Abstract
Background
Platelet concentrates may demonstrate visual, macroscopic clumps immediately after collection following aphaeresis or production from whole blood, independently of the preparation method or equipment used. The relationship between the occurrence of clumping and their effect on in vitro quality of platelets was investigated.
Material and methods
Platelet concentrates, suspended in SSP+ additive solution (Macopharma), were obtained by automated processing and also from routine processing. A total of twelve units were allocated to the test group (n=12) due to the presence of clumps. Platelet concentrates without clumps were used as controls (n=10). All platelet units were treated for pathogen reduction following storage under continuous agitation for in vitro testing over a 9-day storage period.
Results
No significant differences were found throughout storage between the groups. The lactate dehydrogenase levels increased in both groups; this increase was higher in the test group on the last day of testing, without there being a significant difference on day 2. In contrast, pH values on day 2 were significantly different between the test and control groups. Platelet-derived cytokines increased comparably during storage.
Discussion
The results confirm good in vitro quality and storage stability of platelets suspended in SSP+ and treated with the Intercept pathogen reduction system. The presence of “non-compacted” clumps in platelet concentrates does not appear to affect the in vitro quality of the platelets.
Keywords: platelet aggregation, cytokines, blood buffy coat
Introduction
An important goal in transfusion medicine is to ensure an adequate supply of high quality platelets. This goal can be influenced by several variables, some physical such as temperature, throughout the entire process from whole blood donation until fractionation, subsequent preparation of pools and platelet storage. The quality of platelets significantly improved after the introduction of gas-permeable bags1.
In recent decades the introduction of pre-storage leucodepletion, the use of additive solutions and finally the development of pathogen reduction technologies in addition to alternative preparation methods have resulted in variability in platelet concentrates, which may differ in terms of their platelet content, leucocyte count, potential microbial load, and platelet properties. Routine quality control measures, e.g. pH testing and visual inspection, may help to detect abnormalities and products that are out-of-specification.
The aim of pathogen reduction technologies is to minimise an increased potential risk of infection after serological testing, especially in platelet concentrates, which are stored at temperatures that make them more vulnerable to microbial growth. The application of pathogen reduction technologies helps to extend the storage period up to 7 days providing significant advantages, including extended shelf-life and consequent greater product availability. The advantages can also be attributed to the use of platelet additive solutions (PAS) for pathogen reduction technologies, e.g. PAS-C (InterSolTM, Fenwal, Lake Zurich, IL, USA) or PAS-E (SSP+), which have been routinely employed for many years2. PAS reduce the proportion of plasma required to be carried over, which can be used for other purposes, avoiding transfusion of large volumes of plasma and thereby lowering the risk of adverse reactions and circulatory overload and improving the ability of platelet concentrates to survive storage.
From 2008 to 2012 platelet concentrates in our centre were treated with amotosalen+UVA light (Intercept, Cerus, Concord, CA, USA) using PAS-C. In 2012 we began routine use of another additive solution, SSP+ (Macopharma, Tourcoing, France), called modified PAS-III or PAS-E, since approval was obtained in 2009 for this PAS to be used with amotosalen+UVA light and our previous experience3–5 and several studies indicate that it has a positive effect on platelet functionality6–8.
After manufacture of platelet concentrates by pooling buffy coats suspended in PAS-E, the platelet units are maintained for 2 hours at rest and are all visually inspected prior to inactivation. We have observed the presence of clumps, called “non-compact”, as they easily disappear with manual mixing or agitation. Classified by Ringwald et al.9, these clumps are characterised by their large size (>1 mm), visually appear like small flakes, are relatively common (22% average in our experience), and tend to dissipate during rest periods and agitation within 24 hours after collection. A lower rate of spontaneous platelet clumping was previously reported for platelets from platelet-rich plasma (8%)10. Based on our previous experience and attention to quality control, we observed that only a small degree of clumping occurred when we used PAS-C. On this background, the aim of this study was to evaluate metabolic parameters, activation and some biological mediators in the pools showing more evident clumps (test platelet concentrates) and in non-clumped pools (control platelet concentrates), in order to determine whether clumping affects the in vitro properties of platelets over a sustained storage period.
Materials and methods
Buffy coats and preparation of platelet concentrates
Whole blood units (450±45 mL) were collected from volunteer donors meeting the donation criteria set out by national law and European guidelines. The whole blood was collected into quadruple top-and-bottom bags with in-line, red cell filters (Macopharma Leucoflex LCR). The units were cooled on butane-diol cooling plates following donation and processed within the following 14–18 hours. The units were centrifuged (4,497 g, 18 minutes at 22 °C) and processed for component separation using a CompoMat G5® system (Fresenius Kabi, Bad Homburg, Germany). The buffy coats were left to rest a minimum of 2 hours prior to preparation of the platelet concentrates.
For preparation of the platelet concentrates, five buffy coats were pooled (mean buffy coat volume of 52 mL and haematocrit of 37%) with 280 mL PAS including potassium and magnesium (SSP+; Macopharma). The pool of five buffy coats+PAS-E was processed (centrifuged, separated and filtered) with an automatic OrbiSac system (Terumo BCT, Lakewood, CO, USA). The approximate final PAS/plasma ratio was 65/35. The final product (platelet concentrates) was held 2 hours at rest. Two independent technicians visually inspected the concentrates, assessing swirling and the visible presence of clumps following the rest period, before proceeding with Intercept pathogen reduction technology.
The test platelet concentrates were those presenting non-compact clumps after 2 hours at rest (n=12), which disappeared in a few seconds after manual agitation prior to pathogen reduction. Ten platelet concentrates subjected to pathogen reduction treatment were used as controls of a run, in which pre-treatment inspection was correct, i.e. without the presence of macroscopic agglutinates.
Air bubbles were systematically extracted from all platelet concentrates, in order to avoid negative effects on the platelets’ integrity11. The platelet concentrates were stored in polyolefin bags (PL-2410, 1.3L; Fenwal, La Châtre, France) under standard conditions at 22 °C (Helmer PC 3200 Incubator; Helmer Inc., Noblesville, IN, USA) and with continuous agitation. The study samples were aseptically collected on days 2, 5, 7 and 9.
In vitro parameters of the study
The cell content and mean platelet volume were measured using a Sysmex XT-2000i hematology analyser (Sysmex, Kobe, Japan). The pH was measured at 22 °C with a pH-meter Crison Micro pH 2001 (Crison Instruments S.A., Alella -Barcellona-, Spain). The phenomenon of swirling was visually assessed and given a numerical value of 0–2 (0=no swirling, 1=intermediate and 2=patent swirling) based on previous publications12. Residual leucocytes were enumerated using a Leucocount™ FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Glucose, lactate and lactate dehydrogenase were assayed by an Olympus AU400 Chemistry Analyser (Olympus, Tokyo, Japan). The CD62P activation marker and annexin V were measured by flow cytometry. Samples for cytokine analysis were centrifuged twice and frozen at −80 °C until analysed using enzyme-linked immunosorbent assay (ELISA) kits for soluble P-selectin (sCD62p) and regulated and normal T-cell expressed and secreted (RANTES, also known as CCL5 and soluble CD40 ligand [sCD40L]), following the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Samples were analysed in duplicate.
Statistical analysis
Prior to comparative analysis between groups, goodness of fit to the normal distribution of each parameter evaluated in each group of the study was tested (Kolmogorov-Smirnov).
The between groups comparison was performed using the analysis of variance (ANOVA) for repeated measures, after evaluating the homogeneity of samples on day 2 (two-sided t-test for independent groups). Statistical analyses were performed using SPSS 17.0 software for Windows (SPSS Inc., Chicago, IL, USA). Variables are reported as the mean±standard deviation. The differences were considered statistically significant at the 5% level (p<0.05).
Results
In our experience, randomly occurring clumping appears during production of platelet concentrates before inactivation. The comparison between the two groups (Table I) showed no significant differences in any of the parameters studied. However, all showed significant changes along the storage time, except pH, when the two groups were combined (time effect).
Table I.
Characteristics and activity parameters of platelet concentrates with and without clumping.
| Day of storage* | p value | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 2 | 5 | 7 | 9 | Main effect | Time | Interaction term | ||
| Platelets (×109/L) | T | 1,003.1±111.5 | 988.1±107.9 | 972.6±106.4 | 946.9±91.8 | 0.698 | < 0.001 | 0.824 |
| C | 1,024.8±100.4 | 1,009.8±96.9 | 982.6±92.4 | 971.6±93.0 | ||||
|
| ||||||||
| MPV (fL) | T | 8.8±0.4 | 8.9±0.3 | 9.0±0.3 | 9.2±0.5 | 0.604 | < 0.001 | 0.381 |
| C | 8.9±0.4 | 9.0±0.4 | 9.1±0.4 | 9.4±0.5 | ||||
|
| ||||||||
| pH (22 °C) | T | 7.12±0.04a | 7.18±0.07 | 7.17±0.07 | 7.07±0.07 | 0.060 | 0.204 | 0.017 |
| C | 7.18±0.07a | 7.20±0.07 | 7.15±0.07 | 7.01±0.09 | ||||
|
| ||||||||
| CD62p (%) | T | 10.9±3.8 | 20.0±6.2 | 17.1±1.4 | 23.9±7.2 | 0.119 | < 0.001 | 0.015 |
| C | 7.4±5.2 | 17.9±4.2 | 19.7±2.3 | 35.1±14.3 | ||||
|
| ||||||||
| Annexin V (%) | T | 2.0±1.1 | 4.0±0.8 | 3.1±1.0 | 3.9±2.0 | 0.137 | < 0.001 | 0.037 |
| C | 1.7±0.8 | 4.1±1.2 | 3.3±1.9 | 7.2±4.0 | ||||
|
| ||||||||
| “Swirling” | T | 2.0±0.0 | 2.0±0.0 | 2.0±0.0 | 1.9±0.3 | 0.369 | 0.038 | 0.571 |
| C | 2.0±0.0 | 2.0±0.0 | 2.0±0.0 | 1.7±0.6 | ||||
|
| ||||||||
| Glucose (mmol/L) | T | 6.2±0.4 | 4.9±0.6 | 3.5±0.6 | 1.9±0.8 | 0.231 | < 0.001 | 0.120 |
| C | 6.3±0.4 | 4.7±0.7 | 3.0±0.7 | 1.2±0.9 | ||||
|
| ||||||||
| Lactate (mmol/L) | T | 6.0±0.6 | 8.8±1.3 | 12.0 ± 1.1 | 15.5 ± 2.0 | 0.555 | < 0.001 | 0.415 |
| C | 5.6±0.4 | 9.1±1.4 | 12.4 ± 1.3 | 16.5 ± 2.5 | ||||
|
| ||||||||
| LDH (U/L) | T | 97.3±17.2 | 103.1±15.8 | 114.4 ± 14.3 | 129.1 ± 24.1 | 0.937 | < 0.001 | 0.150 |
| C | 97.6±23.4 | 115.5±48.8 | 117.2 ± 35.9 | 116.8 ± 27.0 | ||||
Data are reported as mean±standard deviation
Significant difference on day 2 between groups (p=0.007).
T: Test platelet concentrates (n=12); C: Control platelet concentrates (n=10); MPV: mean platelet volume; LDH: lactate dehydrogenase.
The initial mean volume and mean platelet content per unit for the test platelet concentrates and control platelet concentrates, as measured following pathogen reduction, were 346±8 mL and 3.5±0.4×1011 and 341±11 mL and 3.5±0.4×1011, respectively.
The platelet concentration decreased by 1–3% over the 7 days of storage and by 6% at day 9. The leucocyte content in all units was lower than 0.5/μL.
The pH value dropped during storage in the two groups, but in every case was maintained above 6.8 at day 9. The pH level was significantly different (p=0.007) between groups on day 2 and was included as a covariate in the repeated measures ANOVA. The mean reduction in pH level in the two groups was significantly different (p<0.05) from day 7 to day 9, being higher in the control group (interaction of group and storage time).
The swirling effect was evident and maintained throughout storage in the two groups, indicating that platelets maintain their discoid shape. No macroscopic agglutinates were observed during storage in either group.
The CD62 protein (P-selectin) is located in the alpha granules of platelets and is expressed on the surface of platelets when they are activated. The CD62 level was not significantly different (p=0.105) between groups on day 2. The mean level increased, relative to storage time in the same way in both groups, although it was significantly higher from day 7 to day 9 in the control group (p=0.015).
Phosphatidylserine expression, quantified by the percentage membrane surface binding of annexin V, remained stable in both groups with a slight, but significant increase in the control group (p=0.037) at the end of storage (day 7 vs day 9). The mean glucose level decreased linearly throughout storage in the same way that the lactate increased (p<0.001). Glucose levels at day 9 were ≥1.5 mmol/L in both groups, except in those units with high platelet counts (≥4×1011).
The lactate dehydrogenase level increased throughout storage and was significantly higher in the test group from day 7 to day 9 (p<0.05). As a percentage, the difference (mean±standard error) was 11.6±5.3% between groups.
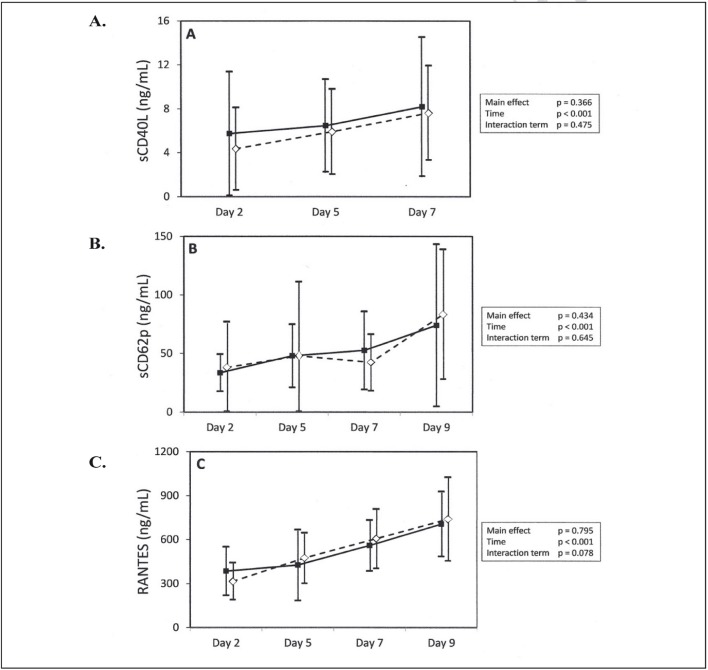
Platelets are the primary source of soluble CD40 ligand (sCD40L, formally known as CD154) and we observed a gradual and moderate increase in levels in both groups of stored platelets (Figure 1A). It was not possible to obtain the values at day 9 for technical reasons.
Figure 1.
Secretion of sCD40L, sCD62p and RANTES/Mean ±95% CI on days 2, 5, 7 and 9 (except sCD40L).
(■) Test platelet concentrates; (⋄) Control platelet concentrates. Statistical analyses performed with repeated-measures ANOVA.
The amounts of sCD40L (A), sCD62p (B) and RANTES (C) increased significantly over time, but the differences between the two groups over time were not significant. CI: confidence interval.
The soluble sCD62 levels eventually increased, as a result of the release of this molecule from the platelet surface. In our study this increase was not different between the two groups of platelet concentrates, being marked from day 2 to 5 and then remaining stable afterwards with the exception of one case on day 9 in the control group (Figure 1B).
The chemokine RANTES, which is stored in alpha granules and released during platelet activation, showed a progressive rise related to the storage time: the increase was similar in both groups (Figure 1C).
Discussion
The use of PAS in platelet concentrates has many advantages including reducing the risk of transfusion complications due to residual substances present in the plasma, saving plasma and improving storage conditions. A good PAS maintains glycolysis at a low level, preventing lactate and hydrogen ion formation and thereby avoiding pH decreases and promoting platelet viability13.
In recent decades, several platelet storage solutions have been developed: these improve platelet function by ensuring a correct metabolic rate, glucose availability and lactate production control. The presence of acetate in PAS (PAS-B) is crucial as it improves the buffering capacity of the medium to produce bicarbonate which facilitates the maintenance of a stable pH level throughout storage14. The addition of phosphate (PAS-C) is associated with higher adenine nucleotide levels which are related to better platelet viability. The inclusion of potassium and magnesium (PAS-E) has a positive effect by decreasing the activation of platelets in addition to other advantages already mentioned. For standard storage conditions, type of bag, PAS and perhaps the pathogen reduction technology, a minimum of 30% residual plasma is needed to ensure optimal platelet quality during storage15.
In the current study PAS-E was used in conjunction with an automated preparation method for the production of platelet concentrates. The presence of clumping was reported in some of the concentrates produced. We think that clumps are basically constituted from platelets and not from plasma proteins because the plasma proportion is quite low (35%). These non-compacted clumps disappeared with manual stirring prior to processing for pathogen reduction. To determine whether the existence of “non-compacted” clumps affects platelet functionality, metabolic and activation parameters were studied in the two groups of platelet concentrates (test and control). Clumping may be influenced by physical, chemical and metabolic factors, related to a more alkaline pH, preferential anaerobic metabolism, a high content of lactate, temperature, volume, agitation, the plastic bag wall elasticity, infections, the use of antibiotics and anti-inflammatory drugs and previous damage caused by the separation process. In fact, the automatic production method has been associated with increased mechanical stress and consequent increased platelet activation16.
Our results confirmed a good in vitro quality of the platelets. The platelets stored in PAS-E maintain their count, glucose levels and low activation during storage, as already reported7.
With regards to lactate dehydrogenase, a marker of platelet disintegration or cell lysis, previous studies confirmed increasing levels of this cytosolic enzyme due to extended storage of platelets17. In our study, the mean difference in levels of this enzyme between the test platelet concentrates and the control concentrates was small but increased along the storage.
Leakage of lactate dehdyrogenase from platelets reveals loss of integrity of the platelet membrane suggesting decreased viability and increased lysis of platelets. After a relatively rapid increase, test platelet concentrates showed higher levels of platelet activation at the beginning of storage (day 2) as evidenced by a greater proportion of platelets expressing CD62P. This may reflect an effect of additional manipulation of test platelet concentrates in order to disperse platelet clumps. However, CD62P expression remained low and lower than reported with PAS-C18. The clinical impact of this phenomenon is not known, as it is not clear whether the level of in vitro activation of stored platelets is correlated with in vivo survival or the haemostatic function of platelets after transfusion.
In accordance with previous studies, annexin V was slightly increased at the end of storage19, but more in the control group. Phosphatidylserine is expressed upon platelet activation and plays an important role in the process of caspase-dependent apoptosis: it has, therefore, been considered a possible indicator of this process20.
The sCD40L increase was comparable to that found in other recent studies in which pathogen reduction technology was applied21. sCD40L is a potent immunomodulator that accumulates in platelets along storage22, acts as inflammatory mediator and could be implicated in some post-transfusion adverse reactions, although this effect has not been demonstrated in all instances23.
A recent study quite similar to ours showed increased release of immunomodulatory factors and platelet activation associated with random aggregates in platelet units24. The differences were observed from day 2 of storage and seemed to be maintained at the same rate throughout storage. This could indicate that the aggregation phenomena occurred early but the differences did not subsequently increase. However, it is difficult to attribute the differences to pathogen reduction technology, because the clumps were present before its application and the increase was similar in our study groups.
The use of PAS-E as a storage medium does not seem to play an important role since the units are randomly affected and clumping does not happen during storage. We think that greater activation of platelets is related to the appearance of clumps but other factors are the cause. Further research involving donor factors or the pooling processing system might shed light on this matter. These issues were beyond the scope of our study.
A major limitation of our study is that no in vivo measurements were done to investigate whether platelet properties were negatively affected during storage. We did not analyse isolated agglutinates either. Future studies should address such issues.
Conclusion
In summary, with PAS-E containing potassium and magnesium, glycolysis activity decreases, glucose is maintained within appropriate levels until the end of extended storage and there is a decrease in platelet activation. The presence of “non-compacted” clumps in platelet concentrates did not appear to significantly affect the quality of platelets suspended in plasma and PAS-E and treated with pathogen reduction technology.
Acknowledgements
This work was supported by a grant from MacoSpania, SL for laboratory testing.
Footnotes
Authorship contributions
AC designed the study. All the Authors were involved in writing the paper.
Conflict of interest
AC declares that she has no conflicts of interest regarding this study. IA and FT are employees of Macopharma SA.
References
- 1.Yuasa T, Ohto H, Yasunaga R, et al. Improved extension of platelet storage in a polyolefin container with higher oxygen permeability. Br J Haematol. 2004;126:153–9. doi: 10.1111/j.1365-2141.2004.04994.x. [DOI] [PubMed] [Google Scholar]
- 2.Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 °C: development and current experience. Transfus Med Rev. 2006;20:158–64. doi: 10.1016/j.tmrv.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Castrillo A, Arcas C, Castro A, et al. In vitro function of PC stored in ssp+ platelet additive solution. Vox Sang. 2009;96:246–7. [Google Scholar]
- 4.Castrillo A, Arcas C, Martínez N, et al. In vitro quality of platelet concentrates treated with Theraflex UVC system. Transfusion. 2009;49(S3):91. [Google Scholar]
- 5.Castrillo A, Arcas C, Castro A, et al. In vitro quality of platelet concentrate from buffy coat and apheresis in additive solution, treated with two pathogen inactivation systems. Vox Sang. 2010;99:249–50. [Google Scholar]
- 6.Tynngård N, Trinks M, Berlin G. In vitro properties of platelets stored in three different additive solutions. Transfusion. 2012;52:1003–9. doi: 10.1111/j.1537-2995.2011.03417.x. [DOI] [PubMed] [Google Scholar]
- 7.Gullikson H, AuBuchon JP, Vesterinen M, et al. Storage of platelets in additive solutions: a pilot in vitro study of effects of potassium and magnesium. Vox Sang. 2002;82:131–6. doi: 10.1046/j.1423-0410.2002.drfgv158.x. [DOI] [PubMed] [Google Scholar]
- 8.Chavarin P, Cognasse F, Argaud C, et al. In vitro assessment of apheresis and pooled buffy coat platelet components suspended in plasma and SSP+ photochemically treated with amotosalen and UVA for pathogen inactivation (INTERCEPT Blood System™) Vox Sang. 2011;100:247–9. doi: 10.1111/j.1423-0410.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- 9.Ringwald J, Antoon M, Eckstein R, Cardoso M. Residual aggregates in platelet products: what do we know? Vox Sang. 2013;20:158–64. doi: 10.1111/vox.12089. [DOI] [PubMed] [Google Scholar]
- 10.Friedlander I, Cook IJ, Hawkey C, Symons C. A laboratory study of spontaneous platelet aggregation. J Clin Path. 1971;24:323–7. doi: 10.1136/jcp.24.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandgren P, Saeed K. Storage of buffy-coat-derived platelets in additive solution: in vitro effects on platelets of the air bubbles and foam included in the final unit. Blood Transfus. 2011;9:182–8. doi: 10.2450/2010.0045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolini F, Murphy S. A multicentre evaluation of reproducibility of swirling in platelet concentrates. Biomedical Excellence for Safer Transfusion (BEST) Transfusion. 1994;34:796–801. doi: 10.1046/j.1537-2995.1994.34994378282.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy S. The efficacy of synthetic media in the storage of human platelets for transfusion. Transfus Med Rev. 1999;13:153–63. doi: 10.1016/s0887-7963(99)80029-7. [DOI] [PubMed] [Google Scholar]
- 14.Gullikson H. Additive solutions for the storage of platelets for transfusion. Transfus Med. 2000;10:257–64. doi: 10.1046/j.1365-3148.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- 15.Van der Meer PF, Pietersz RNI, Reesink HW. Storage of platelets in additive solution for up to 12 days with maintenance of good in vitro quality. Transfusion. 2004;44:1204–11. doi: 10.1111/j.1537-2995.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- 16.Vetlesen A, Mirlashari MR, Ezligini F, Kjeldsen-Kragh J. Evaluation of platelet activation and cytokine release during storage of platelet concentrates processed from buffy coats either manually or by the automated Orbisac system. Transfusion. 2007;47:126–32. doi: 10.1111/j.1537-2995.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 17.Hornsey VS, McColl K, Drummond O, et al. Extended storage of platelets in SSP+ platelet additive solution. Vox Sang. 2006;91:41–6. doi: 10.1111/j.1423-0410.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 18.Diedrich B, Sandgren P, Jansson B, et al. In vitro and in vivo effects of potassium and magnesium on storage up to 7 days of apheresis platelet concentrates in platelet additive solution. Vox Sang. 2007;94:96–102. doi: 10.1111/j.1423-0410.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 19.Saunders C, Rowe G, Wilkins, et al. In vitro storage characteristics of platelet concentrates suspended in 70% SSP+™ additive solution versus plasma over a 14-day storage period. Vox Sang. 2011;101:112–21. doi: 10.1111/j.1423-0410.2011.01468.x. [DOI] [PubMed] [Google Scholar]
- 20.Kile BT. The role of the intrinsic apoptosis pathway in platelet life and death. J Thromb Haemost. 2009;7:214–7. doi: 10.1111/j.1538-7836.2009.03366.x. [DOI] [PubMed] [Google Scholar]
- 21.Vetlesen A, Mirlashari MR, Akkök CA, et al. Biological response modifiers in photochemically pathogen-reduced versus untreated apheresis platelet concentrates. Transfusion. 2013;53:147–55. doi: 10.1111/j.1537-2995.2012.03681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cognasse F, Boussoulade F, Chavarin P, et al. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–9. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 23.Wenzel F, Günter W, Baertl A, et al. Platelet transfusion alters CD40L blood level and release capacity in patients suffering from thrombocytopenia. Transfusion. 2012;52:1213–20. doi: 10.1111/j.1537-2995.2011.03438.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandgren P, Meinke S, Eckert E, et al. Random aggregates in newly produced platelet units are associated with platelet activation and release of immunomodulatory factors sCD40L and RANTES. Transfusion. 2014;54:602–12. doi: 10.1111/trf.12345. [DOI] [PubMed] [Google Scholar]