Abstract
Background
Screening blood donors for the presence of hepatitis B virus surface antigen (HBsAg) has been the backbone of blood safety. However, occult hepatitis B infection (OBI) in donors can be missed when only HBsAg screening is used. Nucleic acid testing (NAT) is capable of detecting OBI among donors. The aim of our study was to analyse the sensitivity of NAT for detecting OBI.
Material and methods
The kits used during the study for serology testing were BioRad Monolisa™ HBsAg Ultra (HBsAg screening), Abbott Architect for anti-HBcAg (total) and anti-HBsAg testing, and Vitros® by Ortho Clinical Diagnostics for anti-HBcAg (IgM). Procleix Ultrio was used for individual donor-NAT (ID-NAT) and Abbott m2000 for estimation of HBV DNA. Out of 28,134 HBsAg non-reactive donors, 25 were ID-NAT-reactive. Of these 25 NAT yield samples, 18 were studied further at different dilutions from 1:2 to 1:16. The doubling dilutions were made with HBV non-reactive AB plasma. Undiluted samples were used for all serological tests and for HBV DNA estimation.
Results
Of the 18 samples studied, nine were NAT-reactive at a dilution of <1:4 and five out of these showed presence of antibody to core antigen (IgG+IgM). Antibody to surface antigen was present in only two of the nine NAT-reactive samples, one with antibody to core antigen and the other without. Six had a viral load in the range from <10 to 38 IU/mL whereas the viral load in the remaining three samples was not determined. Among the other nine samples which were NAT-reactive at dilutions ≥1:4, antibody to core antigen (IgG+IgM) was present in seven.
Discussion
Our study showed that ID-NAT testing along with HBsAg screening could detect most potentially HBV infectious donors (including those with OBI). NAT screening for HBV on diluted samples could compromise blood safety because samples with a low viral load will escape detection.
Keywords: occult hepatitis B infection, nucleic acid testing, hepatitis B virus, transfusion-transmitted infections
Introduction
Screening for hepatitis B virus surface antigen (HBsAg) among blood donors has been the backbone of blood safety for many years. However, even with mandatory HBsAg screening, occult hepatitis B infection (OBI) remains an unresolved challenge. Patients with OBI are HBsAg-negative with persistent hepatitis B virus (HBV) DNA detectable by polymerase chain reaction (PCR), in the presence (80%) or absence (20%) of antibodies to HBV core antigen (anti-HBc) with or without antibodies to HBsAg (anti-HBs). As these infections escape detection when HBsAg screening is used, they are the one of the main sources of post-transfusion hepatitis in India1. The risk of OBI in India is unknown, as few data are available on either prevalence or transmission rate.
With the recent addition of nucleic acid testing (NAT) as an added layer of screening on donated blood, it has become possible to detect OBI at a molecular level among blood donors. The sensitivity of NAT has important implications when the assay is used to screen for infections at a molecular level. The sensitivity of the assays is influenced by the viral load of the agent as well as the size of the pool tested2. The viral load of human immunodeficiency virus (HIV), hepatitis C virus (HCV) and HBV varies during the course of the infections, hence appropriate pool size for optimal detection by NAT needs to be determined in our setting.
The appropriateness of screening for HBV by minipool (MP)-NAT in India is still a topic of debate. It has been established that low titres of HBV DNA (102–104 genome equivalents [geq]/mL) occur during the so-called window period3–5 and these might escape detection by NAT when done in minipools. Busch et al.5 concluded that HBV NAT pooling strategies would detect only a small proportion of donations made in the infectious window period in the USA. The risk of transmission of HBV during the pre-HBsAg-infectious window period is expected to be high in HBV endemic countries such as India.
The aim of our study was to analyse the sensitivity of NAT for detecting OBI. HBV NAT yield samples were studied at different dilutions in order to determine the pool size at which NAT would have the best sensitivity for the detection of donors with OBI as well as donors in the window period.
Material and methods
This study was conducted at the Blood Bank in a Central Government Hospital in India (New Delhi). In addition to mandatory screening by enzyme-linked immunoassay for antibodies to HIV, HCV and HBsAg, all the samples were subjected to individual donor (ID)-NAT. Between January 2012 and December 2013, a total of 28,465 donations were collected, of which 14,927 (52.5%) were from voluntary donors and 13,538 (47.3%) from replacement donors. Of the 28,134 donor samples that were HBsAg non-reactive during the study period, 25 were HBV DNA reactive (NAT yield). These NAT yield samples (ID-NAT reactive, HBsAg non-reactive) form the basis of this study.
Sampling and storage of samples
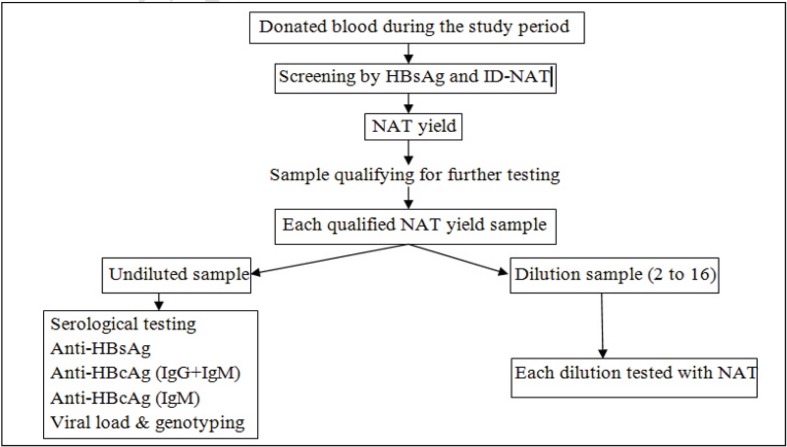
The 25 NAT yield samples, as and when they were detected, were stored at −80 °C for subsequent, further evaluation. The volume of each stored sample was 30 mL of plasma from each collection bag. Of the 25 NAT yield samples stored, only 18 samples qualified for the study and were tested according to the algorithm shown in Figure 1. The other seven samples could not be evaluated because the quality or quantity of the samples was inadequate. All qualified NAT yield samples (n=18) were thawed. Thawed samples were diluted for further testing of each dilution with ID-NAT, whereas undiluted samples were used for serological studies. A maximum of two freeze-thaw cycles was used.
Figure 1.
Algorithm of testing samples.
HBsAg: hepatitis B virus surface antigen; NAT: nucleic acid testing; ID-NAT: individual donor-NAT; HBcAg: hepatitis B virus core antigen; IgG: immunoglobulin G; IgM: immunoglobulin M.
Dilution studies
The 18 NAT yield samples were diluted to simulate the MP-NAT scenario. A doubling dilution method was used with AB plasma that was HBV non-reactive (by both ID-NAT and serology). NAT was performed on samples diluted 1:2, 1:4, 1:8 and 1:16. Undiluted samples were used for quantitative PCR analysis of viral load and genotyping.
Serological studies
All serological tests were done on undiluted samples. Each sample was screened for HBsAg by an ELISA based on a one-step enzyme immunoassay of the sandwich type for detection of surface antigen. Chemiluminescence microparticle immunoassays were used to detect anti-HBc (immunoglobulin G [IgG] and immunoglobulin M [IgM]) and anti- HBs and all samples reactive for anti-HBc (IgG+IgM) were further tested by enhanced chemiluminescence technology to identify IgM-type antibodies against HBc (Table I).
Table I.
Details of principles and kits of the tests performed.
| Test | Principle | Kit |
|---|---|---|
| HBsAg | ELISA | BioRad “MONOLISA™ HBsAg Ultra” |
| ID-NAT | TMA | Procleix Ultrio |
| Anti-HBc (IgG&IgM) | CMIA | Abbott, ARCHITECT |
| Anti-HBc (IgM) | ECi | Ortho Diagnostics, Vitros® |
| Anti-HBsAg | CMIA | Abbott, ARCHITECT |
| HBV DNA | Real-time PCR | Abbott, m2000 |
HBsAg: hepatitis B virus surface antigen; ID-NAT: individual donor-NAT; HBc: hepatitis B virus core; IgG: immunoglobulin G; IgM: immunoglobulin M; HBV: hepatitis B virus; ELISA: enzyme-linked immunoassay; TMA: transcription-mediated amplification; CMIA: chemiluminescence microparticle immunoassay; ECi: enhanced chemiluminescence; PCR: polymerase chain reaction.
Nucleic acid testing
Undiluted samples were subjected to ID-NAT using the Procleix Ultrio kit (Novartis Diagnostics, Cambridge, USA), which is based on manual transcription-mediated amplification (TMA) technology. The assay contains reagents for the simultaneous detection of HBV, HCV and HIV-1. An initial NAT assay was done on the pilot tube sample and if found reactive a sample from the bag was tested. If the repeat sample was found to be reactive, further discriminatory testing for HBV, HCV and HIV-1 was performed. A positive discriminatory test confirmed the presence of the respective virus. The Procleix Ultrio assay has been demonstrated to be sufficiently sensitive to detect HIV-1 and HCV viral RNA concentrations ≥100 copies/mL and HBV viral DNA concentrations ≥15 IU/mL.
Diluted samples (1:2 to 1:16) were tested with the same kit, Procleix Ultrio, as that used for undiluted samples.
Viral load and genotyping
Quantitative real-time PCR (Abbott, Illinois, USA) was done to determine the viral load in undiluted samples. The linear reporting range of the assay was 10 to 1×109 IU/mL. The conversion factor used for 1 IU/mL was 3.41 copies/mL. Results <10 IU/mL were below the lower limit of the linear range of the assay whereas “target not detected” signified a sample which do not contain HBV DNA.
HBV genotyping was done on reactive cases if the viral load was >5,000 copies/mL.
Results
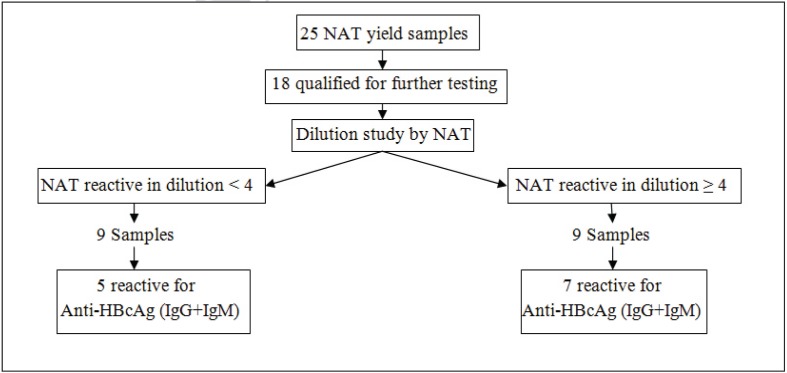
Eighteen NAT yield samples were studied in undiluted form as well as in doubling dilutions (1:2 to 1:16). Of these 18 samples, nine were reactive to NAT at dilutions of ≥1:4) (Figure 2). The other nine samples were reactive at dilutions of <1:4 and five of these showed the presence of antibodies to core antigen (IgG+IgM). Antibodies to HBsAg were present in only two of the nine samples, one with antibody to core antigen and the other without. Six samples had a viral load ranging from <10 to 38 IU/mL (Table II) whereas the viral load was not determined in the remaining three samples.
Figure 2.
Results of NAT and anti-HBcAg testing on OBI samples.
OBI: occult hepatitis B infection; NAT: nucleic acid testing; HBcAg: hepatitis B virus core antigen; IgG: immunoglobulin G; IgM: immunoglobulin M.
Table II.
Details of further studies done on OBI samples.
| Sample | NAT reactive at dilution | Anti-HBcAg (IgG+IgM) | Anti-HBcAg (IgM) | Anti-HBsAg | Viral load (IU/mL) | Genotype | Percentage of total NAT yield | Donor demographic details | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Age | Type | ||||||||
| Samples reactive at dilution <1:4 | |||||||||
|
| |||||||||
| 1 | 1 | NR | NR | NR | ND | ND | 22.22% | 29 | RD |
| 2 | 1 | NR | NR | NR | ND | ND | 23 | RD | |
| 3 | 1 | NR | NR | NR | 38 | ND | 23 | RD | |
| 4 | 1 | NR | NR | R | <10 | ND | 32 | VD | |
|
| |||||||||
| 5 | 1 | R | NR | R | ND | ND | 27.77% | 40 | VD |
| 6 | 1 | R | NR | NR | <10 | ND | 28 | RD | |
| 7 | 1 | R | NR | NR | 13 | ND | 48 | VD | |
| 8 | 1 | R | NR | NR | 38 | ND | 29 | RD | |
| 9 | 2 | R | NR | NR | 22 | ND | 30 | VD | |
|
| |||||||||
| Samples reactive at dilution ≥1:4 | |||||||||
|
| |||||||||
| 10 | 8 | NR | NR | NR | 16 | ND | 11.11% | 21 | RD |
| 11 | 4 | NR | NR | NR | 505 | ND | 27 | VD | |
|
| |||||||||
| 12 | 8 | R | R | NR | 38 | ND | 38.88% | 19 | RD |
| 13 | 4 | R | NR | R | 115 | ND | 22 | RD | |
| 14 | 8 | R | NR | NR | 2,711 | ND | 20 | RD | |
| 15 | 16 | R | NR | R | 15,849 | A | 32 | RD | |
| 16 | 16 | R | NR | NR | 177,827,941 | A | 21 | RD | |
| 17 | 16 | R | NR | NR | 154,881,662 | A | 22 | RD | |
| 18 | 16 | R | NR | NR | 5,011,872 | A | 30 | VD | |
OBI: occult hepatitis B infection; NAT: nucleic acid testing; HBcAg: hepatitis B virus core antigen; IgG: immunoglobulin G; IgM: immunoglobulin M; HBsAg: hepatitis B virus surface antigen; R: reactive; NR: non-reactive; ND: not determined.
Of the nine samples reactive at dilutions ≥1:4, seven had anti-HBc (IgG+IgM) and of these seven samples, one had IgM-type antibodies to the core antigen. Antibody to surface antigen was detected in two of the seven samples that were anti-Hbc-reactive. The viral load of the nine samples ranged from 16 to 1×108 IU/mL. Genotype estimation was possible in four samples and all were found to be genotype A (Table II).
In our study two samples which had a viral load less than 50 IU/mL were detected at a dilution of 1:8 whereas the rest of them (n=9) with a viral load of <50 IU/mL were not detected at a dilution of 1:4 or higher. Three samples which were detected at a dilution <1:4 did not show the presence of viral particles. This disparity between viral load and detection of the virus at different dilutions occurred at very low concentrations of the viral particles and is due to the Poisson distribution of viral particles in the donated blood.
Discussion
The role of NAT is well established in our country for the detection of transfusion-transmissible infections in donors during the window period which would escape detection by serology6–8. In India, both ID-NAT and MP-NAT methodologies are practiced in blood banks. Minipool testing (MP-NAT) strategies are preferred in the western world where the prevalence of the viruses being searched for is low9.
There is currently a debate on whether ID-NAT, MP-NAT or detection of anti-HBc should be performed along with mandatory HBsAg screening. This debate is fuelled by the financial implications of ID-NAT for donor screening along with the knowledge that a infected donor with a low viral load of HBV can escape detection by MP-NAT and that some core antibody-negative units can have detectable HBV DNA (window period)10,11.
Despite mandatory screening for HBV by HBsAg in India, HBV continues to be transmitted by blood and blood components, mainly because of OBI among donors as well as window period donations. Genotype identification was possible in four samples from donors with OBI and was genotype A in all of them. Genotype D is more common in this part of Asia but genotype A has also been reported in India.
HBV NAT yield samples with detectable anti-HBc are considered as OBI whereas anti-HBc-negative NAT yield donations are window period donations. Anti-HBcAg testing as an approach to exclude OBI from the blood supply was carefully examined because it is cheaper than ID-NAT. However, when considered for use in areas with a 10–60% prevalence of anti-HBc (whole Asian continent), the costs related to deferring large numbers of blood units and the unaffordable impact on an already insufficient blood supply impeded countries from implementing such screening12.
Screening with HBsAg and anti-HBc could offer a suitable serological combination of tests but in our study six out of 18 HBV NAT yield samples were negative for antibody to core antigen but were detected by ID-NAT. Out of these six samples negative for anti-HBc, four would have escaped detection if a dilution of 1:4 had been used for the NAT. The combination of screening for HBsAg and antibody to core antigen would miss 30% of potentially infectious HBV donors. If MP4-NAT screen was added to the serological tests (HBsAg and anti-HBc), 22.22% of total HBV ID-NAT yield samples would still be missed (Table II).
Many studies have been conducted on HBsAg-negative samples among blood donors in our country but almost all have studied13–15 the seroprevalance of antibody to core antigen. The incidence of anti-HBc has been reported to range from 8.4 to 30.2%. Anti-HBc is long-lasting and can be detected later than HBsAg. The presence of anti-HBc in association with antibody to surface antigen is reported to be associated with lower rates of transfusion transmission than in the absence of HBsAg16–18.
The concentration of HBV DNA is low early in acute infections when both MP-NAT and tests for HBsAg would be non-reactive; ID-NAT during the early phase of an infection has a much higher yield rate, reduces the window-period and consequently offers greater benefits (as shown in our study). HBsAg tests with high sensitivity (<0.1 ng/mL) are predicted to have a comparable yield to MP-NAT. Finally, given the relatively low yield of MP-NAT and comparing this with the safety afforded by HBsAg and anti-HBc assays, coupled with low rates of chronic HBV infection and clinical disease, HBV MP-NAT offers only marginal cost-effectiveness over ID-NAT19.
Kleinman et al.20 tried to address the question of whether screening for HBV should be done through HBsAg or HBV NAT (MP-NAT or ID-NAT). They tested 581,790 samples out of which 23 were MP-NAT-positive and HBsAg-negative and 16 were MP-NAT-negative and HBsAg-positive, of which ten were positive by ID-NAT. They reported that HBV DNA was detected by MP-NAT in 84% of the HBsAg-positive, anti-HBc-reactive donations whereas the detection rate by ID-NAT was 94%. They also showed that HBsAg screening offers most chances of detecting an HBV-infected donor. In the cases not detected by MP-NAT, the viral loads ranged from less than 100 to 5,900 copies/mL.
Although very few anti-HBc-reactive donations that were HBsAg-non-reactive were detected as being HBV-DNA-positive by MP-NAT (0.03%), the detection rate increased with ID-NAT to 0.41%, which was higher than a previously reported rate of 0.24%20 but lower than the 0.63% in a study performed by the American Red Cross21. In all studies, the vast majority of samples identified by ID-NAT had viral loads too low for quantitation (i.e., less than 100 copies/mL).
Conclusion
Despite mandatory HBsAg screening, HBV is still the most frequent cause of post-transfusion hepatitis in India1. Viral dynamics and serological markers in HBV-infected people are highly variable. HBsAg screening alone is not sufficient to detect HBV in all phases of the infection. A combination of screening with HBsAg and antibody to core antigen or NAT (MP or ID) is desirable. Multicentre analysis of more numerous samples is required to establish the most efficient screening model for HBV in blood donors in India.
Our study showed that ID-NAT testing along with HBsAg screening could detect most of the potentially infectious donors (including those with OBI). NAT screening for HBV on diluted samples could compromise blood safety as samples with a low viral load will escape detection.
Footnotes
Autorship contributions
SA conducted the study and formulated the manuscript. VD critically reviewed the manuscript and suggested invaluable inputs. TK helped with testing and collection of the data.
The Authors declare no conflicts of interest.
References
- 1.Chaudhuri V, Nanu A, Panda SK, Chand P. Evaluation of serologic screening of blood donors in India reveals a lack of correlation between anti-HBc titer and PCR-amplified HBV DNA. Transfusion. 2003;43:1442–8. doi: 10.1046/j.1537-2995.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman S, Strong DM, Tegtmeier G, et al. Hepatitis B virus (HBV) DNA screening of blood donations in minipools with the COBAS AmpliScreen HBV test. Transfusion. 2005;45:1247–57. doi: 10.1111/j.1537-2995.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- 3.Busch MP, Stramer SL, Kleinman SH. Evolving applications of nucleic acid amplification assays for prevention of virus transmission by blood components and derivatives. In: Garratty G, editor. Applications of Molecular Biology to Blood Transfusion Medicine. Bethesda: American Association of Blood Banks; 1997. pp. 123–76. [Google Scholar]
- 4.Sacher RA, Schreiber GB, Kleinman SH. Prevention of transfusion-transmitted hepatitis. Lancet. 2000;355:331–2. doi: 10.1016/S0140-6736(99)00391-8. [DOI] [PubMed] [Google Scholar]
- 5.Committee report. Nucleic acid amplification testing of blood donors for transfusion-transmitted infectious diseases. Report of the Interorganizational Task Force on Nucleic Acid Amplification Testing of Blood Donors. Transfusion. 2000;40:143–59. doi: 10.1046/j.1537-2995.2000.40020143.x. [DOI] [PubMed] [Google Scholar]
- 6.Makroo RN, Choudhury N, Jagannathan L, et al. Multicenter evaluation of individual donor nucleic acid testing (NAT) for simultaneous detection of human immunodeficiency virus -1 & hepatitis B & C viruses in Indian blood donors. Indian J Med Res. 2008;127:140–7. [PubMed] [Google Scholar]
- 7.Chaterjee K, Coshic P, Borgohain M, et al. Individual donor nucleic acid testing for blood safety against HIV-1 and hepatitis B and C viruses in a tertiary care hospital. Natl Med J India. 2012;25:207–9. [PubMed] [Google Scholar]
- 8.Agarwal N, Chatterjee K, Coshic P, Borgohain M. Nucleic acid testing for blood banks: An experience from a tertiary care centre in New Delhi, India. Transf Apheres Sci. 2013;49:482–4. doi: 10.1016/j.transci.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Stramer SL, Notari EP, Krysztof DE, Dodd RY. Hepatitis B virus testing by mini pool nucleic acid testing: does it improve blood safety? Transfusion. 2013;53:2449–58. doi: 10.1111/trf.12213. [DOI] [PubMed] [Google Scholar]
- 10.Schuttler CG, Caspari G, Jursh CA, et al. Hepatitis C transmission by a blood donation negative in nucleic acid amplification tests for viral RNA. Lancet. 2000;355:41–2. doi: 10.1016/S0140-6736(99)04719-4. [DOI] [PubMed] [Google Scholar]
- 11.Stramer SL, Chambers L, Page PL, et al. Third reported US case of breakthrough HIV transmission from NAT screened blood. Transfusion. 2003;43(Suppl 9S) abstract S134. [Google Scholar]
- 12.Allain JP, Candotti D ISBT HBV Safety Collaborative Group. Hepatitis B virus in transfusion medicine: still a problem? Biologicals. 2012;40:180–6. doi: 10.1016/j.biologicals.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri V, Tayal R, Nayak B, et al. Occult hepatitis B virus infection in chronic liver disease: full-length genome and analysis of mutant surface promoter. Gastroenterology. 2004;127:1356e71. doi: 10.1053/j.gastro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Panigrahi R, Biswas A, Datta S, et al. Anti-hepatitis B core antigen testing with detection and characterization of occult hepatitis B virus by an in-house nucleic acid testing among blood donors in Behrampur, Ganjam, Orissa in southeastern India: implications for transfusion. Virol J. 2010;7:204–10. doi: 10.1186/1743-422X-7-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhawan HK, Marwhaha N, Sharma RS, et al. Anti-HBc screening in Indian blood donors: still an unresolved issue. World J Gastroenterol. 2008;14:5327–30. doi: 10.3748/wjg.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48:1001–26. doi: 10.1111/j.1537-2995.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 17.Satake M, Taira R, Yugi H, et al. Infectivity of blood components with low hepatitis B virus DNA levels identified in a lookback program. Transfusion. 2007;47:1197–205. doi: 10.1111/j.1537-2995.2007.01276.x. [DOI] [PubMed] [Google Scholar]
- 18.Katz L, Strong DM, Tegtmeier G, Stramer S. Performance of an algorithm for the reentry of volunteer blood donors deferred due to false-positive test results for antibody to hepatitis B core antigen. Transfusion. 2008;48:2315–22. doi: 10.1111/j.1537-2995.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- 19.Jackson BR, Busch MP, Stramer SL, AuBuchon JP. The cost-effectiveness of NAT for HIV, HCV and HBV in whole-blood donations. Transfusion. 2003;43:721–9. doi: 10.1046/j.1537-2995.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- 20.Kleinman SH, Kuhns MC, Todd DS, et al. Frequency of HBV DNA detection in US blood donors positive for anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 21.Busch MP. Should HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors? Transfus Clin Biol. 2004;11:26–32. doi: 10.1016/j.tracli.2003.12.003. [DOI] [PubMed] [Google Scholar]