplasia, in which the antibody response is depressed, or for the detection of recently discovered variant erythroviruses in human disease33.
Finally, as far as the identification of B19V genotypes 2 and 3 is concerned, many commercial and in-house PCR methods have shown lower sensitivity or failure to detect either or both of these genotypes12,15.
Clinical features and therapy
Many individuals with B19V infection are asymptomatic (about 25% of adults and 50% of children during an outbreak), or manifest mild, non-specific, cold-like symptoms that are never linked to the virus34,35 (Figure 2). Most patients with B19V infection do not require laboratory tests or therapy because symptoms are mild and the illness resolves over 5–7 days.
The pattern of clinical disease is strongly influenced by the haematological and immunological status of the host36. B19V infection may have a serious clinical outcome in three categories of susceptible recipients: (i) patients with shortened red cell survival (e.g. those with thalassaemia major, sickle cell disease, or other haemolytic diseases); (ii) immunocompromised patients (whether previously exposed to B19V or not); and (iii) pregnant women.
The most common clinical manifestation in children, especially those aged 4–11 years, is erythema infectiosum causing a “slapped-cheek” facial rash, extending later to the trunk and limbs (“gloves and socks” syndrome) and accompanied, in a few cases, by fever, headache, coryza, nausea, and diarrhoea. Joint involvement, transient haemolytic anaemia, and encephalitis may also occur.
In adults, polyarthropathy characterised by sudden symmetrical arthralgia is more common than rash. Some patients may present acute anaemia but those with underlying haemolytic disorders may develop a transient aplastic crisis37. Persistent B19V infection can also cause severe anaemia. The first patient recognised to have this condition had an underlying combined B- and T-cell immunodeficiency with low immunoglobulin levels (Nezelof syndrome)38. Patients with human immunodeficiency virus (HIV) infection can also develop pure red blood cell aplasia due to B19V infection; in these patients, B19V titres can be as high as 1012 copies/mL.
During pregnancy (weeks 9 to 20) B19V infection is associated with an approximately 10% incidence of foetal loss, which is rare after the 20th week. B19V infection of foetal cardiac tissue may lead to myocarditis and heart failure, which can result in a hydrops foetalis, neurological foetal abnormalities, or congenital anaemia34.
Other complications associated with B19V infection include encephalopathy, epilepsy, meningitis, myocarditis, dilated cardiomyopathy, and autoimmune hepatitis34.
There are no specific antiviral drugs or vaccines against human parvovirus infection. The (aplastic) anaemia can be treated through intravenous immunoglobulin therapy, possibly repeated in chronic and persistent infections25, or red blood cell transfusion, when necessary39. On the other hand, in immunocompetent patients, treatment is unnecessary and infections are self-clearing.
Virus transmission and blood product safety
Human B19V circulates worldwide, is mildly contagious, and may occur sporadically or in epidemics. In temperate climates, epidemic manifestations occur more commonly in late winter, spring or early summer36. B19V is mainly transmitted through the respiratory route and its DNA is contained in respiratory secretions at the time of viraemia, when infectivity is highest40. Transmission in the household, at day care, and in school is common, although the virus can also be transmitted vertically41 and via blood transfusion and organ/bone marrow transplantation14.
The risk of vertical transmission varies between 33% and 51% following acute infection of pregnant women with reported adverse foetal outcomes in 3% to 12% of cases41. There is evidence that transmission of severe B19V infection may occur at the time of solid organ or haematopoietic transplantation and cause serious complications such as aplastic crises, pneumonia, and multiorgan failure42–45. Iatrogenic transmission of B19V through blood products is possible because high-level viraemia regularly occurs during primary infection and more than 1012 geq/mL are often found in the blood of asymptomatic individuals during the early phase of acute infection46.
Plasma-derived medicinal products
Although there have been very few reports of clinically relevant B19V infection resulting from the transfusion of a blood component containing the virus47, B19V is a frequent contaminant of blood and plasma donations and has been transmitted by plasma-derived medicinal products13,48,49. Interestingly, both a temporal correlation between infusion of plasma-derived medicinal products and viraemia, seroconversion and/or non-clinically significant infections and blood product viral contamination have been documented. Other studies showed that the prevalence of B19V-specific antibodies was much higher in groups receiving clotting factors than in control groups50.
Transmission via plasma-derived medicinal products can occur because of incomplete physiological clearance of virus, high-level viraemia in acutely infected individuals14, and the resistance of B19V to most viral inactivation procedures used in the manufacturing of blood-derived products51–55.
B19V is a good model for new emergent viruses capable of infecting blood products because of their properties of physical resistance. In fact, B19V DNA is detectable in 50% to 80% of non-virally inactivated factor VIII concentrates and in 30% to 50% of solvent/detergent-inactivated factor IX concentrates, respectively12,56. B19V was also detected in two of three unheated batches of clotting factor preparations and in 20% to 25% of solvent/detergent-treated batches, while the fractionation process used to obtain albumin preparations is apparently more efficient at eliminating the virus57–62. B19V DNA sequences were also detected in 16%63 to 28%64 of factor VIII concentrates. A more recent German study detected B19V DNA in 26% of different coagulation factor concentrates produced between 2007 and 200865. Soucie and colleagues found that compared to populations not exposed to blood or blood products, the studied population receiving plasma-derived medicinal products was 1.7 times more likely to have IgG antibodies to B19V66. Another study demonstrated that, overall, 25% of albumin samples, 100% of factor VIII concentrates, and 20% of intravenous and 75% of intramuscular immunoglobulin preparations contained B19V DNA67. The viral load in those samples ranged from 1×102 to 1×106 geq/mL. Alter et al. reported a high prevalence (over 60%) of B19V DNA in factor IX, factor VIII, and prothrombin complex concentrates, as well as plasma pools with viral loads of 1×102 to 1×108 geq/mL9.
In conclusion, the multiple reports of B19V transmission by pooled-plasma products were almost always documented by recipient seroconversion in asymptomatic cases and less frequently by clinical diagnosis of B19V-related disease associated with positive B19V test results68. It is also worth noting that according to a recent interim report from a prospective clinical study on the incidence of factor VIII inhibitors in previously untreated patients during prophylaxis, on-demand treatment, and surgical procedures with a plasma-derived, human, von Willebrand factor-stabilised FVIII product, the 57 adverse events rated “serious” probably or possibly related to factor VIII treatment reported in 24 subjects also included 14 cases of asymptomatic parvovirus B19 seroconversion69.
Inactivation/removal steps and B19V
In order to obtain safe plasma-derived medicinal products, the processes to produce these products include steps to inactivate or remove viruses. B19V has been shown to be susceptible to chromatography, pasteurisation (10 hours at 60 °C), steam (vapour heat), exposure to pH 4 (occasionally used during the manufacture of immunoglobulins), and (only partially)55 to small-pore-size nanofiltration70. It was shown that porcine parvovirus (a model for human parvovirus B19) could be effectively inactivated with riboflavin/UV light suggesting that B19V could also be inactivated. On the other hand, amotosalen/UVA light is not effective on porcine parvovirus; its limited effectiveness against certain of these viruses was demonstrated when transmission of hepatitis E virus occurred via transfusion of a plasma product. Levels of viral load of non-lipid-enveloped viruses, such as B19V, could be reduced by 4 log or more through methylene blue treatment, while other viruses, such as hepatitis A virus, are not affected70. Transfusion transmission of B19V was also reported with solvent/detergent-treated plasma54.
Relationship between viral load in plasma and probability of transmission by blood products
To date, no B19V transmission from pooled-plasma products has been documented when less than 103 to 104 IU/mL B19V DNA are present in an infused product13. There is still some doubt as to the reason for this lack of infectivity. It may be due to an inadequate amount of infused infectious virions, a neutralising effect from B19V antibodies present in other plasma units contained in the plasma pool, or a combination of these factors. Recipient factors may also play a role because it has been reported that B19V antibodies protect against B19V re-infection, and most of the adult population is B19V-seropositive as a result of previous infection71.
To reduce the potential risk of transmission, the US Food and Drug Administration proposed a limit of 104 geq/mL for the production of plasma pools destined for all plasma derivatives72. Similarly, the European Pharmacopoeia has imposed a limit of 104 UI/mL of B19V in anti-D immunoglobulins and pooled virus-inactivated plasma73.
Labile blood products
As far as cellular blood products are concerned, B19V DNA levels lower than 104 IU/mL might not be clinically significant while the transfusion of labile blood products with B19V titres greater than 107 IU/mL has been associated with transmission of B19V infection13,25. However, susceptibility to infection could be highly dependent on the presence or absence of neutralising antibodies in the recipient74. Symptomatic infections have been reported in a few case series and linked donor-recipient studies have confirmed that in most cases B19V transfusion-transmitted infections are clinically irrelevant68 while vulnerability to serious B19V-related haematological disorders is dependent on the patients’ underlying diseases50.
Studies in different countries found B19V DNA in 1% of all blood cell preparations and blood products transfused to patients in a haematology ward49, in 0.9% of standard blood components49 (in 2% of pooled-plasma products and in 0.7% of single-donor products)49, in 0.006% of blood donations75, in 0.14% of single-donor blood products76, and in 0.16% of plasma samples77. The prevalence of B19V DNA in plasma pools ranged from 0.024%78 to 97%79. Interestingly, another study demonstrated that, overall, 85% (60% to 100% depending on the manufacturer) of plasma pools contained B19V DNA67. The percentage of the plasma pools positive for B19V DNA that were also positive for IgG was 100%79,80, while the percentage of the same plasma pools also positive for IgM ranged from 23%80 to 65%79.
There are several reasons for the very different figures in diverse studies: first, the epidemiological settings are different; second, the rates of detectable viral DNA are related to the sensitivity of the methods used; and third, there are seasonal variations in transmission and, therefore, in viraemia49.
Strategies for screening donors or plasma pools
Since April 2000, all blood donations started to be screened by a B19V minipool real-time NAT in German Red Cross Centres and four areas in Austria51. Since 2004, Polish blood donors have also been tested for B19V DNA. Screening has been performed in donors of plasma for fractionation for anti-D and anti-HBs production, and donors of erythrocytes used for immunisation81. In 2008, to reduce the risk of B19V transmission through contaminated blood for transfusion and plasma-derived medicinal products, Japanese Red Cross Blood Centres introduced B19V antigen screening by chemiluminescent enzyme immunoassay for all donated blood. This test has a sensitivity of approximately 107 IU/mL. Positive samples are then excluded from the 20-pool-screening triple NAT to reduce the risk of cross-contamination during NAT82. There has been a subsequent expansion of B19V DNA screening of pools of plasma used to manufacture plasma derivatives in many countries83. The combined strategy of high-titre-B19V PCR screening and viral decontamination during the plasma manufacturing process has significantly increased the margin of B19V safety of plasma-derived medicinal products84.
Prevalence of B19V in blood donors and patients
Blood donor screening for B19V is feasible using B19V antigen assays or NAT. Many commercial or in-house real-time NAT systems are available83. The risk of exposure to a high-load B19V viraemia during a window period is relatively small, but during epidemic periods, the incidence of parvovirus in the blood can be as high as one in 260 donors85.
Several studies published between 1995 and 201416,51,68,78–82,84,86–117 show that the prevalence of B19V in blood donor populations ranges from 6%92 to 79.1%112 for IgG (92% in donors older than 61 years101), from 0.72%98 to 7.53%96 for IgM, from 0.01%82 to 15.3%87 for IgM+IgG, and from 0%89 to 1.3%16,89,102 for B19V DNA (see Table Ia and Table Ib).
Table Ia.
Prevalence of parvovirus B19 reported in blood donors, blood donations and plasma pools.
| Continent | Country | Prevalence | Subject of study | Study author and year of publication | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| IgG (%) | IgM (%) | IgM+IgG (%) | B19V DNA (%) | ||||
| AFRICA | Ghana | - | - | - | 0.8 | Blood donors | Compston LI, 200886 |
|
| |||||||
| AMERICA | Canada | 24.3 | 4.7 | 15.3 | - | Blood donors | Wasfi S, 199687 |
| Brazil | 60 | - | - | 1 | Blood donors | Slavov SN, 201288 | |
| Brazil | 57.4 | - | - | 0 | Blood donors | Slavov SN, 201289 | |
| Chile | - | - | - | 0.84 | Blood donors | Lévican J, 201190 | |
| Chile | 54.8 | - | - | - | Blood donors | Gaggero A, 200791 | |
| USA | 6 | - | - | - | Blood donors | O’Bryan TA, 201092 | |
| - | - | - | 0.84 | Blood donors | Kleinman SH, 200968 | ||
| - | 1 | - | - | Blood donors | Doyle S, 200093 | ||
| - | - | - | 0.88 (100: IgG+ 23: IgM+) | Blood donations | Kleinman SH, 200780 | ||
|
| |||||||
| ASIA | China | - | - |
|
|
Zhang W, 201279 | |
| 24.6 | 6.9 | 2.5 | 0.58 | Blood donors | Ke L, 201194 | ||
| 55.43 | - | - | - | Blood donors | Wei Q, 200695 | ||
| India | 27.96 | 7.53 | 2.40 | - | Blood donors | Kumar S, 201396 | |
| 39.9 | - | - | - | Blood donors | Kishore J, 201097 | ||
| Japan | - | - | 0.01 | 0.04 | Blood donors | Sakata H, 201382 | |
| 67.9 | 0.72 | - | 0.2 | Blood donors | Ihara T, 201398 | ||
| - | - | - | 0.01 | Blood donors | Matsukura H, 200899 | ||
| - | - | 0.11 | - | Blood donors | Wakamatsu C, 1999100 | ||
| 40 (donors 16–30 years old) 92 (donors >61 years old) |
- | - | 0.250 | Blood donors | Tsujimura M, 1995101 | ||
| Saudi Arabia | 39.3 | 0 | 44.6 | 0 | Blood donors | Obeid OE, 2011102 | |
| South Korea | - | - | 60.1 | 0.1 | Blood donors | Oh DJ, 2010103 | |
| Thailand | 20.16 | - | - | - | Blood donors, children | Poovorawan Y, 2000104 | |
B19V: parvovirus B19; IgG+: immunoglobin G positivity; IgM+: immunoglobin M positivity; IgG&IgM+: immunoglobin G and immunoglobin M positivity; geq/mL: genome equivalents/mL.
Table Ib.
Prevalence of parvovirus B19 reported in blood donors, blood donations and plasma pools.
| Continent | Country | Prevalence | Subject of study | Study author and year of publication | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| IgG (%) | IgM (%) | IgM+IgG (%) | B19V DNA (%) | ||||
| EUROPE | Belgium | - | - | - | 0.16 | Blood donors | Thomas I, 2003105 |
| France | - | - | - | 0.024 | Plasma pools | Petermann R, 201078 | |
| Germany | 0.88 | - | - | 0.014 | Blood donations | Hitzler WE, 2002106 | |
| - | - | - | 0.125 | Blood donations | Weimer T, 200184 | ||
| - | - | - | 0.66 | Blood donors | Juhl D, 2014107 | ||
| The Netherlands | - | - | - | 0.006 (0: IgG+ 24: IgM+ 6: IgG&IgM+) | Blood donors, plasma pools | Kooistra K, 2010108 | |
| - | - | - | 0.005 (70: IgG&IgM+) | Blood donors | Corcoran A, 2007109 | ||
| - | - | - | 47 | Plasma pools | Zaaijer HL, 2004110 | ||
| Italy | - | - | - | 0.89 | Mini-pools | Gessoni G, 2007111 | |
| 79.1 | - | - | - | Blood donors | Manaresi E, 2004112 | ||
| Poland | 0.10 | Blood donors | Grabarczyck P, 201281 | ||||
| Portugal | - | - | - | 0.12 | Blood donors | Henriques I, 2005113 | |
| Russia | - | - | - | 1 (100: IgG+ 1 case: IgM+) | Blood donors | Filatova EV, 2010114 | |
| Spain | 64.7 | 0 | - | - | Blood donors | Munoz S, 1998115 | |
| 9.78 | - | - | - | Blood donors | Mata Rebon M, 1998116 | ||
|
| |||||||
| OTHER COUNTRIES | UK, Ghana, South Africa, and Malawi | - | - | - | 0.9 UK 1.3 Ghana 0.55 S. Africa 1.25 Malawi |
Blood donors | Candotti D, 200416 |
| Germany and Austria | - | - | - | 0.0127 >105 geq/mL 0.26<105 geq/mL |
Blood donors | Schmidt M, 200751 | |
| Belgium and Tunisia | Belgium: 74 Tunisia: 65 |
- | - | - | Blood donors | Letaïef M, 1997117 | |
B19V: parvovirus B19; IgG+: immunoglobin G positivity; IgM+: immunoglobin M positivity; IgG&IgM+: immunoglobin G and immunoglobin M positivity; geq/mL: genome equivalents/mL.
Table II lists several studies, published between 1988 and 2013, on the prevalence of B19V in patients with congenital bleeding disorders66,118–127. The prevalence of B19V in these patients ranged from 31%126 to 97%123. Interestingly, the range for each specific disorder was from 21%120 to 93%123 for haemophilia A, from 35.5%66 to 97%123 for haemophilia B, and from 37.9%66 to 80%123 for von Willebrand disease.
Table II.
Prevalence of parvovirus B19 reported in blood product recipients with bleeding disorders.
| Prevalence of B19V antibody (%) | Subjects of study | Study author and year of publication |
|---|---|---|
|
Patients with bleeding disorders | Soucie JM, 201366 |
| Haemophilia: 64.2 | Patients with haemophilia, malignant disease, immunodeficiency diseases, common gynaecological ailments, pregnant women and children with malignant diseases. | Reinheimer C, 2010118 |
| 91.8 | Patients with haemophilia | Langara H, 2005119 |
| 21 to 48.5 | Patients with haemophilia A | Soucie JM, 2004120 |
No B19V DNA was found. |
Patients with haemophilia | Brojer E, 1999121 |
| 76.7 | Patients with bleeding disorders | Canales MA, 1998122 |
|
Patients with bleeding disorders | Mauser-Bunschoten EP, 1998123 |
| IgG: 84 IgM: 0 |
Patients with bleeding disorders | Aguilar Franco C 1997124 |
| 81.6 | Patients with haemophilia | Ragni MV, 1996125 |
|
Patients with haemophilic arthritis | Zakrzewska K, 2001126 |
|
Patients with haemophilia and healthy donors | Bartolomei Corsi O, 1988127 |
B19V: parvovirus B19; vWD: von Willebrand disease; IgG: immunoglobin G; IgM: immunoglobin M.
Other parvoviruses, blood donors, and blood products
B19V was considered to be the only human pathogenic parvovirus until the recent discovery of Bocaparvoviruses and Parvovirus 4 (PARV4), whose epidemiology and disease association are still poorly understood128.
PARV4 is a member of the Primate Tetraparvovirus 1 species10, Parvoviridae family, discovered in 2005 in plasma from an intravenous drug user, with symptoms consistent with acute HIV infection but who was confirmed to be HIV-RNA negative129. A related virus variant Parvovirus 5 (PARV5) was identified in plasma pools used in the manufacturing process of plasma-derived medicinal products130. Later, the name PARV5 was changed to PARV4 genotype 2. In 2008, a third genotype of PARV4 was found in two patients in sub-Saharan Africa with acquired immune deficiency syndrome131; non-parenteral transmission might contribute to its transmission in this area132. The virus was detected in plasma pools used in the manufacturing process of plasma-derived medicinal products, particularly those from the United States129,133 and Asia134, and also in clotting factor concentrates, namely preparations of factor VIII and IX134,135. In contrast, three studies carried out in France and Germany did not detect any PARV4 DNA positive samples in a large number of plasma donations, minipools or coagulation factor concentrates65,136; it is still unclear whether these negative results have seasonal or geographical explanations12.
Interestingly, products manufactured in the early 1970s were found to be positive for PARV4, and in general, older concentrates were found to be more frequently contaminated with PARV4137. This could be due either to differences in population-based hazard of infection over time or to improvements in manufacturing processes12.
Several studies have subsequently found PARV4 in intravenous drug users in Europe, Asia, and the United States as well as in men who have sex with men and in febrile patients138–141. Such cases are indicative of blood-borne transmission of PARV4. The virus has also been detected in blood donors in the United States, South East Asia, and Europe65,130,133–135,138,141–150 (Table III). The prevalence of PARV4 in blood donor populations is not clear; studies carried out in France found that the prevalence of PARV4-DNA ranged from 0%145 to 24%148 in donors and from 4%134 to 26.15%133 in plasma-derived medicinal products. The high frequency of detection of PARV4 DNA reported in some articles65,133,148,149 may be the result of seasonal and/or geographical epidemiological variation or lack of standardisation of detection methods.
Table III.
PARV4 DNA findings by PCR in blood donors or plasma-derived medicinal products.
| PARV4 DNA (%) | Subjects of study | Study author and year of publication |
|---|---|---|
| 5.1 | Plasma donations | Fryer JF, 2006142 |
|
|
Fryer JF, 2007130 |
| 16 pre-1990 23% 1990–2005 2% |
Factor VIII concentrates collected from 1974 to 2005 | Fryer JF, 2007134 |
| 11.5 | HCV positive subjects and/or IDU | Fryer JF, 2007138 |
| 5.3 | IDU, blood donors negative for HIV, HCV and HBV | Lurcharchaiwong W, 2008141 |
| 12.4 | Coagulation factor concentrates | Schneider B, 2008135 |
|
|
Vallerini D, 2008143 |
| 3.8 | Healthy blood and skin donors, skin donors with dermatological disease | Botto S, 2009144 |
| 0 | Transfused immunocompetent patients, blood donors | Servant-Delmas A, 2009145 |
| 1.7 | HCV, HIV, and HBV positive subjects | Tuke PW, 2010146 |
| 8.6 | Infants (Ghana) | Panning M, 2010147 |
| 24 | Blood donors | Touinssi M, 2010148 |
| 24.5 | Haemodialysis patients positive or not for HBV markers, lung transplant recipients | Touinssi M, 2011149 |
| 26 | Plasma donations | Modrow S, 201165 |
| 26.15 | Blood donors | Ma YY, 2012133 |
| 0 (4.76 IgG) | Blood donors | Maple PA, 2013150 |
PCR: polymerase chain reaction; IDU: intravenous drug users; HIV: human immunodeficiency virus; HCV: hepatitis C virus; HBV: hepatitis B virus; BM: bone marrow; PBSC: peripheral blood stem cell; IgG: immunoglobin G.
Very little information is available regarding the clinical significance of infection with PARV4; so far, in a study of acutely infected persons with haemophilia the only repeatedly observed clinical presentation was a rash in three subjects and unexplained hepatitis in two patients151.
Among Bocaparvoviruses, the Primate Bocaparvovirus 1 species includes human bocavirus 1 (HBoV1)10, which was identified in 2005 in nasopharyngeal aspirates of children with respiratory tract infections152. Although the routes of transmission of Primate Bocaparvoviruses are unknown, many parvoviruses are transmitted by inhalation or contact with infectious sputum, faeces, or urine. HBoV1 is predominantly a respiratory pathogen, whereas three additional species (HBoV2, HBoV3, and HBoV4) have been found mainly in stool. A variety of signs and symptoms have been described in patients with HBoV infection, including rhinitis, pharyngitis, cough, dyspnoea, wheezing, pneumonia, acute otitis media, fever, nausea, vomiting, and diarrhoea153. The rate of nosocomial respiratory acquisition may be as high as 18% in hospitalised HBoV1 cases, and up to 19% of nosocomial acute respiratory tract infections are HBoV1 positive. Intrauterine infection is unlikely because of the high degree of immunity in pregnant women153. The seroprevalence of HBoV1 has been reported to be more than 90% in adults. However, the HBoV1-4 viral-like particles used in the ELISA have shown cross-reactivity, which might affect serological assays. Norja et al. found that the seroprevalence of HBoV1 was 94.9% but after removing cross-reacting antibodies the rate dropped to 68.4%154. Similar results were obtained by Kantola et al.155, who observed that the seroprevalence of HBoV1 in adults decreased, from 96% to 59%, after removing the cross-reacting antibodies. The Kantola study found that the seroprelavence of HBoV in adults was 34% for HBoV2, 15% for HBoV3, and 2% for HBoV4155.
Interestingly, three studies in blood donors and plasma-derived medicinal products failed to detect HBoV1 DNA65,133,136. This may be due to the higher frequency of HBoV1 infections among young children than in blood donors or to low-level viraemia undetectable in large plasma pools12. As seroprevalence studies on blood donors and blood products are limited, this issue could be an interesting and useful subject of investigation for the near future.
Conclusion
Transfusion-transmitted human B19V is a classic example of an unresolved issue for the transfusion medicine community. The strategies used by plasma fractionators and competent authorities to ensure the safety of plasma-derived medicinal products include NAT screening of single donations and mini-pools and the adoption of multiple steps of viral inactivation and removal with solvent/detergent, super-heating (at 80 °C for 3 days), pasteurisation, and nano-filtration156.
The current strategy of B19V-NAT plasma mini-pool screening might not be completely effective at preventing the transmission of B19V and, more importantly, would not detect other new or emerging viruses with similar characteristics that could pose a hazard to the users of these products.
The universal screening of donated blood for B19V by NAT-based algorithms is currently carried out in Poland81, Germany (German Red Cross Centres)51, Austria51 and Japan82. The detection limit set at 105 IU/mL is undoubtedly contributing to the decrease not only of the viral load in pooled source plasma but also of the frequency of seroconversion or symptomatic infection after treatment with blood products.
The implementation of NAT screening with a much higher sensitivity for B19V is unlikely as it would result in a considerable number of components being discarded, thus jeopardising the capacity of blood systems to ensure self-sufficiency of blood and blood products.
In the near future the transmissibility of B19V by transfusion could be better clarified by taking into account not only the level of B19V in the blood product and its overall transfused dose but also the presence of anti-B19V antibodies, their potency, and titre. Donors with persistent IgG anti-B19V might be considered “B19V-safe” for single-donor blood components157. Another strategy currently recommended in the Netherlands is based on the identification of negligible B19V infectivity and the definition of selected indications for the transfusion of a “B19V-safe” blood component (i.e. a blood component donated by a donor in whom “IgG antibodies against B19V have been detected in two separate blood samples, one taken at least six months after the other”)157.
Other factors to be considered are the immune and anti-B19V status of the recipient as well as his/her B19V infection history, which can influence viral persistence. In addition, the approach to high-risk patients requires particular care.
However, as the extent of clinical disease due to transfusion transmission is unknown and reported infrequently, the benefits of (universal) B19V-blood-donor screening may be minimal and, at the moment, not justified, especially in countries with low endemicity158.
For the immediate future PARV4 is likely to remain under suspicion as a cause of different symptoms in subsets of infected individuals. Continued evaluation of the incidence of PARV4 in treated individuals and disease associations of PARV4 infections is also required to support decision making on whether costly measures such as testing and excluding PARV4-positive donations from fresh blood inventories should be implemented.
At the moment, the pathological role, clinical relevance, and epidemiology of HBoV1 remain unclear thus making any assessment of its possible role in blood-product safety speculative.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Pupella S, Pisani G, Cristiano K, et al. West Nile virus in the transfusion setting with a special focus on Italian preventive measures adopted in 2008–2012 and their impact on blood safety. Blood Transfus. 2013;11:563–74. doi: 10.2450/2013.0077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grazzini G, Liumbruno GM, Pupella S, et al. West Nile virus in Italy: a further threat to blood safety, a further challenge to the blood system. Blood Transfus. 2008;6:235–7. doi: 10.2450/2008.0065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liumbruno GM, Calteri D, Petropulacos K, et al. The chikungunya epidemic in Italy and its repercussion on the blood system. Blood Transfus. 2008;6:199–210. doi: 10.2450/2008.0016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobo CA, Cursino-Santos JR, Alhassan A, Rodrigues M. Babesia: an emerging infectious threat in transfusion. PLoS Pathog. 2013;9:e1003387. doi: 10.1371/journal.ppat.1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med. 2009;19:66–77. doi: 10.1111/j.1365-3148.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marano G, Vaglio S, Pupella S, et al. Hepatitis E: an old infection with new implications. Blood Transfus. 2014 doi: 10.2450/2014.0063-14. [DOI] [Google Scholar]
- 7.Calizzani G, Vaglio S, Vetrugno V, et al. Management of notifications of donors with Creutzfeldt-Jakob disease (post-donation information) Blood Transfus. 2014;12:22–7. doi: 10.2450/2013.0035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd RY. B19: benign or not? Transfusion. 2011;51:1878–9. doi: 10.1111/j.1537-2995.2011.03274.x. [DOI] [PubMed] [Google Scholar]
- 9.Alter HJ, Stramer SL, Dodd RY. Emerging infectious diseases that threaten the blood supply. Semin Hematol. 2007;44:32–41. doi: 10.1053/j.seminhematol.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotmore SF, Agbandje-McKenna M, Chiorini JA, et al. The family Parvoviridae. Arch Virol. 2014;159:1239–47. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1:72–3. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- 12.Norja P, Lassila R, Makris M. Parvovirus transmission by blood products - a cause for concern? B J Haematol. 2012;159:385–93. doi: 10.1111/bjh.12060. [DOI] [PubMed] [Google Scholar]
- 13.Blümel J, Burger R, Drosten C, et al. Parvovirus B19 - revised. Transfus Med Hemother. 2010;37:339–50. doi: 10.1159/000322190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppelman MH, Rood IG, Fryer JF, et al. Parvovirus B19 genotype 1 and 2 detection with real-time polymerase chain reaction assays. Vox Sang. 2007;93:208–15. doi: 10.1111/j.1423-0410.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 15.Baylis SA. Standardization of nucleic acid amplification technique (NAT)-based assays for different genotypes of parvovirus B19: a meeting summary. Vox Sang. 2008;94:74–80. doi: 10.1111/j.1423-0410.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 16.Candotti D, Etiz N, Parsyan A, Allain JP. Identification and characterization of persistent human erythrovirus infection in blood donor samples. J Virol. 2004;78:12169–78. doi: 10.1128/JVI.78.22.12169-12178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hübschen JM, Mihneva Z, Mentis AF, et al. Phylogenetic analysis of human parvovirus B19 sequences from eleven different countries con rms the predominance of genotype 1 and suggests the spread of genotype 3b. J Clin Microbiol. 2009;47:3735–8. doi: 10.1128/JCM.01201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–7. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 19.Weigel-Kelley KA, Yoder MC, Srivastava A. Recombinant human parvovirus B19 vectors: erythrocyte P antigen is necessary but not sufficient for successful transduction of human hematopoietic cells. J Virol. 2001;75:4110–6. doi: 10.1128/JVI.75.9.4110-4116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown KE, Hibbs JR, Gallinella G, et al. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen) N Engl J Med. 1994;330:1192–6. doi: 10.1056/NEJM199404283301704. [DOI] [PubMed] [Google Scholar]
- 21.Yaegashi N, Shiraishi H, Takeshita T, et al. Propagation of human parvovirus B19 in primary culture of erythroid lineage cells derived from fetal liver. J Virol. 1989;63:2422–6. doi: 10.1128/jvi.63.6.2422-2426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz TF, Serke S, Hottentrager B, et al. Replication of parvovirus B19 in hematopoietic progenitor cells generated in vitro from normal human peripheral blood. J Virol. 1992;66:1273–6. doi: 10.1128/jvi.66.2.1273-1276.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava CH, Zhou S, Munshi NC, et al. Parvovirus B19 replication in human umbilical cord blood cells. Virology. 1992;189:456–61. doi: 10.1016/0042-6822(92)90569-b. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Ozawa K, Takahashi K, et al. Susceptibility of human erythropoeitic cells to B19 parvovirus in vitro increases with differentiation. Blood. 1990;75:603–10. [PubMed] [Google Scholar]
- 25.Corcoran A, Doyle S. Advances in the biology, diagnosis and host-pathogen interactions of parvovirus B19. J Med Microbiol. 2004;53:459–75. doi: 10.1099/jmm.0.05485-0. [DOI] [PubMed] [Google Scholar]
- 26.Erdman DD, Usher MJ, Tsou C, et al. Human parvovirus B19 specific IgG, IgA, and IgM antibodies and DNA in serum specimens from persons with erythema infectiosum. J Med Virol. 1991;35:110–5. doi: 10.1002/jmv.1890350207. [DOI] [PubMed] [Google Scholar]
- 27.Bluth MH, Norowitz KB, Chice S, et al. Detection of IgE anti parvovirus B19 and increased CD23+ B cells in parvovirus B19 infection: relation to Th2 cytokines. Clin Immunol. 2003;108:152–8. doi: 10.1016/s1521-6616(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 28.Anderson LJ, Tsou C, Parker RA, et al. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:522–6. doi: 10.1128/jcm.24.4.522-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kühl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–93. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 30.Mouthon L, Guillevin L, Tellier Z. Intravenous immunoglobulins in autoimmune- or parvovirus B19-mediated pure red-cell aplasia. Autoimmun Rev. 2005;4:264–9. doi: 10.1016/j.autrev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Bredl S, Plentz A, Wenzel JJ, et al. False-negative serology in patients with acute parvovirus B19 infection. J Clin Virol. 2011;51:115–20. doi: 10.1016/j.jcv.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Cassinotti P, Siegl G. Quantitative evidence for persistence of human parvovirus B19 DNA in an immunocompetent individual. Eur J Clin Microbiol Infect Dis. 2000;19:886–7. doi: 10.1007/s100960000384. [DOI] [PubMed] [Google Scholar]
- 33.Baylis SA, Shah N, Minor PD. Evaluation of different assays for the detection of parvovirus B19 DNA in human plasma. J Virol Methods. 2004;121:7–16. doi: 10.1016/j.jviromet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373–7. [PubMed] [Google Scholar]
- 35.van Beers-Tas MH, Heidema J. Pathogenesis of parvovirus infections in children. Virol Mycol. 2013;2:1. [Google Scholar]
- 36.Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Exindari M, Chatzidimitriou D, Melidou A, et al. Epidemiological and clinical characteristics of human parvovirus B19 infections during 2006–2009 in Northern Greece. Hippokratia. 2011;15:157–60. [PMC free article] [PubMed] [Google Scholar]
- 38.Kurtzman GJ, Ozawa K, Cohen B, et al. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987;317:287–94. doi: 10.1056/NEJM198707303170506. [DOI] [PubMed] [Google Scholar]
- 39.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown KE. The expanding range of parvoviruses which infect humans. Rev Med Virol. 2010;20:231–44. doi: 10.1002/rmv.648. [DOI] [PubMed] [Google Scholar]
- 41.Puccetti C, Contoli M, Bonvicini F, et al. Parvovirus B19 in pregnancy: possible consequences of vertical transmission. Prenatal Diagnosis. 2012;32:897–902. doi: 10.1002/pd.3930. [DOI] [PubMed] [Google Scholar]
- 42.Eid AJ, Chen SF AST Infectious Diseases Community of Practice. Human parvovirus B19 in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):201–5. doi: 10.1111/ajt.12111. [DOI] [PubMed] [Google Scholar]
- 43.Heegaard ED, Laub Petersen B. Parvovirus B19 transmitted by bone marrow. Br J Haematol. 2000;111:659–61. doi: 10.1046/j.1365-2141.2000.02407.x. [DOI] [PubMed] [Google Scholar]
- 44.Carraturo A, Catalani V, Ottaviani D, et al. Parvovirus B19 infection and severe anemia in renal transplant recipients. Scientific World Journal. 2012;2012:102829. doi: 10.1100/2012/102829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahiala J, Koskenvuo M, Norja P, et al. Human parvoviruses B19, PARV4 and bocavirus in pediatric patients with allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:1308–12. doi: 10.1038/bmt.2013.63. [DOI] [PubMed] [Google Scholar]
- 46.Siegl G, Cassinotti P. Presence and significance of parvovirus B19 in blood and blood products. Biologicals. 1998;26:89–94. doi: 10.1006/biol.1998.0138. [DOI] [PubMed] [Google Scholar]
- 47.Satake M, Hoshi Y, Taira R, et al. Symptomatic parvovirus B19 infection caused by blood component transfusion. Transfusion. 2011;51:1887–95. doi: 10.1111/j.1537-2995.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 48.Eis-Hubinger AM, Sasowski U, Brackmann HH. Parvovirus B19 DNA contamination in coagulation factor VIII products. Thromb Haemost. 1999;81:476–7. [PubMed] [Google Scholar]
- 49.Laub R, Strengers P. Parvoviruses and blood products. Pathol Biol. 2002;50:339–48. doi: 10.1016/s0369-8114(02)00303-6. [DOI] [PubMed] [Google Scholar]
- 50.Plentz A, Hahn J, Knöll A, et al. Exposure of hematologic patients to parvovirus B19 as a contaminant of blood cell preparations and blood products. Transfusion. 2005;45:1811–5. doi: 10.1111/j.1537-2995.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt M, Themann A, Drexler C, et al. Blood donor screening for parvovirus B19 in Germany and Austria. Transfusion. 2007;47:1775–82. doi: 10.1111/j.1537-2995.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 52.Willkommen H, Schmidt I, Lower J. Safety issues for plasma derivatives and benefit from NAT testing. Biologicals. 1999;27:325–31. doi: 10.1006/biol.1999.0227. [DOI] [PubMed] [Google Scholar]
- 53.Koenigbauer UF, Eastlund T, Day JW. Clinical illness due to parvovirus B19 infection after infusion of solvent/detergent-treated pooled plasma. Transfusion. 2000;40:1203–6. doi: 10.1046/j.1537-2995.2000.40101203.x. [DOI] [PubMed] [Google Scholar]
- 54.Liumbruno GM, Franchini M. Solvent/detergent plasma: pharmaceutical characteristics and clinical experience. J Thromb Thrombolysis. 2015;39:118–28. doi: 10.1007/s11239-014-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burnouf-Radosevich M, Appourchaux P, Huart JJ, et al. Nanofiltration, a new specific virus elimination method applied to high-purity factor IX and factor XI concentrates. Vox Sang. 1994;67:132–8. doi: 10.1111/j.1423-0410.1994.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 56.Azzi A, Morfini M, Mannucci PM. The transfusion-associated transmission of parvovirus B19. Transfus Med Rev. 1999;13:194–204. doi: 10.1016/s0887-7963(99)80033-9. [DOI] [PubMed] [Google Scholar]
- 57.Yee TT, Cohen BJ, Pasi KJ, Lee CA. Transmission of symptomatic parvovirus B19 infection by clotting factor concentrate. Br J Haematol. 1996;93:457–9. doi: 10.1046/j.1365-2141.1996.5161062.x. [DOI] [PubMed] [Google Scholar]
- 58.Lefrère JJ, Mariotti M. Use of digoxigenin-labelled probes for the detection of B19 parvovirus DNA in batches of blood products. Cell Mol Biol. 1995;41:985–8. [PubMed] [Google Scholar]
- 59.Lefrère JJ, Mariotti M, de la Croix I, et al. Albumin batches and B19 parvovirus DNA. Transfusion. 1995;35:389–91. doi: 10.1046/j.1537-2995.1995.35595259148.x. [DOI] [PubMed] [Google Scholar]
- 60.Lefrere JJ, Mariotti M, Thauvin M. B19 parvovirus DNA in solvent/detergent-treated anti-haemophilia concentrates. Lancet. 1994;343:211–2. doi: 10.1016/s0140-6736(94)90993-8. [DOI] [PubMed] [Google Scholar]
- 61.Rollag H, Solheim BG, Svennevig JL. Viral safety of blood derivatives by immune neutralization. Vox Sang. 1998;74:213–7. doi: 10.1111/j.1423-0410.1998.tb05475.x. [DOI] [PubMed] [Google Scholar]
- 62.Saldanha J, Minor P. Detection of human parvovirus B19 DNA in plasma pools and blood products derived from these pools: implications for efficiency and consistency of removal of B19 DNA during manufacture. Br J Haematol. 1996;93:714–9. doi: 10.1046/j.1365-2141.1996.d01-1679.x. [DOI] [PubMed] [Google Scholar]
- 63.Corcioli F, Zakrzewska K, Fanci R, et al. Human parvovirus PARV4 DNA in tissues from adult individuals: a comparison with human parvovirus B19 (B19V) Virol J. 2010;7:272. doi: 10.1186/1743-422X-7-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geng Y, Wu C-G, Bhattacharyya SP, et al. Parvovirus B19 DNA in factor VIII concentrates: effects of manufacturing procedures and B19 screening by nucleic acid testing. Transfusion. 2007;47:883–9. doi: 10.1111/j.1537-2995.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 65.Modrow S, Wenzel JJ, Schimanski S, et al. Prevalence of nucleic acid sequences specific for human parvoviruses, hepatitis A and hepatitis E viruses in coagulation factor concentrates. Vox Sang. 2011;100:351–8. doi: 10.1111/j.1423-0410.2010.01445.x. [DOI] [PubMed] [Google Scholar]
- 66.Soucie JM, Monahan PE, Kulkarni R, et al. Evidence for the continued transmission of parvovirus B19 in patients with bleeding disorders treated with plasma-derived factor concentrates. Transfusion. 2013;53:1143–4. doi: 10.1111/trf.12153. [DOI] [PubMed] [Google Scholar]
- 67.Saldanha J. Validation and standardisation of nucleic acid amplification technology (NAT) assays for the detection of viral contamination of blood and blood products. J Clin Virol. 2001;20:7–13. doi: 10.1016/s1386-6532(00)00149-9. [DOI] [PubMed] [Google Scholar]
- 68.Kleinman SH, Glynn SA, Lee TH, et al. National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (NHLBI REDS-II) A linked donor-recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood. 2009;114:3677–83. doi: 10.1182/blood-2009-06-225706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klukowska A, Komrska V, Jansen M, Laguna P. Low incidence of factor VIII inhibitors in previously untreated patients during prophylaxis, on-demand treatment and surgical procedures, with Octanate®: interim report from an ongoing prospective clinical study. Haemophilia. 2011;17:399–406. doi: 10.1111/j.1365-2516.2010.02428.x. [DOI] [PubMed] [Google Scholar]
- 70.Mundt JM, Rouse L, Van den Bossche J, Goodrich RP. Chemical and biological mechanisms of pathogen reduction technologies. Photochem Photobiol. 2014;90:957–64. doi: 10.1111/php.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goeyvaerts N, Hens N, Ogunjimi B, et al. Estimating infectious disease parameters from data on social contacts and serological status. J Royal Stat Soc. 2010;13:255. [Google Scholar]
- 72.US Food Drug Administration. Guidance for Industry: Nucleic Acid Testing (NAT) to Reduce the Possible Risk of Parvovirus B19 Transmission by Plasma-Derived Products. 2009. [Accessed on 25/06/2014]. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm071592.htm.
- 73.Council of Europe. European Pharmacopoeia. 6th ed. Strasbourg: Council of Europe Publishing; 2008. Human plasma (pooled and treated for virus inactivation), monograph 1646. [Google Scholar]
- 74.Hourfar MK, Mayr-Wohlfart U, Themann A, et al. Recipients potentially infected with parvovirus B19 by red blood cell products. Transfusion. 2011;51:129–36. doi: 10.1111/j.1537-2995.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- 75.Kooistra K, Mesman HJ, De Waal M, et al. Epidemiology of high-level parvovirus B19 viremia among Dutch blood donors, 2003–2009. Vox Sang. 2010;100:261–6. doi: 10.1111/j.1423-0410.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 76.Jordan J, Tiangco B, Kiss J, et al. Human parvovirus B19: prevalence of viral DNA in volunteer blood donors and clinical outcomes of transfusion recipients. Vox Sang. 1998;75:97–102. [PubMed] [Google Scholar]
- 77.Broliden K, Tolfvenstam T, Norbeck O. Clinical aspects of parvovirus B19 infection. J Internal Med. 2006;260:285–304. doi: 10.1111/j.1365-2796.2006.01697.x. [DOI] [PubMed] [Google Scholar]
- 78.Petermann R, Piquet Y, Lapeyre M, et al. Detection of B19 parvovirus in plasma pools before solvent-detergent treatment of plasma: AFSSAPS and EFS Aquitaine-Limousin’s experience. Transfus Clin Biol. 2010;17:54–62. doi: 10.1016/j.tracli.2010.04.002. [In French] [DOI] [PubMed] [Google Scholar]
- 79.Zhang W, Ke L, Changqing L, et al. Parvovirus B19V DNA contamination in Chinese plasma and plasma derivatives. J Transl Med. 2012;10:194. doi: 10.1186/1479-5876-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kleinman SH, Glynn SA, Lee TH, et al. National Heart, Lung, Blood Institute Retrovirus Epidemiology Donor Study (REDS-II) Prevalence and quantitation of parvovirus B19 DNA levels in blood donors with a sensitive polymerase chain reaction screening assay. Transfusion. 2007;47:1756–64. doi: 10.1111/j.1537-2995.2007.01341.x. [DOI] [PubMed] [Google Scholar]
- 81.Grabarczyk P, Korzeniowska J, Liszewski G, et al. Parvovirus B19 DNA testing in Polish blood donors, 2004–2010. Przegl Epidemiol. 2012;66:7–12. [PubMed] [Google Scholar]
- 82.Sakata H, Matsubayashi K, Ihara H, et al. Impact of chemiluminescent enzyme immunoassay screening for human parvovirus B19 antigen in Japanese blood donors. Transfusion. 2013;53:2556–66. doi: 10.1111/j.1537-2995.2012.03949.x. [DOI] [PubMed] [Google Scholar]
- 83.Koppelman MH, Cuijpers HT, Wessberg S, et al. Multicenter evaluation of a commercial multiplex polymerase chain reaction test for screening plasma donations for parvovirus B19 DNA and hepatitis A virus RNA. Transfusion. 2012;52:1498–508. doi: 10.1111/j.1537-2995.2012.03705.x. [DOI] [PubMed] [Google Scholar]
- 84.Weimer T, Streichert S, Watson C, Gröner A. High-titer screening PCR: a successful strategy for reducing the parvovirus B19 load in plasma pools for fractionation. Transfusion. 2001;41:1500–4. doi: 10.1046/j.1537-2995.2001.41121500.x. [DOI] [PubMed] [Google Scholar]
- 85.Lefrère JJ, Servant-Delmas A, Candotti D, et al. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood. 2005;106:2890–5. doi: 10.1182/blood-2005-03-1053. [DOI] [PubMed] [Google Scholar]
- 86.Compston LI, Sarkobie F, Li C, et al. Multiplex real-time PCR for the detection and quantification of latent and persistent viral genomes in cellular or plasma blood fractions. J Virol Methods. 2008;151:47–54. doi: 10.1016/j.jviromet.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 87.Wasfy S, Nishikawa J, Petric M. Seroprevalence of immunoglobulin G antibody to parvovirus B19 in Ontario. Can J Infect Dis. 1996;7:313–6. doi: 10.1155/1996/941356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slavov SN, Haddad SK, Silva-Pinto AC, et al. Molecular and phylogenetic analyses of human Parvovirus B19 isolated from Brazilian patients with sickle cell disease and β-thalassemia major and healthy blood donors. J Med Virol. 2012;84:1652–65. doi: 10.1002/jmv.23358. [DOI] [PubMed] [Google Scholar]
- 89.Slavov SN, Kashima S, Silva-Pinto AC, Covas DT. Genotyping of Human parvovirus B19 among Brazilian patients with hemoglobinopathies. Can J Microbiol. 2012;58:200–5. doi: 10.1139/w11-119. [DOI] [PubMed] [Google Scholar]
- 90.Lévican J, Torres M, Gaggero N, et al. Parvovirus B19 among blood donors from three hospitals in Santiago, Chile. Rev Med Chil. 2011;139:143–9. [PubMed] [Google Scholar]
- 91.Gaggero A, Rivera J, Calquín E, et al. Seroprevalence of IgG antibodies against parvovirus B19 among blood donors from Santiago, Chile. Rev Med Chil. 2007;135:443–8. doi: 10.4067/s0034-98872007000400005. [DOI] [PubMed] [Google Scholar]
- 92.O’Bryan TA, Wright WF. Parvovirus B19 and C-reactive protein in blood bank donors: implications for hygiene hypothesis research. Lupus. 2010;19:1557–60. doi: 10.1177/0961203310375438. [DOI] [PubMed] [Google Scholar]
- 93.Doyle S, Kerr S, O’Keeffe G, et al. Detection of parvovirus B19 IgM by antibody capture enzyme immunoassay: receiver operating characteristic analysis. J Virol Methods. 2000;90:143–52. doi: 10.1016/s0166-0934(00)00227-5. [DOI] [PubMed] [Google Scholar]
- 94.Ke L, He M, Li C, et al. The prevalence of human parvovirus B19 DNA and antibodies in blood donors from four Chinese blood centers. Transfusion. 2011;51:1909–18. doi: 10.1111/j.1537-2995.2011.03067.x. [DOI] [PubMed] [Google Scholar]
- 95.Wei Q, Li Y, Wang JW, et al. Prevalence of anti-human parvovirus B19 IgG antibody among blood donors in Jilin province. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2006;20:60–2. [PubMed] [Google Scholar]
- 96.Kumar S, Gupta RM, Sen S, et al. Seroprevalence of human parvovirus B19 in healthy blood donors. Med J Armed Forces India. 2013;69:268–72. doi: 10.1016/j.mjafi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kishore J, Srivastava M, Choudhary N. Standardization of B19 IgG ELISA to study the seroepidemiology of parvovirus B19 in North Indian voluntary blood donors. Asian J Transfus Sci. 2010;4:86–90. doi: 10.4103/0973-6247.67022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ihara T, Furusyo N, Hayashi T, et al. A population-based epidemiological survey of human parvovirus B19 infection: a project of the Kyushu and Okinawa Population Study (KOPS) Arch Virol. 2013;158:2465–72. doi: 10.1007/s00705-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 99.Matsukura H, Shibata S, Tani Y, et al. Persistent infection by human parvovirus B19 in qualified blood donors. Transfusion. 2008;48:1036–7. doi: 10.1111/j.1537-2995.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 100.Wakamatsu C, Takakura F, Kojima E, et al. Screening of blood donors for human parvovirus B19 and characterization of the results. Vox Sang. 1999;76:14–21. doi: 10.1159/000031014. [DOI] [PubMed] [Google Scholar]
- 101.Tsujimura M, Matsushita K, Shiraki H, et al. Human parvovirus B19 infection in blood donors. Vox Sang. 1995;69:206–12. doi: 10.1111/j.1423-0410.1995.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 102.Obeid OE. Molecular and serological assessment of parvovirus B19 infections among sickle cell anemia patients. J Infect Dev Ctries. 2011;5:535–9. doi: 10.3855/jidc.1807. [DOI] [PubMed] [Google Scholar]
- 103.Oh DJ, Lee YL, Kang JW, et al. Investigation of the prevalence of human parvovirus B19 DNA in Korean plasmapheresis donors. Korean J Lab Med. 2010;30:58–64. doi: 10.3343/kjlm.2010.30.1.58. [DOI] [PubMed] [Google Scholar]
- 104.Poovorawan Y, Theamboonlers A, Suandork P, Hirsch P. Prevalence of antibodies to parvovirus B 19 in Thailand. Southeast Asian J Trop Med Public Health. 2000;31:422–4. [PubMed] [Google Scholar]
- 105.Thomas I, Di Giambattista M, Gerard C, et al. Prevalence of human erythrovirus B19 DNA in healthy Belgian blood donors and correlation with specific antibodies against structural and non-structural viral proteins. Vox Sang. 2003;84:300–7. doi: 10.1046/j.1423-0410.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 106.Hitzler WE, Runkel S. Prevalence of human parvovirus B19 in blood donors as determined by a haemagglutination assay and verified by the polymerase chain reaction. Vox Sang. 2002;82:18–23. doi: 10.1046/j.0042-9007.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 107.Juhl D, Steppat D, Görg S, Hennig H. Parvovirus b19 infections and blood counts in blood donors. Transfus Med Hemother. 2014;41:52–9. doi: 10.1159/000357650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kooistra K, Mesman HJ, de Waal M, et al. Epidemiology of high-level parvovirus B19 viraemia among Dutch blood donors, 2003–2009. Vox Sang. 2011;100:261–6. doi: 10.1111/j.1423-0410.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 109.Corcoran A, Kerr S, Elliott G, et al. Improved detection of acute parvovirus B19 infection by immunoglobulin M EIA in combination with a novel antigen EIA. Vox Sang. 2007;93:216–22. doi: 10.1111/j.1423-0410.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- 110.Zaaijer HL, Koppelman MH, Farrington CP. Parvovirus B19 viraemia in Dutch blood donors. Epidemiol Infect. 2004;132:1161–6. doi: 10.1017/s0950268804002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gessoni G, Barin P, Di Natale C, Marchiori G. NAT screening for Parvovirus B19: a feasibility study. Blood Transfus. 2006;4:67–80. [Google Scholar]
- 112.Manaresi E, Gallinella G, Morselli Labate AM, et al. Seroprevalence of IgG against conformational and linear capsid antigens of parvovirus B19 in Italian blood donors. Epidemiol Infect. 2004;132:857–62. doi: 10.1017/s0950268804002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Henriques I, Monteiro F, Meireles E, et al. Prevalence of parvovirus B19 and hepatitis A virus in Portuguese blood donors. Transfus Apher Sci. 2005;33:305–9. doi: 10.1016/j.transci.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 114.Filatova EV, Zubkova NV, Novikova NA, et al. Detection of parvovirus B19 markers in blood samples of donors. Zh Mikrobiol Epidemiol Immunobiol. 2010;5:67–70. [PubMed] [Google Scholar]
- 115.Muñoz S, Alonso MA, Fernández MJ, et al. Seroprevalence versus Parvovirus B19 in blood donors. Enferm Infecc Microbiol Clin. 1998;16:161–2. [PubMed] [Google Scholar]
- 116.Mata Rebón M, Bartolomé Husson C, Bernárdez Hermida I. Seroprevalence of anti-human parvovirus B19 antibodies in a sample of blood donors in Galicia. Enferm Infecc Microbiol Clin. 1998;16:25–7. [PubMed] [Google Scholar]
- 117.Letaïef M, Vanham G, Boukef K, et al. Higher prevalence of parvovirus B19 in Belgian as compared to Tunisian blood donors: differential implications for prevention of transfusional transmission. Transfus Sci. 1997;18:523–30. doi: 10.1016/s0955-3886(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 118.Reinheimer C, Allwinn R, Doerr HW, Wittek M. Seroepidemiology of parvovirus B19 in the Frankfurt am Main area, Germany: evaluation of risk factors. Infection. 2010;38:381–5. doi: 10.1007/s15010-010-0035-y. [DOI] [PubMed] [Google Scholar]
- 119.Langara H, Trikia H, Gouiderb E, et al. Blood-transmitted viral infections among haemophiliacs in Tunisia. Transfus Clin Biol. 2005;12:301–5. doi: 10.1016/j.tracli.2005.07.001. [In French] [DOI] [PubMed] [Google Scholar]
- 120.Soucie JM, Siwak EB, Hooper WC, et al. Universal Data Collection Project Working Group. Human parvovirus B19 in young male patients with hemophilia A: associations with treatment product exposure and joint range-of-motion limitation. Transfusion. 2004;44:1179–85. doi: 10.1111/j.1537-2995.2004.04029.x. [DOI] [PubMed] [Google Scholar]
- 121.Brojer E, Grabarczyk P, Łopaciuk S, et al. Prevalence of human parvovirus B19 DNA and IgG/IgM antibodies in Polish haemophilia patients. Vox Sang. 1999;77:107. doi: 10.1159/000031085. [DOI] [PubMed] [Google Scholar]
- 122.Canales MA, Pinilla J, Mateos P. Human parvovirus B19 antibodies are less frequent among patients treated with factor IX concentrate inactivated by ultrafiltration: a report from a single Spanish institution. Vox Sang. 1998;74:260–1. [PubMed] [Google Scholar]
- 123.Mauser-Bunschoten EP, Zaaijer HL, van Drimmelen AA. High prevalence of Parvovirus B19 lgG antibodies among Dutch hemophilia patients. Vox Sang. 1998;74:225–7. [PubMed] [Google Scholar]
- 124.Aguilar Franco C, Lucía Cuesta JF, Ferrer Torres J, Omeñaca Teres M. Parvovirus B19 infection in patients with congenital blood coagulation disorders. Med Clin (Barc) 1997;108:641–6. Erratum in: Med Clin (Barc) 1997; 109: 5 [In Spanish] [PubMed] [Google Scholar]
- 125.Ragni MV, Koch WC, Jordan JA. Parvovirus B19 infection in patients with hemophilia. Transfusion. 1996;36:238–41. doi: 10.1046/j.1537-2995.1996.36396182142.x. [DOI] [PubMed] [Google Scholar]
- 126.Zakrzewska K, Azzi A, De Biasi E, et al. Persistence of parvovirus B19 DNA in synovium of patients with haemophilic arthritis. J Med Virol. 2001;65:402–7. doi: 10.1002/jmv.2048. [DOI] [PubMed] [Google Scholar]
- 127.Bartolomei Corsi O, Azzi A, Morfini M, et al. Human parvovirus infection in haemophiliacs first infused with treated clotting factor concentrates. J Med Virol. 1988;25:165–70. doi: 10.1002/jmv.1890250206. [DOI] [PubMed] [Google Scholar]
- 128.Manning A, Willey SJ, Bell JE, Simmonds P. Comparison of tissue distribution, persistence, and molecular epidemiology of parvovirus B19 and novel human parvoviruses PARV4 and human bocavirus. J Infect Dis. 2007;195:1345–52. doi: 10.1086/513280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones MS, Kapoor A, Lukashov VV, et al. New DNA viruses identified in patients with acute viral infection syndrome. J Virol. 2005;79:8230–6. doi: 10.1128/JVI.79.13.8230-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fryer JF, Delwart E, Hecht FM, et al. Frequent detection of the parvoviruses, PARV4 and PARV5, in plasma from blood donors and symptomatic individuals. Transfusion. 2007;47:1054–61. doi: 10.1111/j.1537-2995.2007.01235.x. [DOI] [PubMed] [Google Scholar]
- 131.Simmonds P, Douglas J, Bestetti G, et al. A third genotype of the human parvovirus PARV4 in sub-Saharan Africa. J Gen Virol. 2008;89:2299–302. doi: 10.1099/vir.0.2008/001180-0. [DOI] [PubMed] [Google Scholar]
- 132.Drexler JF, Reber U, Muth D, et al. Human parvovirus 4 in nasal and fecal specimens from children, Ghana. Emerg Infect Dis. 2012;18:1650–3. doi: 10.3201/eid1810.111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma YY, Guo Y, Zhao X, et al. Human parvovirus PARV4 in plasma pools of Chinese origin. Vox Sang. 2012;103:183–5. doi: 10.1111/j.1423-0410.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 134.Fryer JF, Hubbard AR, Baylis SA. Human parvovirus PARV4 in clotting factor VIII concentrates. Vox Sang. 2007;93:341–7. doi: 10.1111/j.1423-0410.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 135.Schneider B, Fryer JF, Oldenburg J, et al. Frequency of contamination of coagulation factor concentrates with novel human parvovirus PARV4. Haemophilia. 2008;14:978–86. doi: 10.1111/j.1365-2516.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- 136.Eis-Hübinger AM, Drexler JF, Reber U, et al. Absence of detection of novel human parvoviruses in German plasma donations. Transfusion. 2010;50:266–7. doi: 10.1111/j.1537-2995.2009.02433.x. [DOI] [PubMed] [Google Scholar]
- 137.Baylis SA, Tuke PW, Miyagawa E, Blümel J. Studies on the inactivation of human parvovirus 4. Transfusion. 2013;53:2585–92. doi: 10.1111/trf.12372. [DOI] [PubMed] [Google Scholar]
- 138.Fryer JF, Lucas SB, Padley D, Baylis SA. Parvoviruses PARV4/5 in hepatitis C virus-infected persons. Emerg Infect Dis. 2007;13:175–6. doi: 10.3201/eid1301.060856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Simmonds P, Manning A, Kenneil R, et al. Parenteral transmission of the novel human parvovirus PARV4. Emerg Infect Dis. 2007;13:1386–8. doi: 10.3201/eid1309.070428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Longhi E, Bestetti G, Acquaviva V, et al. Human parvovirus 4 in the bone marrow of Italian patients with AIDS. AIDS. 2007;21:1481–3. doi: 10.1097/QAD.0b013e3281e38558. [DOI] [PubMed] [Google Scholar]
- 141.Lurcharchaiwong W, Chieochansin T, Payungporn S, et al. Parvovirus 4 (PARV4) in serum of intravenous drug users and blood donors. Infection. 2008;36:488–91. doi: 10.1007/s15010-008-7336-4. [DOI] [PubMed] [Google Scholar]
- 142.Fryer JF, Kapoor A, Minor PD, et al. Novel parvovirus and related variant in human plasma. Emerg Infect Dis. 2006;12:151–4. doi: 10.3201/eid1201.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vallerini D, Barozzi P, Quadrelli C, et al. Parvoviruses in blood donors and transplant patients, Italy. Emerg Infect Dis. 2008;14:185–6. doi: 10.3201/eid1401.070610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Botto S, Bergallo M, Sidoti F, et al. Detection of PARV4, genotypes 1 and 2, in healthy and pathological clinical specimens. New Microbiol. 2009;32:189–92. [PubMed] [Google Scholar]
- 145.Servant-Delmas A, Laperche S, Mercier M, et al. Human parvovirus 4 in recipients of cellular products and in blood donors: epidemiologic similarity with B19 parvovirus. Transfusion. 2009;49:1771–3. doi: 10.1111/j.1537-2995.2009.02286.x. [DOI] [PubMed] [Google Scholar]
- 146.Tuke PW, Parry RP, Appleton H. Parvovirus PARV4 visualization and detection. J Gen Virol. 2010;91:541–4. doi: 10.1099/vir.0.014852-0. [DOI] [PubMed] [Google Scholar]
- 147.Panning M, Kobbe R, Vollbach S, et al. Novel human parvovirus 4 genotype 3 in infants, Ghana. Emerg Infect Dis. 2010;16:1143–6. doi: 10.3201/eid1607.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Touinssi M, Brisbarre N, Picard C, et al. Parvovirus 4 in blood donors, France. Emerg Infect Dis. 2010;16:165–6. doi: 10.3201/eid1601.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Touinssi M, Reynaud-Gaubert M, Gomez C, et al. Parvovirus 4 in French in-patients: a study of hemodialysis and lung transplant cohorts. J Med Virol. 2011;83:717–20. doi: 10.1002/jmv.22003. [DOI] [PubMed] [Google Scholar]
- 150.Maple PA, Beard S, Parry RP, et al. Testing UK blood donors for exposure to human parvovirus 4 using a time-resolved fluorescence immunoassay to screen sera and Western blot to confirm reactive samples. Transfusion. 2013;53:2575–84. doi: 10.1111/trf.12278. [DOI] [PubMed] [Google Scholar]
- 151.Sharp CP, Lail A, Donfield S, et al. Virologic and clinical features of primary infection with human parvovirus 4 in subjects with hemophilia: frequent transmission by virally inactivated clotting factor concentrates. Transfusion. 2012;52:1482–9. doi: 10.1111/j.1537-2995.2011.03420.x. [DOI] [PubMed] [Google Scholar]
- 152.Allander T, Tammi MT, Eriksson M, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–96. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jartti T, Hedman K, Jartti L, et al. Human bocavirus-the first 5 years. Rev Med Virol. 2012;22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 154.Norja P, Hedman L, Kantola K, et al. Occurrence of human bocaviruses and parvovirus 4 in solid tissues. J Med Virol. 2012;8:1267–73. doi: 10.1002/jmv.23335. [DOI] [PubMed] [Google Scholar]
- 155.Kantola K, Hedman L, Arthur J, et al. Seroepidemiology of human bocaviruses 1–4. J Infect Dis. 2011;204:1403–12. doi: 10.1093/infdis/jir525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Di Minno G, Canaro M, Ironside JW, et al. Pathogen safety of long-term treatments for bleeding disorders: still relevant to current practice. Haematologica. 2013;98:1495–8. doi: 10.3324/haematol.2013.084145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Groeneveld K, van der Noordaa J. Blood products and parvovirus B19. Neth J Med. 2003;61:154–6. [PubMed] [Google Scholar]
- 158.AABB Special Issue: Emerging Infectious Disease Agents and their Potential Threat to Transfusion Safety. Human Parvovirus B19. Transfusion. 2009;49:107S–9S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]