Abstract
Background
Over the last 50 years, clinical research investigating new treatments has been transforming the care of patients with haemophilia but we still have a long way to go and most clinical investigators are facing difficulties in recruiting appropriate candidates. A survey was conducted to evaluate what motivates people with haemophilia to participate in clinical research and to identify factors that might influence their willingness to participate.
Material and methods
A specific questionnaire concerning motivation and barriers to participation in clinical trials was sent to 135 adults with haemophilia. A classification tree was used to identify predictors of willingness to participate.
Results
Sixty-two patients returned the completed questionnaire, of whom 51 declared a potential willingness to participate in a clinical trial, although many were concerned about the new treatments’ possible side effects or about time away from work. Predictors of willingness to participate were evaluated using a classification tree and four groups were established. Group 1 comprised patients aged ≤45 years old. Group 2 comprised patients >45 years old who reported having no knowledge of clinical research modalities. The two other groups comprised patients >45 years old who reported having some knowledge of clinical research modalities, with group 3 being ≤59 years old and group 4 being >59 years old. The rate of willingness to participate was 96.6%, 28.6%, 70.6% and 100.0%, respectively.
Discussion
The rate of willingness to participate in clinical research was significantly lower in patients who reported having no knowledge of clinical trial modalities, highlighting the relevance of providing improved knowledge about the modalities, risks, and benefits of clinical research to enhance participation in haemophilia trials.
Keywords: clinical research, patients’ motivation, haemophilia, survey
Introduction
Over the last 50 years, clinical research and development of new treatments have been instrumental in transforming the care of patients with haemophilia. Life expectancy for people with severe haemophilia has increased from 16–23 years in the first-half of the 20th century to more than 65 years nowadays, and many such people are now able to lead active and fulfilled lives1,2. All this has been possible largely through the commitment of people with haemophilia to participate actively in clinical research. Despite major achievements, there are still several issues to resolve, such as the development of longer-acting clotting factor concentrates, new bypassing agents, gene therapy, as well as new treatment strategies for preventing or reducing the risk of developing inhibitors2,3. Although numerous clinical trials are ongoing worldwide right now, most investigators face difficulties in recruiting appropriate candidates, particularly in developed countries4–7.
There is thus a major unmet need for improved patient awareness regarding the relevance of clinical haemophilia research and promotion of opportunities to participate actively in clinical trials4–7. A survey was launched to evaluate the motivation of people with haemophilia to enter clinical trials in a developed country (Belgium) in which they have unlimited and unrestricted access to efficient treatments, and to identify factors that may influence their willingness to participate.
Materials and methods
Participants
A specific questionnaire was sent to 135 adults with haemophilia regularly attending the Haemophilia Comprehensive Centre of the Saint-Luc University Hospital in Brussels, Belgium, between June 2011 and December 2012. The questionnaire was first sent by post, with an email reminder sent using the online version. Overall, 62 patients completed the questionnaire (n=62/135, 45.9%). All the people who did not complete the questionnaire were contacted by phone in order to determine the reasons why they did not return the questionnaire.
Study measures
The questionnaire addressed socio-demographic status, family context, current haemophilia treatment, knowledge of principles and of perceived benefits and risks of clinical research, as well as positive and negative factors influencing motivation to participate. Socio-demographic data including age, country of residence, time spent in Belgium, country of origin, educational background, and primary occupation, were also collected.
Family data covered family context, number of children, number of affected males in the family and entourage, carriers in the family and entourage, as well as haemophilia mortality instances in males of the family and entourage.
Haemophilia treatment and care data included haemophilia type (A or B) and severity (severe, moderate, or mild), medication (recombinant or plasma-derived factor VIII or factor IX, or desmopressin), treatment regimen (prophylaxis or on-demand), total number of years on prophylaxis, current clotting factor concentrate consumption, inhibitor development in the past, estimated number of hospital admissions because of haemophilia, use of a notebook to record injections, and distance from the haemophilia centre.
Clinical research data included knowledge about clinical trials, the different phases of new drug development prior to marketing, number of participants needed, relevance of participation in clinical trials, awareness of clinical research, prior participation in a clinical trial, general interest in clinical trials, willingness to participate in a clinical trial, and which study phase they would agree to participate in, as well as perceived risks and benefits of participating in a clinical trial. A clinical trial was defined as a study aiming to develop or validate new treatments for haemophilia.
The study was approved by the local Ethical Committee, and informed written consent was obtained from all participants.
Statistical analysis
Continuous variables were expressed as means and standard deviations when they were normally distributed and as medians (p25 and p75) when they were not normally distributed. Continuous variables were compared using an independent Student’s t-test, Mann-Whitney U test, or the Kruskal-Wallis test depending on the validity conditions of each test. Categorical variables were compared using the Pearson’s chi-squared test or Fisher’s exact test, as appropriate. A classification tree analysis was used to analyse predictors of willingness to participate in clinical trials. The classification tree-based models were non-linear and non-parametric alternatives to linear models for classification problems8. Classification tree models were fitted by recursive partitioning of a multidimensional covariate space, in which the dataset was successively split into homogeneous subgroups. The selected split was the one that maximised the homogeneity of the two resulting nodes with respect to the response variable. The minimum-cost tree was selected as the best tree and the Gini index was used to measure the homogeneity of each node. A random forest was used as an additional tool to provide a variable ranking based on the overall contribution to the tree construction. Statistical analyses were performed using R software version 2.15.1 (Free Software Foundation, Inc., Boston, MA, USA) and Salford Predictive Modeler Builder Version 6.6 (Salford Systems, San Diego, CA, USA). A p value <0.05 was considered statistically significant.
Results
A total of 135 questionnaires were sent to adults with haemophilia (≥18 years), and 62 completed questionnaires were returned (n=62/135, 45.9%).
Reasons for not completing the questionnaire
Among the 73 patients who did not complete the questionnaire, ten patients did not remember having received it, one patient had died, three patients had moved and had not received the questionnaire, three patients thought they had returned it, four patients declared that it was an oversight not having completed it, one patient stated that he did not have the time to complete it, and 45 patients did not respond, despite several attempts. Four patients thought their participation was not necessary because they have mild or moderate haemophilia treated by on-demand therapy with minor impact on their life and two patients declared they did not want to complete the questionnaire, including one who do not accept his haemophilia status.
Patients who completed the questionnaire
The age of patients who completed the questionnaire was higher than that of those who did not (median age: 48.0 vs 42.0 years, p=0.045) (Table I). The proportion of patients on prophylaxis was not significantly different between patients who completed the questionnaire and those who did not (46.8% [n=29/62] vs 30.6% [n=22/73]; p=0.054). The distribution of types of haemophilia was similar in the two groups (p=0.300), with a total of 80.0% of individuals having haemophilia A and 20.0% having haemophilia B.
Table I.
Characteristics of the 135 patients who were contacted to complete the questionnaire.
| Variables | Total (N=135) n (%) or median [p25; p75] |
Non-respondent (n=73) n (%) or median [p25; p75] |
Respondent (n=62) n (%) or median [p25; p75] |
p |
|---|---|---|---|---|
| Haemophilia type | 0.300 | |||
| Haemophilia A | 108 (80.0) | 56 (76.7) | 52 (83.9) | |
| Haemophilia B | 27 (20.0) | 17 (23.3) | 10 (16.1) | |
|
| ||||
| Haemophilia severity | 0.230 | |||
| Severe | 69 (51.1) | 34 (46.6) | 35 (56.5) | |
| Moderate | 21 (15.6) | 10 (13.7) | 11 (17.7) | |
| Mild | 45 (33.3) | 29 (39.7) | 16 (25.8) | |
|
| ||||
| Treatment regimen* | ||||
| On-demand | 83 (61.9) | 50 (69.4) | 33 (53.2) | 0.054 |
| Prophylaxis | 51 (38.1) | 22 (30.6) | 29 (46.8) | |
|
| ||||
| Age, years | 44.0 [31.0; 57.5] | 42.0 [27.0; 56.0] | 48.0 [36.0; 59.0] | 0.045 |
1 missing value (0.7%);
p: p value for the comparison of patients’ characteristics between non-respondents and respondents to the questionnaire.
Among the 62 patients who responded, 11 (17.7%) declared they would refuse to participate in a clinical trial (Table II). The 11 patients who did not want to participate in a clinical trial were older than those who were willing to participate, with a mean age of 57.6 years vs 45.3 years, respectively (p=0.017). The proportion of people willing to participate in a clinical trial did not differ significantly between educational levels, although a significant trend was observed (chi-squared test for trend; p=0.048). Among people who did not graduate from high school, 40% (n=4/10) indicated that they were not willing to participate in a clinical trial, against 9.5% (n=2/21) of the people who have a college degree (Table II).
Table II.
Characteristics of the 62 patients who returned the questionnaire.
| Total respondents to the questionnaire [N=62] | Willing to participate in a clinical trial [n=51] | Not willing to participate in a clinical trial [n=11] | p | |
|---|---|---|---|---|
|
|
||||
| n (%) or mean±SD | n (%) or mean±SD | n (%) or mean±SD | ||
| Type of haemophilia | 0.674 | |||
| Haemophilia A | 52 (83.9) | 42 (82.4) | 10 (90.9) | |
| Haemophilia B | 10 (16.1) | 9 (17.6) | 1 (9.1) | |
|
| ||||
| Haemophilia severity | 0.733 | |||
| Severe | 35 (56.5) | 30 (58.8) | 5 (45.4) | |
| Moderate | 11 (17.7) | 9 (17.7) | 2 (18.2) | |
| Mild | 16 (25.8) | 12 (23.5) | 4 (36.4) | |
|
| ||||
| Treatment regimen | 0.569 | |||
| Prophylaxis | 29 (46.8) | 23 (45.1) | 6 (54.5) | |
| On-demand | 33 (53.2) | 28 (54.9) | 5 (45.5) | |
|
| ||||
| Age, years | 47.5±14.6 | 45.3±14.0 | 57.6±13.9 | 0.017 |
|
| ||||
| Educational level* | 0.239 | |||
| Did not graduate from high school | 10 (16.4) | 6 (12.0) | 4 (36.4) | |
| High school graduate | 15 (24.6) | 12 (24.0) | 3 (27.3) | |
| Some college or 3-year degree | 15 (24.6) | 13 (26.0) | 2 (18.2) | |
| College graduate | 21 (34.4) | 19 (38.0) | 2 (18.2) | |
|
| ||||
| Family context* | 0.404 | |||
| Single | 14 (23.0) | 12 (23.5) | 2 (18.2) | |
| Couple | 45 (73.7) | 37 (72.5) | 8 (72.7) | |
| Widower | 2 (3.3) | 1 (2.0) | 1 (9.1) | |
|
| ||||
| Having daughters** | 0.739 | |||
| No | 30 (50.0) | 24 (49.0) | 6 (54.5) | |
| Yes | 30 (50.0) | 25 (51.0) | 5 (45.5) | |
|
| ||||
| Having sons** | 0.317 | |||
| No | 30 (50.0) | 26 (53.1) | 4 (36.4) | |
| Yes | 30 (50.0) | 23 (46.9) | 7 (63.6) | |
|
| ||||
| Notebook for injections** | ||||
| No | 21 (35.0) | 18 (36.7) | 3 (27.3) | 0.731 |
| Yes | 39 (65.0) | 31 (63.3) | 8 (72.7) | |
1 missing value (1.6%);
2 missing values (3.2%);
p: p value for the comparison of patients’ characteristics between patients who declared a certain willingness to participate in a clinical trial and patients who were not willing to do so; SD: standard deviation.
Impact of family variables
The fact that patients were acquainted with other people with haemophilia (54.1%), had one or several haemophilia carriers in the family or entourage (72.1%), or had had at least one haemophilic male death in the family or entourage (34.4%) had no impact on the patients’ willingness to participate in a clinical trial.
Impact of treatment modalities
Approximately half of the patients were on prophylaxis (46.8%), among whom 44.8% were given prophylaxis three times a week, 44.8% twice a week, and 10.4% once a week (Table I). Only one of the ten patients with haemophilia B was on prophylaxis (10.0%) whereas 28 of the 52 patients with haemophilia A were on prophylaxis (53.8%). Three patients (4.8%) had been on secondary prophylaxis for under a year. Seven patients reported having developed an inhibitor in the past (11.9%); in six of them this occurred between the ages of 5 and 20 years, and in the remaining one at the age of 70. Nine patients (14.5%) did not know whether they had ever developed an inhibitor in the past. Thirteen patients (21.7%, n=13/60; two missing values) reported that they had never been admitted to hospital on account of haemophilia; these 13 individuals included four who had severe haemophilia. The majority of patients (58.3%, n=35/60, two missing values) reported between one and ten hospital admissions related to haemophilia, and 20.0% reported more than ten hospital admissions linked to haemophilia.
Knowledge and awareness of clinical trials
Approximately 70% of the patients (n=43/62) reported having some knowledge of clinical research principles, but only 30.2% of them fully understood the different phases of a clinical trial (Table III). Among the 43 patients who declared knowing what a clinical trial is, two-thirds (n=27/43) stated that they have received their information from a medical doctor, 14.0% (n=6/43) from people around them working in the medical field or from the media and 23.3% (n=10/43) from none of the above sources. However, patients’ knowledge about clinical trials appeared to be very limited, as the majority reported being unaware of the different phases of such trials or of the number of participants required for the clinical development of a new drug. Twenty-four patients (40.0%; two missing values) had already participated in a clinical trial; among these, 87.5% (n=21) were satisfied and 12.5% were not. In addition, 30.5% of patients (n=18/59) knew someone who had participated in a clinical trial; of these acquaintances, 13 would participate again, two would not, and the remaining three were unsure.
Table III.
Patients’ knowledge about clinical trials (N=62).
| Yes n (%) |
No n (%) |
I do not know n (%) |
|
|---|---|---|---|
| Do you know what a clinical trial is? | 43 (69.4) | 7 (11.3) | 12 (19.4) |
| Do you know the different phases of a clinical trial?* | 13 (21.3) | 34 (55.7) | 14 (23.0) |
| In your opinion, should each factor VIII or IX concentrate be evaluated in a clinical trial prior to marketing authorisation?* | 43 (70.5) | 3 (4.9) | 15 (24.6) |
| Do you have an idea of the number of participants required to develop new clotting factor concentrates for haemophilia treatment?* | 7 (11.5) | 47 (77.0) | 7 (11.5) |
| Could you estimate the number of participants needed to develop new clotting factor concentrates?* | 12 (19.7) | 44 (72.1) | 5 (8.2) |
| Do you consider your involvement in clinical trials important?** | 50 (87.7) | 7 (12.3) | / |
1 missing value (1.6%);
5 missing values (8.1%)
With respect to level of knowledge about clinical trials, 48.3% felt they were not sufficiently informed, with 96.4% wanting more information on trials. Conversely, 51.7% felt they were sufficiently informed, although 70.0% wanted more information.
The majority of patients considered their involvement in clinical trials as relevant (87.7%) (Table III).
The reasons why patients considered their involvement in clinical trials as unimportant were: limited severity of haemophilia (on-demand treatment, n=2/7); old age (n=1/7); lack of experience with clinical trials (n=1/7); concomitant hepatitis C and perceived risk of inhibitor development (n=1/7). Two patients provided no explanations.
Perception of the need for new drug development
Thirty-seven patients (n=37/52, 71.2%, 10 missing values) indicated that the development of new haemophilia drugs was necessary, 19.2% were neutral, and 9.6% did not consider this a necessity. However, 87.0% of patients stated that the development of new treatments was desirable.
The expected improvements quoted were better and safer treatments (less risk of infection), reduction in the number of weekly injections, availability of more effective drugs to control bleeding, development of gene therapy, as well as subcutaneous administration. Patients expressed a desire for oral tablets (n=3), better conservation conditions for transport abroad (n=2), and a cure for haemophilia (n=1). Before deciding to participate, the majority of patients would discuss the decision with their doctor (59.7%), their wife (53.2%), their parents (12.9%), or other patients (8.1%), while 20.0% would make the decision alone.
Although 29.0% of patients reported that participating in a clinical trial would be a source of anxiety or incompatible with their personal/professional life, and 25.8% reported that it would be an additional constraint for them, the majority (59.7%) considered it to be an opportunity. One of the major reasons for fearing participation was a new drug’s potential side-effects (Table IV). Other reasons for potential non-participation were also stated, such as the purpose and type of study (n=3), doctor’s approval (n=2), allergies to prior treatments (n=1), the perception that the disease was not sufficiently severe in one patient with mild haemophilia A (n=1), the concern that the tested drug should be developed by the same company manufacturing the patient’s current treatment (n=1), or multiple stays abroad incompatible with study participation (n=1). Two patients reported having no fears.
Table IV.
Reasons that could be an obstacle to an active participation in a clinical trial in the 51 patients who would have been willing to participate in such a trial.
| n (%) | |
|---|---|
| Reasons for non-participation in clinical trials | |
| Clinical trials imply too many visits to the hospital | 23 (45.1) |
| I care about the risks (side-effects) of a new drug. I do not want to take these risks | 20 (39.2) |
| I do not want to change my coagulation factor concentrate | 4 (7.8) |
| I do not want to change my actual treatment scheme. I do not want to have more frequent infusions | 3 (5.9) |
|
| |
| Questions assessing flexibility and tolerance of patients agreeing to take part in a clinical trial | |
| Spending a day at the hospital for a kinetic study | 31 (60.8) |
| Spending a night at the hospital | 28 (54.9) |
| Filling out a notebook regarding infusions several times a week | 28 (54.9) |
| Going to the hospital once a month for the trial | 25 (49.0) |
| Receiving a placebo and a real drug | 17 (31.5) |
| Change of treatment scheme (more frequent infusions) | 13 (25.5) |
| None of these propositions | 2 (3.9) |
Among the 51 patients who were interested in participating in a clinical trial, 11 (22.4%, two missing values) would accept being the first people with haemophilia to receive a new drug, 20 (40.8%) would agree to participate in a clinical trial provided the new drug had already been tested in other patients and volunteers, and 18 (36.7%) would accept taking a new drug if it was already on the market in order to help to confirm the drug’s efficacy and safety. The patients displayed some flexibility as regards participating in a clinical trial (Table IV). Patients indicated that visits to a hospital could pose a problem, mainly because of professional concerns (49.0%), long waiting times at the hospital (29.4%) and transport issues (23.5%). An interesting result was that only 11.8% (n=6/51) of the patients who were interested in participating in a clinical trial would do so if they got financial compensation.
Predictors of willingness to participate in clinical trials
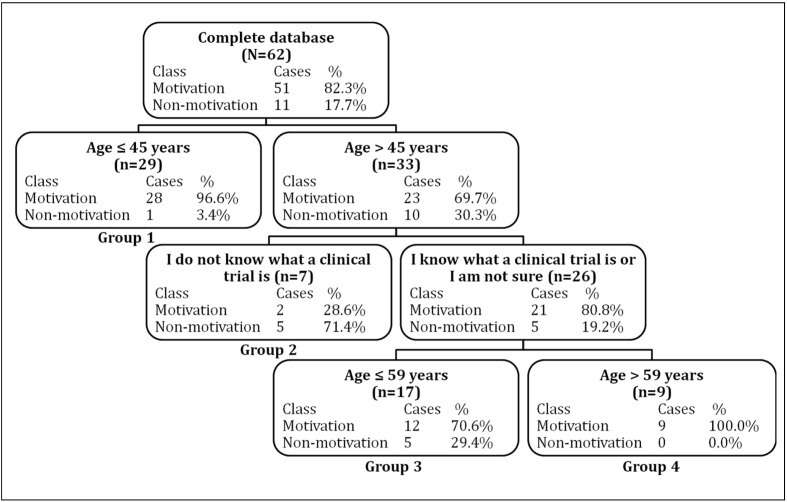
A major finding was that the treatment regimen (prophylaxis vs on-demand) had no impact on willingness to participate (Table II), nor did the type or severity of haemophilia. Predictors of willingness to participate in a clinical trial were evaluated using a classification tree, which established four groups (Figure 1). Group 1 comprised patients aged ≤45 years: their rate of willingness to participate (with 95% confidence interval [95% CI]) was 96.6% (82.8–99.4%), (n=28/29). Group 2 comprised patients aged >45 years old who reported no knowledge of clinical research modalities (rate of willingness to participate: 28.6% [95% CI: 5.1–69.7%]; n=2/7). Group 3 comprised patients aged between 46 and 59 years old who reported some knowledge of clinical research modalities (rate of willingness to participate: 70.6% [95% CI: 46.9–86.7%]; n=12/17). Finally, group 4 comprised patients aged >59 years who reported some knowledge of clinical research modalities (rate of willingness to participate: 100.0% [95% CI: 62.9–100.0%]; n=9/9). The proportions of patients who were willing to take part in a clinical trial differed significantly between group 1 and groups 2 and 3 and between group 2 and group 4. Table V shows other variables that were important in the construction of the classification tree. Other variables had no impact on willingness to participate (importance of 0.0).
Figure 1.
Classification tree representing the important predictors of willingness to participate in clinical trials (n=62).
The selected splitting variables (patients’ reported knowledge of clinical trials and age) are shown in the nodes. Motivation: rate of willingness to participate in clinical trials; non-motivation: rate of non-willingness to participate in clinical trials.
Table V.
Ranking of variables for willingness to participate in clinical trials by overall power as discriminant (random forest).
| Variable | Power |
|---|---|
| Age, years | 100.0 |
| Patient’s interest in having more information about clinical trials | 36.0 |
| Patient’s knowledge about clinical trials | 26.3 |
| Profession | 14.1 |
| Severity of haemophilia | 12.7 |
| Type of haemophilia | 10.3 |
| Family context | 4.4 |
| Patient’s awareness of clinical trials | 0.2 |
Discussion
Various studies reported in the scientific literature mention the importance of developing further research and new drugs in the field of haemophilia. Since the participation of patients in clinical research is essential for the advance of science, and the recruitment of a sufficient number of participants in clinical trials is crucial for health care to progress, we need to increase the number of patients willing to participate in clinical trials by enhancing their motivation2–7.
To the best of our knowledge, this is the first study that has evaluated the factors influencing the willingness of people with haemophilia to participate in clinical research. The authors are not aware of any studies that have assessed the knowledge that adults with haemophilia have about clinical trials or motivation for participating in such trials, rendering it difficult to compare the findings of the present study with literature data. Given that haemophilia is a rare and chronic disease, now well-tolerated because of new treatments that are available in developed countries, such as Belgium, it is difficult to compare the willingness of people with haemophilia to participate in clinical research with that of patients suffering from other diseases, such as cancer or diabetes. In contrast to other prevalent diseases, the relatively low number of potential participants is a relevant barrier for a rare disease such as haemophilia. However, several studies, essentially evaluating patients’ willingness to participate in clinical trials, as well as barriers to the patients’ participation, have been carried out in other medical fields, such as oncology9,10. In line with our study, these studies found that fear of a new drug’s potential side-effects, additional procedures and appointments needed for clinical trials, as well as travel problems and related costs were potential barriers to active participation in the trials9,10.
The main finding of our study was that patients who were >45 years old and who declared having no knowledge about clinical trials were less motivated to participate (rate of willingness to participate: 28.6%; n=2/7). These findings support the view that physicians should provide patients with clear and unbiased information on clinical research, such as information leaflets explaining clinical trials. A website was developed at the Haemophilia Comprehensive Centre of the Saint-Luc University Hospital in Brussels, Belgium, in order to increase patients’ awareness of the importance of participating in clinical research on haemophilia (http://participatetoinnovate.com), explaining the different clinical research steps, why patients’ participation is required, what challenges remain in the field of haemophilia therapy, and the expected benefits of participating in a study. A leaflet was also made available to patients, inviting them to consult the website. Our study also suggested that willingness to participate is affected by educational level, given the significant trend of willingness to participate in a clinical trial observed with increasing educational level.
Three major biases of our study must be noted. First, there may have been a bias due to the participation rate, with less that half of the patients returning the questionnaire (46.7%; n=62/135). Among the 73 patients who did not complete the questionnaire, one patient had died, three patients had moved and did not receive the questionnaire, ten patients did not remember having received the questionnaire, three patients thought they had returned it and 45 patients did not respond, despite several attempts. Secondly, there may have been a bias related to the fact that patients who completed the questionnaire were older than those who did not. Only 11.3% of patients who completed the questionnaire were aged ≤30 years compared to 32.9% for the others; suggesting that younger patients were less interested in their disease, possibly because they benefitted from current well-tolerated and effective haemophilia therapies, whereas older patients were likely infected following blood transfusions in the 1980s. Another reason could be that not all patients aged ≤30 years have children, so the question of haemophilia transmission to their daughters is not of immediate interest. The third potential bias was related to the higher proportion of patients being treated on-demand among those who did not return the questionnaire (69.4% for non-respondents vs 53.2% for respondents) even if these proportions were not statistically significantly different (p=0.054). Patients receiving on-demand therapy probably felt less concerned about their haemophilia, as indicated by the four patients with mild or moderate haemophilia treated by on-demand therapy who did not complete the questionnaire because haemophilia has a minor impact on their life. Moreover, patients on prophylaxis are more likely to have regular contact with the treatment centre, probably resulting in the higher proportion of respondents to the questionnaire among these patients.
Given the significant non-response rate, the reported findings may represent the basis for further larger studies. As an example, non-respondents could be asked to fill out the questionnaire at a routine clinical follow-up at the haemophilia centre. We also encourage this approach for other questionnaire-based studies in order to increase participation rate. Moreover, multicentre collaborative studies could be conducted in the near future. In addition, this study should be repeated on a larger scale in order to confirm our findings and to underline potential differences between countries and between patients with various levels of access to treatment, such as a prophylaxis scheme. Results from patients in our developed country who have access to optimal treatment could differ from those in other countries, both developed and developing, in which patients do not have access to optimal treatment or have no access at all.
Conclusion
In conclusion, this survey highlights the relevance of increasing awareness and providing improved knowledge of clinical research modalities, risks, and benefits in order to increase the number of potential participants in clinical haemophilia trials. The conclusion of this study cannot, however, be extrapolated to all people with haemophilia, because of different access rates to prophylaxis depending on the country. We recommend that similar surveys be conducted in other developed and developing countries.
Footnotes
Authorship contributions
SH, NS and CH designed the study, analysed the data, wrote and edited the manuscript and approved its final version.
The Authors state that they had no interests which might be perceived as posing a conflict or bias.
References
- 1.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–25. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 2.Young G. New challenges in hemophilia: long-term outcomes and complications. ASH Education Program Book. 2012;(1):362–8. doi: 10.1182/asheducation-2012.1.362. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AD, Soucie JM, Peyvandi F, et al. Knowledge and therapeutic gaps: a public health problem in the rare coagulation disorders population. Am J Prev Med. 2011;41:S324–31. doi: 10.1016/j.amepre.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 4.DiMichele DM, Blanchette V, Berntorp E. Clinical trial design in haemophilia. Haemophilia. 2012;18(Suppl 4):18–23. doi: 10.1111/j.1365-2516.2012.02824.x. [DOI] [PubMed] [Google Scholar]
- 5.Lassila R, Armstrong E. Current challenges of pharmacovigilance in bleeding disorders: converting the burden to benefit. Haemophilia. 2010;16:231–7. doi: 10.1111/j.1365-2516.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- 6.Mannucci PM. Evolution of the European guidelines for the clinical development of factor VIII products: little progress towards improved patient management. Haemophilia. 2013;19:344–8. doi: 10.1111/hae.12041. [DOI] [PubMed] [Google Scholar]
- 7.Ragni MV, Moore CG, Bias V, et al. Challenges of rare disease research: limited patients and competing priorities. Haemophilia. 2012;18:e192–4. doi: 10.1111/j.1365-2516.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 8.Speybroeck N. Classification and regression trees. Int J Public Health. 2012;57:243–6. doi: 10.1007/s00038-011-0315-z. [DOI] [PubMed] [Google Scholar]
- 9.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–8. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 10.Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]