Abstract
Background
Dental extractions in haemophiliacs may cause secondary bleeding, requiring repeated surgical and haematological interventions. As a local haemostatic, fibrin glue has recognised efficacy but, as a plasma-derived product, it carries the risk of viral infections. We, therefore, compared fibrin glue with an autologous haemostatic, plasma rich in growth factors (PRGF), in a controlled trial.
Material and methods
One hundred and twenty patients with different blood disorders were randomised into two cohorts to undergo dental extraction procedures without hospitalisation. Prior to the extractions, patients underwent systemic haematological treatment. Complications were defined as secondary bleeding after the 7-day follow-up period or protracting after the repair procedure.
Results
There were 106 extractions (7 retained 3rd molars) in the group managed with fibrin glue: secondary bleeding affected 3/60 patients (5%) on the third day after extraction and necessitated additional surgery and systemic treatment (in one case the procedure had to be repeated on the 7th day). In the PRGF arm there were 98 extractions (23 retained 3rd molars): secondary bleeding affected two patients (3.3%) on the first day after extraction and was arrested with surgery without systemic treatment. Four out of the five secondary bleeds occurred in patients with haemophilia A. Concomitant diabetes or liver disease significantly increased the bleeding risk.
Discussion
The bleeding rates in the study and control arm prove that PRGF works as well as fibrin glue as a local haemostatic. Further assets are that PRGF has autologous origin, does not require additional systemic treatment in post-extraction repair surgery, is associated with an earlier onset of neo-angiogenesis and, overall, can reduce patients’ distress and costs to the health system.
Keywords: platelet-rich plasma, fibrin tissue adhesive, blood coagulation disorders, tooth extraction
Introduction
Teeth extractions in people affected by hereditary bleeding disorders pose several problems to both oral surgeons and haematologists because of possible post-procedural bleeding, which may go on for days, requiring repeated surgical and haematological interventions, and in several cases even prolonged hospitalisation and transfusions with considerable costs and aggravation for the patients. The few studies on this issue agree on the fact that the surgical dental management of patients with hereditary bleeding disorders requires close cooperation between haematologists and oral surgeons, with a multidisciplinary approach including pre-surgical haematological preparation and appropriate peri-operative local haemostasis1,2.
Currently there are a range of treatment options for the pre-surgical haematological preparation, such as recombinant and plasma-derived factor concentrates, desmopressin and antifibrinolytic agents.
Local haemostatic treatment is always necessary as a supplement to systemic replacement therapy and in mild bleeding disorders may be sufficient in itself to ensure adequate haemostasis. Its scope is to seal the wound and promote clotting and wound healing. There are many possible approaches to local haemostasis: the choice depends on the clinical history of the patient and the associated presumed bleeding risk, and must balance different and often contrasting factors. Oxidized cellulose works well in several low-risk cases; fibrin glue (FG), which acts as a scaffold for the cells that will form the clot, is advised for high-risk patients or patients in whom other haemostatic measures fail1–3.
The drawback of FG is that is a plasma-derived product, and thus may carry the risk of viral infections. Indeed, there is at least one documented case of human immunodeficiency virus transmission from using a homologous cryoprecipitate-based form of FG4. In spite of the fact that nowadays blood products, including FG, are subjected to measures of viral inactivation sufficiently secure against viruses such as human immunodeficiency virus, hepatitis C virus and hepatitis B virus, the risk of transmission of and infection by pathogens such as parvovirus and prions remains a potential threat that cannot be eliminated by existing technologies. To increase viral safety, international guidelines recommend preferring, when available, recombinant clotting factors over plasma-derived products, particularly in the treatment of previously untreated children. Many doctors tend to ignore the fact that patients treated life-long with recombinant coagulation factors get exposed sporadically to FG, which, being a plasma-derived blood product, may undermine the benefits of recombinant products5.
In the last few years the Oral Surgery Section of our local joint Hospital-University Institution has accrued considerable experience in dealing with patients prone to abnormal bleeding or difficult healing because of specific pathologies (diabetes, thrombocytopenia) or bone problems (bisphosphonate-related osteonecrosis of the jaw, history of radiotherapy for head and neck cancer)6–9; all these cases were successfully managed using preparations of plasma rich in growth factors (PRGF) as the local haemostatic. This platelet gel is a highly concentrated form of autologous platelets, which are known to have a crucial role in haemostasis; they prevent blood loss at sites of vascular injury by adhering, aggregating, and forming a pro-coagulant surface, leading to thrombin generation and fibrin formation. Platelets also release growth factors and active metabolites that promote tissue repair and influence the reactivity of vascular cells and other blood cells in angiogenesis and inflammation, so are useful in clinical situations requiring rapid healing and tissue regeneration. Exogenous bovine thrombin is not used as an activator of PRGF, thus avoiding the risk of immunological reactions and disease transmission7,10.
After PRGF became the standard approach used in our structure for patients with acquired coagulation disorders, the next natural step was to wonder whether it could be viable also for patients with congenital disorders who were all being routinely treated with FG according to a protocol established jointly some years ago by the Oral Surgery Section and the Haemophilia Care Centre in an attempt to minimise bleeding and hospital admissions.
We, therefore, planned a prospective study aimed at testing our hypothesis that patients treated with PRGF could have an outcome comparable to that of patients treated with FG as far as concerns incidence of severe secondary bleeds, defined as blood loss not manageable by the patient, which continued in the days following the extraction and required re-intervention by the surgeon in order to remove the built up necrotic clot and reseal the wound.
The primary goal of our investigation was answering the following questions. (i) Does PRGF yield the same results as FG in preventing secondary bleeding and promoting tissue repair? (ii) Is PRGF effective also in the most severe forms of blood disorders? (iii) What happens in the presence of clotting factor inhibitors? (iv) In the case of protracted bleeding necessitating a second surgical procedure, can PRGF avoid the administration of the additional haematological preparation used with FG?
Secondary goals of the trials were to determine whether severe bleeding is affected differently in one blood disorder over others, or in one kind of dental extraction (single/multiple, retained/not retained, molar/not molar) over others and, finally, whether the concomitant presence of pathologies such as diabetes, liver diseases and chronic renal failure might represent an additional risk factor.
Materials and methods
Study design and patients’ samples
Our independent prospective study was designed to recruit 120 consecutive patients referred by the Haemophilia Care Centre to the Oral Surgery Section who met precise inclusion and exclusion criteria. Patients with all types of inherited bleeding disorders were to be accepted, but to be enrolled in the study patients had to require one or more dental extractions because of root or crown fractures, not restorable caries, residual roots and periodontal and endodontic abnormalities. Since blood samples must be taken to produce PRGF, patients positive for hepatitis B or C virus were excluded from the trial. No exclusion criteria were applied regarding age, sex or presence of comorbidities such as diabetes, kidney failure and liver diseases, as long as these last were not related viral hepatitis.
Prior to surgical teeth extractions, the Haemophilia Care Centre administered systemic haematological treatment to all the patients. This treatment was specifically designed for their blood diseases according to the guidelines approved by the Italian Association of Haemophilia Centres and by the World Federation of Haemophilia.
At their first attendance at the Oral Surgery Unit, patients were randomly assigned to one of the two arms of the trial in which they were to be managed with either FG or PRGF. Patients needing more than one surgical procedure were to retain the original protocol assignation for all the procedures.
All patients were informed about the nature of the study and had to explicitly express willingness to cooperate with the study protocol and follow-up programme and sign an informed consent form. Patients who were to be treated with FG were given the standard consent form used in the last years in all cases of teeth extractions in haemophiliacs, since there was no change to the extraction protocol followed in the last years in our Dental School for these patients. Patients who were to be treated with PRGF received extensive information on the approach, its features and its positive performance in patients with acquired blood coagulation problems and were given the possibility to withdraw from the study and be treated as usual with FG. None of them refused the PRGF approach and afterwards all expressed satisfaction with the procedure and its outcome.
The study conformed with the Helsinki Declaration of 1975 and subsequent modifications.
Pre-operative haematological management
Systemic haemostatic treatment included oral antifibrinolytic agents [tranexamic acid: Tranex (Malesci, Florence, Italy), Ugurol, (Bayer, Berkeley, CA, USA)] at a dose of 25 mg/kg every 8 hours for at least 5 days. In patients with mild/moderate haemophilia A or type I von Willebrand’s disease with a prior favourable response, the treatment was complemented with desmopressin (Emosint®; Kedrion, Castelvecchio Pascoli, Barga [Lucca] Italy) given as a single subcutaneous injection about 30–60 minutes before the dental procedure at a dose of 0.3 μg/kg. In severe congenital bleeding disorders desmopressin was substituted by specific replacement therapy with a plasma-derived or recombinant form of the deficient factor. The dosage of the deficient factor was calculated to ensure a peak value greater than 50% in the peri-operative period and in the 3 days following the surgical procedure.
Patients with haemophilia A with inhibitors were protected before the procedure by administration of recombinant activated factor VII (rFVIIa) (NovoSeven®; Novo Nordisk A/S, Bagsvaerd, Denmark). rFVIIa was administered as a bolus dose of 90 μg/kg in close temporal proximity with respect to the single dental extraction.
Surgical procedure
All teeth extractions were carried out as outpatient procedures by the same oral surgeon who had great expertise with both local haemostatic approaches.
In all cases, local anaesthesia (mepivacaine 3%) was achieved: nerve root trunk infiltration was chosen for the extraction of inferior second premolars and molars whereas plexus infiltration was chosen in all other cases. Adrenaline was used as a vasoconstrictor in cases of plexus infiltration. The teeth were extracted in a non-traumatic manner, with rotation and traction movements using dental forceps and an elevator. The alveolar sockets were sutured with 4/0 silk thread, with the suture to be removed after 7 days. A non-resorbable suture was used to prevent inflammatory reactions due to the resorption, which may enhance the fibrinolytic response according to Franchini et al.
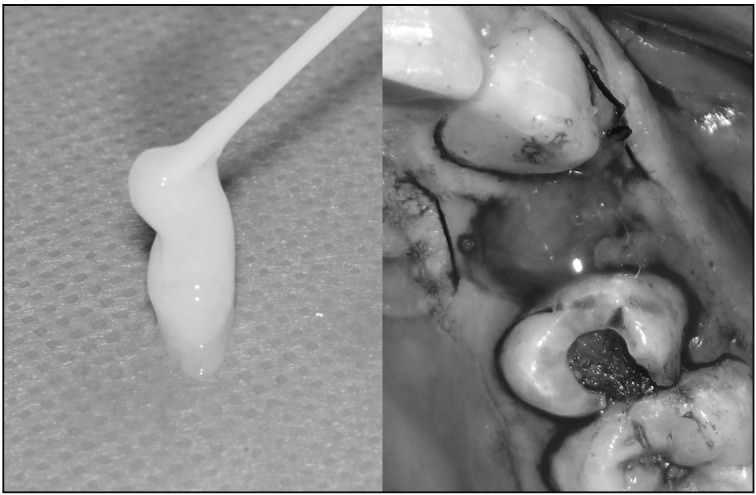
Patients assigned to the FG group had their alveolar sockets closed with FG (TISSEEL, Baxter AG, Rome, Italy). This is a two-component mixture, in which concentrated fibrinogen, factor XIII and fibronectin are added to thrombin, calcium chloride, and an inhibitor of fibrinolysis to form a fibrin clot. Figure 1 shows the two phases of applying FG.
Figure 1.
Fibrin glue and its application in an alveolar socket.
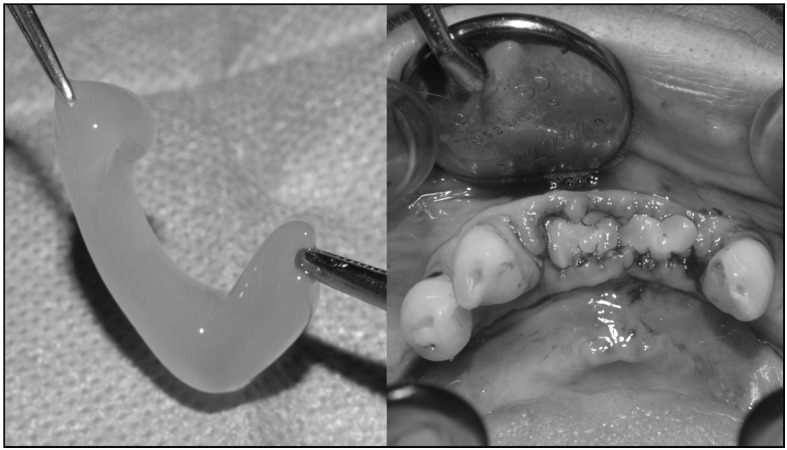
The PRGF to be used for filling sockets was obtained as follows. Prior to the tooth extraction, 10–20 mL of blood were drawn from each patient using 5 mL tubes containing 3.8% trisodium citrate solution as anticoagulant. The tubes were centrifuged at 1,800 rpm for 8 minutes (PRGF System, BTI Biotechnology Institute, Milan, Italy) at room temperature. The blood separated into its three basic components: red blood cells at the bottom of the tube, PRGF in the middle of the tube, and plasma poor in growth factors at the top of the tube. The fraction (0.5 mL) located immediately above the erythrocytes was collected from each tube and transferred to sterile tubes. To each 1 mL fraction of PRGF, 30 mL of calcium chloride 10% were added. After 15 to 20 minutes, the PRGF gel was formed and was ready to be introduced into the post-extraction socket. The time between PRGF gel formation and socket filling was standardised to be between 5 and 10 minutes. Figure 2 shows the two phases of applying PRGF.
Figure 2.
PRGF and its application in alveolar sockets.
Post-operative follow-up
The same post-operative instructions were given to all patients, regardless of the protocol adopted. Patients were advised to avoid hot food and drinks until normal feeling had returned and possibly avoid smoking as this can hinder healing. Vigorous mouthwashes were to be avoided since they could dissolve the clot and take away the haemostatic gel (either PRGF or FG), placed to protect the wound. Gentle mouthwashes were allowed to spread and swallow the antifibrinolytic agent. The analgesic therapy included paracetamol 1,000 mg (1 tablet, twice daily for 2 days). Patients were told to report immediately to the haematologist in the case of prolonged bleeding and/or difficulty in speaking, swallowing, or breathing.
The dental surgeon who performed the procedures was in charge of the follow-up visits on days 1, 3 and 7 after the extractions, to assess the healing and manage any possible bleeding and/or hematoma. In the case of severe bleeding, FG patients were to receive an additional infusion of their haematological preparation and surgical intervention with local application of FG, in accordance with Franchini et al.1; in contrast, PRGF patients were to be treated only locally, with a second application of PRGF to the wound.
Complications were defined as a need for surgical interventions after the standard 7-day follow-up period or work on the same socket more than once in order to arrest bleeding.
Statistics
The predictor variable was the treatment status: PRGF vs FG. Within each cohort, the primary outcomes of interest were incidence of severe secondary bleeds at one or more than one of the three follow-up sessions and complications. Adverse events were studied as a function of baseline variables: severity of the congenital blood disorder, presence of factor inhibitors, age, gender, teeth to be extracted (3rd molar/retained), and comorbid conditions (diabetes, liver diseases and chronic renal failure).
Continuous variables, reported as mean±standard deviation, were compared with the non-parametric Mann-Whitney test. Binary and categorical variables, reported as counts and percentages, were arranged in R×C contingency tables and studied with the chi square test (with Yates’ correction for 2×2) or, when appropriate, with Fisher’s exact test. The risk ratio was computed with its 95% confidence interval (95% CI). Statistical significance was set at a two-tailed p value <0.05.
Results
The recruitment target of 120 patients, which corresponded to 149 surgical procedures (38 multiple and 111 single ones) and 204 extractions, was reached in about 2 years. Table I reports and compares the details of the baseline features of the populations enrolled in the two arms of the study (FG and PRGF protocols) divided according to the four types of blood disorders: type A haemophilia (47/120=39.2%), von Willebrand’s disease (53/120=44.2%), type B haemophilia (11/120=9.2%) and factor V/XI deficiency (9/120=7.5%).
Table I.
Baseline variables.
| Type A haemophilia | Von Willebrand’s disease | Type B haemophilia | Factor V/XI deficiency | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| FG | PRGF | FG | PRGF | FG | PRGF | FG | PRGF | |
| Overall number | 26 | 21 | 26 | 27 | 4 | 7 | 4 | 5 |
| mild | 10 (38%) | 10 (48%) | 18 (69%) | 23 (85%) | 2 (50%) | 3 (42%) | 2 (50%) | 2 (40%) |
| moderate | 7 (27%) | 5 (24%) | 6 (23%) | 4 (15%) | 1 (25%) | 2 (29%) | 1 (25%) | 2 (40%) |
| severe | 9 (35%) | 6 (28%) | 2 (8%) | 0 (0%) | 1 (25%) | 2 (29%) | 1 (25%) | 1 (20%) |
| Factor VIII inhibitor | 0 | 2 (9.5%) | n.a. | n.a. | n.a. | n.a | n.a. | n.a. |
| Age range (years) | 10–71 | 17–74 | 6–77 | 17–77 | 22–57 | 13–65 | 16–78 | 18–71 |
| Females n. (%) | n.a. | - | 12 (46%) | 18/27 (6) | 1 (25%) | 1 (20) | 3 (75) | 2 (40%) |
| Comorbidities | 10/26 (38) | 3/21 (14) | 2/26 (8) | 2/27 (7) | 0 | 0 | 0 | 0 |
| Single procedures | 19 | 19 | 26 | 24 | 4 | 6 | 5 | 8 |
| Multiple procedures | 8 | 5 | 12 | 8 | 0 | 3 | 0 | 2 |
| Extractions | 41 | 29 | 56 | 41 | 4 | 13 | 5 | 15 |
| 3rd molars (retained) | 7 (2)* | 13 (8)* | 16 (4)* | 23 (6)* | 2 | 4 (1) | 3 (1) | 8 (1) |
| Extractions/procedure | 1–9 | 1–2 | 1–3 | 1–2 | 1 | 1–3 | 1 | 1–3 |
Statistically significant (p<0.05).
n.a: not applicable; FG: fibrin glue; PRFG: plasma rich in growth factors.
The patients in the two arms were comparable as far as concerns age, gender and presence of comorbid conditions (diabetes, liver diseases and kidney failure), but the randomisation procedure assigned the two patients with type A haemophilia and factor VIII inhibitors to the PRGF arm.
The numbers of single and multiple dental procedures were 54 and 20, respectively, in the FG arm and 57 and 18 in the PRGF arm. There were 106 extractions in the FG arm and 98 in the PRGF arm: the latter however included significantly more third molars (48 against 28) and, furthermore, one-third of them were retained (p=0.001).
All the surgical procedures were carried out as outpatient interventions and included, on average, one to three extractions. One patient with severe type A haemophilia in the FG arm had to undergo nine dental extractions in one procedure (no 3rd molars) because of his urgent need to undergo prosthetic rehabilitation.
Additional surgical interventions for severe and protracted secondary bleeding were required by three patients in the FG cohort and two in the PRGF cohort. The relevant features of the five bleeding cases are shown in Table II.
Table II.
Cases of secondary bleeding.
| Bleeding disorder | type A haemophilia | type A haemophilia | type A haemophilia | type A haemophilia | von Willebrand’s disease |
|---|---|---|---|---|---|
|
|
|||||
| FG | PRGF | ||||
| Severity | mild | severe | severe | severe | type 2 |
| Age | 31 | 53 | 60 | 74 | 52 |
| Comorbidity | none | liver disease | liver disease | diabetes | none |
| Tooth extracted | 3rd molar (retained) | molar | 3rd molar (retained) | 3rd molar | premolar |
| Procedure | single | single | single | multiple | single |
| Bleeding occurred on | day 3 | day 3 | days 3–7 | day 1 | day 1 |
| Complications | none | none | 2 interventions | none | none |
FG: fibrin glue; PRFG: plasma rich in growth factors.
The bleeding rate in the FG arm was 3/60 (5.0%) computed over patients, 3/74 (4.1%) over surgical procedures and 3/106 (2.8%) over extractions. In one case (retained 3rd molar and liver comorbidity) bleeding continued well after administration of additional haematological preparations and surgical intervention on the 3rd day and was still present on the 7th day, when it was finally arrested after a third surgical intervention and third administration of haematological systemic therapy.
The bleeding rate in the PDGF arm was 2/60 (3.3%) over patients, 2/75 (2.7%) over surgical procedures and 2/98 (2.0%) over extractions. The 74-year-old bleeding patient had another double third molar extraction soon after with no bleeding problem. Both bleeds were arrested immediately after the surgical intervention on day 1, without administration of a second haematological preparation.
Low bleeding and complication rates, while being good for patients, are bad for statistical tests, which lose power and may become blinded even to real differences. So, the statistical comparison of the rates of major bleeding for the two protocols yielded a p value >0.99, and the situation was similar for the complication rates; however the test power was below 10%, and the sample size necessary to reach the desired 80% power is preposterously high, being around 3,000 patients per arm.
The pathology that caused four out of the five secondary bleeds observed in the trial was type A haemophilia. Considering the number of patients, this means 4/47 cases (8.5%) vs 1/73 cases (1.4%) for the other blood diseases; considering the number of extractions, the rate was 4/70 (5.7%) vs 1/136 (0.7%). The bleeding rate for type A haemophilia appear definitely higher than the rates for the other three types of blood disorders, although this difference lacks statistic confirmation (p=0.15 for patients and p=0.09 for extractions).
As far as regards the type of dental extraction, the bleeding rate following all third molar extractions was 3/76 (3.9%), whereas that following extraction of other teeth was 2/130 (1.5%) (p=0.53). Within the third molar extractions, the bleeding rate for retained ones was 2/23 (8.7%) whereas that for not retained ones was 1/53 (1.9%) (p=0.45). Finally the bleeding rate for multiple procedures was 1/38 (2.6%), while that for single procedures was 4/111 (3.6%) (p=0.81). Needless to say, the power of all these tests was always <10%, thus absolutely insufficient to allow statistically sound conclusions to be drawn.
The bleeding rate for patients with diabetes or liver disease was 3/22 (13.6%), which was higher than the rate of 2/98 (2.0%) for patients without these comorbid conditions (p=0.06, borderline significance) but the risk ratio was 6.7 and the whole of the 95% CI was above 1 (1.2–37.6), indicating that the presence of either one of these two comorbidities may actually be associated with a higher (more than 6-fold) risk of bleeding.
Discussion
The main goal of our trial was to determine the validity of our hypothesis that PRGF could be used as local haemostatic for dental extractions in haemophiliac patients as an alternative to FG, historically the standard in our structure for its good haemostatic performance but with possible risks of viral infections.
The trial ran for 2 years and involved 120 patients randomised into two arms according to the local haemostatic used, for a total of 149 surgical procedures and 204 dental extractions. The trial also included patients with factor inhibitors (2 out of 120) and patients who required extraction of retained third molars (23 out of 204). The same surgeon carried out all surgical procedures in order to assure uniform surgical know-how. All procedures, including re-interventions for secondary bleeding, were performed in an outpatient setting with local anaesthesia.
The randomisation process led to a slightly unbalanced division of patients and extractions against the PRGF arm, which happened to include the only two patients with factor VIII inhibitors, while none was randomised to the FG arm; the number of extractions of third molars was also larger in the PRGF arm than in the FG arm (48/98 [49%] vs 28/106 [26%]), aggravated by a higher proportion of retained third molars (16/98 [16%] vs 7/106 [7%]). All these differences were statistically significant (p<0.001)
Despite these unfavourable baseline characteristics, the rate of severe secondary bleeding among patients treated with PRGF was 2/60 (3.3%) (2/98 [2.0%] over extractions), whereas it was 3/60 (5.0%) (3/106 [2.8%] over extractions) for patients treated with FG.
A relevant finding is that neither of the two patients with severe type A haemophilia with factor VIII inhibitors treated locally with PRGF developed secondary bleeding. This is particularly significant when one considers the specific vulnerability of rFVIIa to oral mucosal bleeding. We also underline the good performance of PRGF in the 27 patients with von Willebrand’s disease. In fact, a characteristic of this disease is impaired platelet adhesiveness related to a deficit of von Willebrand factor. In our cohort, PRGF obtained from patients with von Willebrand’s disease maintained good haemostatic properties.
Even if sound statistical conclusions on the equivalence of the bleeding rates in the two cohorts are precluded by the scarcity of the adverse events, we feel that we can affirm that the haemostatic efficacy of PRGF is at least equivalent to that of FG, with two differences that were highly appreciated by patients.
First of all, in the patients with secondary bleeding treated with PRGF, the bleeding occurred in the 24 hours after extraction, while in those treated with FG it occurred 3 days after surgery. Early bleeding is generally better managed by patients, who are still in a delicate stage of post-operative recovery; several days after surgery patients have normally resumed their normal activities and find having to face severe bleeding and outpatient visits for new interventions more disruptive.
The risk of haemorrhage for haemophiliac patients is maximum when neo-angiogenesis occurs. Normally this is presumed to happen a few days after surgery which is also when the impact of the dosage of factor, at its maximum in the peri-operative period and in the 3 days following the surgical procedure, starts to fade away. The time of onset of bleeding in our patients treated with FG is consistent with this hypothesis and with what reported by Franchini et al. for their patients, also treated with fibrin glue1.
The earlier bleeding in the patients treated with PRGF might indicate an earlier onset of neo-angiogenesis, triggered by the ability of PRGF to accelerate the healing process of tissues greatly, an effect already proven in several studies11–15. Secondly, the two bleeds in the patients treated with PRGF were arrested with second surgical interventions with reapplication of PRGF, without need for an additional systemic therapy which was, instead, required for the three cases of secondary bleeding treated with fibrin glue. Indeed, in one of the latter cases, the bleeding initiated on the 3rd day was not solved by systemic therapy and surgical re-intervention, but continued until the 7th day, when it was finally arrested after a third surgical intervention and third additional haematological systemic therapy.
Considering the ensemble of the two arms, we obtain an overall success rate of 115/120 (95.8%; 95% CI: 90.5–98.6) over patients, 144/149 (96.6%; 95% CI 92.3–98.9) over surgical procedures (107/111 [96.4%] for single and 38/39 [97.4%] for multiple ones) and 199/204 (97.5%; 95% CI: 94.4–99.2) over extractions. The idea that type A haemophilia was the blood disorder with the highest bleeding rate could not be supported by statistics because of the small number of adverse events involved. We did, however, find a borderline significance for an additional bleeding risk connected to the presence of concomitant pathologies (liver disease and diabetes).
Our overall results on secondary bleeding are not significantly different from those found in the normal healthy population and concordant with those reported in the scarce literature on this subject, with due attention to the different conditions of the various studies.
Benoit et al.16 reported four bleeding complications after 103 extractions in 93 patients (4%); their study did, however, exclude patients with inhibitors, and all patients were hospitalised for a period of 1.41±0.7 days, except for the two patients who required additional treatment (2.5 days).
Franchini et al.1 included patients with inhibitors and used local therapy with antifibrinolytic drugs (tranexamic acid) and FG, as in our FG group. They reported eight bleeding complications out of 121 single dental extractions, 98 multiple dental extractions and 21 retained teeth extractions, with the bleeding events developing about 5–7 days after surgery. All bleeding complications were managed with additional FG and systemic therapy (DDAVP or coagulation factor concentrates) without hospitalisation. Their bleeding incidence over surgical procedures is equivalent to that observed in our FG cohort: 8/240 (3.3[1.5–6.5]%) vs 3/74 (4.1[0.8–11.4]%).
In the study by Peisker et al.17 secondary bleeding occurred in two patients with mild haemophilia out of 15 patients (13%) who underwent 58 dental extractions (3%). All patients were hospitalised for a mean period of 6 days, but it is not clear whether patients with inhibitors and/or those undergoing extraction of retained third molars were included.
Conclusion
Our study allows us to encourage the use of PRGF as an effective and innovative haemostatic agent for the local treatment of patients with congenital coagulation disorders, since the capacity of PRGF to prevent secondary bleeding is definitely not inferior to that of FG, and the product has several advantages: (i) it is an autologous material that does not expose the patient to a risk of infection, thus satisfying the requirement of viral safety; (ii) it actually accelerates wound healing and tissue repair; even the bleeding of the 74-year old patient with severe type A haemophilia and diabetes who underwent extraction of two third molars was easily arrested after the second intervention with application of PRGF (and no systemic treatment) and, last but not least; (iii) it costs significantly less than FG, both as a haemostatic material in itself and as a way to avoid additional administration of haematological preparations in the case of secondary bleeds. The cost of a dedicated centrifuge is in fact rapidly amortised by the money saved from using a product that avoids the purchase of large quantities of FG and indirectly reduces the consumption of coagulation factors.
We would like to make a final consideration on preventive measures to reduce the need for dental extractions, which in patients with blood disorders is even higher than in the normal population, is needed. Dental problems are very often an unfortunate consequence of the fact that patients with coagulopathies suffer from spontaneous gingival bleeding and may, therefore, limit daily oral hygiene for fear of inducing extra bleeding. The fact that, in recent years, both dentists and haematologists have been taking particular care in instructing patients about effective, safe oral hygiene as prevention for the onset of caries and other pathologies should, it is hoped, lead to a reduction in the need for dental extractions.
Footnotes
Authorship contributions
NC designed and coordinated the study, performed the operations, supervised collection of the data and wrote a large part of the manuscript. FP assisted NC in the surgical procedures, collected and organised data. LB designed the study, analysed and interpreted the data, performed the statistical analysis and wrote a large part of the manuscript. MMo conceived the study and critically reviewed the final manuscript. MMe was in charge of the haematological aspects of the patients’ care and critically reviewed the final manuscript. BP was in charge of the haematological aspects of the patients’ care, wrote part of the paper and critically reviewed the final manuscript.
The Authors declare no conflicts of interest.
References
- 1.Franchini M, Rossetti G, Tagliaferri A, et al. Dental procedures in adult patients with hereditary bleeding disorders: 10 years experience in three Italian Hemophilia Centers. Haemophilia. 2005;11:504–9. doi: 10.1111/j.1365-2516.2005.01132.x. [DOI] [PubMed] [Google Scholar]
- 2.Rakocz M, Mazar AL, Taicher S, et al. Dental extraction in patients with bleeding disorders. The use of fibrin glue. Oral Surg Oral Med Oral Pathol. 1993;75:280–2. doi: 10.1016/0030-4220(93)90135-q. [DOI] [PubMed] [Google Scholar]
- 3.Frachon X, Pommereuil M, Berthier AM, et al. Management options for dental extractions in haemophiliacs: a study of 55 extractions (2000–2002) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:270–5. doi: 10.1016/j.tripleo.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SM, Pell P, Donegan EA. HIV-transmission following the use of cryoprecipitated fibrinogen as gel/adhesive. Transfusion. 1991;31s:51s. [Google Scholar]
- 5.Zanon E, Martinelli F, Bacci C, et al. Proposal of a standard approach to dental extraction in haemophilia patients. A case-control study with good results. Haemophilia. 2000;6:533–6. doi: 10.1046/j.1365-2516.2000.00423.x. [DOI] [PubMed] [Google Scholar]
- 6.Mozzati M, Gallesio G, DiRomana S, et al. Efficacy of PRGF in the healing of post-extractive sockets in patients affected by insulin-dependent diabetes mellitus. JOMS. 2014;72:456–62. doi: 10.1016/j.joms.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Cocero N, Bergamasco L, Mozzati M. Oral surgery procedures in patients with severe thrombocytopenia. The use of platelet concentrates instead of platelet transfusion. Casecontrol comparison between the two methods. Int Dent Res. 2012;2:27–32. [Google Scholar]
- 8.Mozzati M, Gallesio G, Arata V, et al. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncol. 2012;48:469–74. doi: 10.1016/j.oraloncology.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Mozzati M, Gallesio G, Gassino G, et al. Can plasma rich in growth factors improve healing in patients who underwent radiotherapy for head and neck cancer? A split-mouth study. J Craniofac Surg. 2014;25:938–43. doi: 10.1097/SCS.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 10.Pol R, Cristiano A, Cocero N. Concentrati piastrinici nella profilassi strutturale. In: Mozzati M, editor. Chirurgia Stomatologica Biologicamente Guidata. Complementi per la Chirurgia e la Biostimolazione. Torino: UTET; 2008. pp. 117–60. [Google Scholar]
- 11.Sanchez M, Anitua E, Azofra J, et al. Comparison of surgically repaired Achilles tendon tears using platelet-rich-fibrin matrices. Am J Sports Med. 2007;35:245–51. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 12.Anitua E, Prado R, Orive G. Bilateral sinus elevation evaluating plasma rich in growth factor technology: a report of five cases. Clin Implant Dent Relat Res. 2012;14(1):51–60. doi: 10.1111/j.1708-8208.2009.00233.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez M, Anitua E, Azofra J, et al. Ligamentization of tendon graft treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy. 2010;26:470–80. doi: 10.1016/j.arthro.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Jornet P, Camacho-Alonso F, Molina-Monano F, et al. Effects of plasma rich in growth factors on wound healing of the tongue. Experimental study on rabbits. Med Oral Patol Cir Bucal. 2009;14:e425–28. [PubMed] [Google Scholar]
- 15.Anitua E, Aguirre J, Algorta J. Effectiveness of autologous preparation rich in growth factors for the treatment oh chronic cutaneous ulcers. J Biomed Mater Res Part B. 2008;84b:415–21. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- 16.Benoit P, Marianne SF, Pascal H, et al. Management of dental extractions in patients with bleeding disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:247–50. doi: 10.1067/moe.2002.121431. [DOI] [PubMed] [Google Scholar]
- 17.Peisker A, Raschke GF, Schultze-Mosgau S. Management of dental extraction in patients with haemophilia A and B: a report of 58 extractions. Med Oral Patol Oral Cir Bucal. 2014;19:e55–60. doi: 10.4317/medoral.19191. [DOI] [PMC free article] [PubMed] [Google Scholar]