Introduction
Haemophilia A is a congenital bleeding disorder due to the lack of coagulation factor VIII (FVIII). The standard treatment is based on administration of plasma-derived or recombinant FVIII concentrates. The natural history of the disease includes recurrent bleeding into joints and the major long-term disability in affected patients is chronic arthropathy. The life expectancy of patients with haemophilia is now nearing that of the normal population thanks to replacement therapy and major surgery has also become feasible1. Unfortunately, after treatment with FVIII concentrates, a subgroup of patients become sensitised, developing neutralising antibody inhibitors which render standard replacement treatment ineffective. Surgery becomes a challenge for these patients and even more so when haemostasis is urgently necessary, as in the case of fractures.
When treatment with FVIII is unfeasible in patients with high titres of inhibitor, haemostasis may be achieved by using the so-called bypassing agents such as activated recombinant factor VII (rFVIIa; NovoSeven, Novo Nordisk, Denmark) or activated prothrombin complex (aPCC; Feiba, Baxter, Deerfield, IL, USA)2,3. The advantages of rFVIIa are the possibility of concurrently using antifibrinolytic agents such as tranexamic acid and avoiding an anamnestic response to FVIII, which has been reported with aPCC. There have now been a few descriptions of major surgery carried out with the use of rFVIIa in patients with haemophilia A and high-titre FVIII antibodies, including a single joint replacement and a case of concurrent double prostheses4,5. Although achieving haemostasis is the main challenge in these instances, once obtained, the critical and complex picture of the chronic, multiple haemophilic arthropathy must be tackled by orthopaedists6.
Case report
We present the case of a 52-year old Caucasian male, admitted with post-traumatic supracondylar fracture of the left femur (Figure 1) and severe haemophilia A (FVIII:C<1%, F9 mutation: int22inv) with high-titre inhibitors. On admission the patient had pain and oedema of the left femur (WHO 3)7. The inhibitor titre was 3.8 Bethesda units (BU), and had peaked in the past at 78 BU. Before the fracture, the range of movement of both knees was limited in extension (−10°) and in flexion (30°) (Figure 1) but the patient was able to walk unaided. The fracture was first treated with a plastic splint and traction with 3 kg apart for 3 days and then, following extensive discussion with the orthopaedists given the high risk of bleeding, it was decided to proceed to surgical reduction of the fracture. The operation involved a longitudinal incision along the distal femoral axis and reduction of the fracture under fluoroscopy, because the fracture was found to have multiple fragments. Nevertheless, the knee remained approximately 20° flexed. Consequently, a shortening osteotomy (about 1 cm) was performed making it possible to compact and reduce the fracture restoring complete extension of the leg. The bone gap was filled with a synthetic support (Tecnoss Sp-Block, Torino, Italy) and demineralized bone matrix (DBX® Putty, Paste & Mix; Musculoskeletal Transplant Foundation, Edison, NJ, USA) and complete stability was obtained by a Zimmer angular modified plate blocked with compression screws.
Figure 1.
The pre-fracture knee flexion contracture was 30º (first left).
The X-ray second from the left shows the multiple fragments of the femoral fracture (and the original flexion of the knee). The frontal and lateral pictures in the right panel show the Zimmer plate and complete extension of the leg. Bone healing occurred at 3 months and at 10 months (last follow-up) the patient was able to walk without aid.
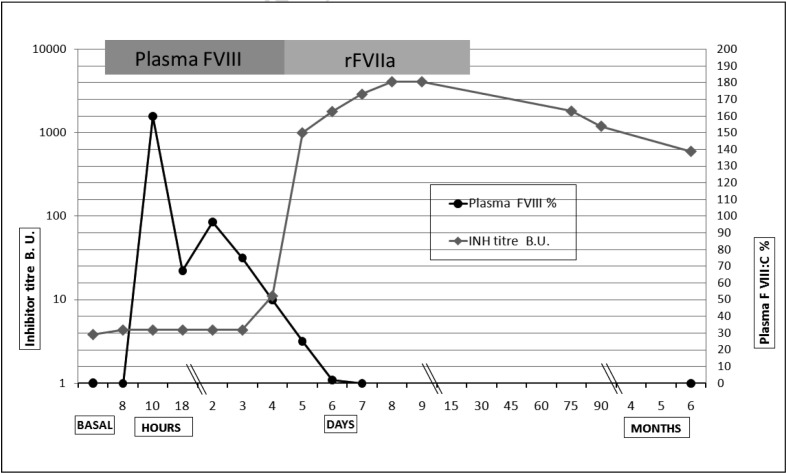
Pro-coagulation replacement treatment was initially based on FVIII concentrates (Emoclot D.I., Kedrion, Barga [Lucca], Italy) when the anti-FVIII inhibitor titre was low and a neutralising dose plus an incremental dose (FVIII was dosed calculating a neutralising dose of BU×40×kg/bw plus the dose needed for the targeted increment) were able to normalise coagulation, restoring a normal activated partial thromboplastin time (aPTT) and measurable plasma FVIII levels (Figure 2). The treatment with Emoclot was started on the day of surgery at a dose of 10,000 U pre-operatively and 3,000 U post-operatively, then 6,000 U twice daily on the first post-operative day and 4,000 U twice daily on days 3 and 4, but was interrupted on day 5 (Figure 2) because of the prolonged aPTT (ratio up to 3.34). The pre-operative plasma FVIII was increased up to 159% and then decreased to 90%, 67%, 50% and 20% from day 1 to day 5 and on day 6 FVIII plasma levels became unmeasurable (Figure 2) due to an increase of the anti-FVIII titre up to 95. Treatment with NovoSeven was initiated with a bolus dose of 120 μg/kg (body weight 67 kg) and then with continuous infusion at a dose of 40 μg/kg/hour administered for 2 days and then 25 μg/kg/hour until post-operative day 12, when treatment was interrupted, with complete haemostatic control (Figure 2).
Figure 2.
Time course of haemostatic replacement treatment, first plasma FVIII and then rFVII (horizontal bars above the graphs), with plots of the plasma FVIII levels (until measurable) and the anti-FVIII titre which increased rapidly from +5 days post-operatively.
B.U.: Bethesda units.
Unfortunately, during the early post-operative period the patient complained of a motor deficit of his left foot and although X-ray excluded fractures, the neurologist diagnosed a flexed foot deficit (35°) with compression damage to the left toe extensors with tibialis anterior and longus associated with superficial peroneal paraesthesia. This was confirmed by electromyography which showed sufferance of the common trunk proximal to the left popliteal sciatic nerve with lack of excitability of sensory branches (superficial peroneal and sural), nerve pain and, to a less extent, deficits of the external and internal popliteal sciatic nerves (0°). These complications were probably iatrogenic (not secondary to the fracture), due to forced extension of the knee. However, the neurological deficit improved over time and the patient started assisted physiotherapy 60 days after the operation, when his leg was able to support his body weight. At discharge, 3 months after the operation, the patient had no pain, was able to walk with crutches and his upright stability was improving (WHO 1)7. At present, 10 months later, he is able to walk without any additional help.
Discussion
Major surgery in haemophilic patients with inhibitors is still a challenge because of the haemostatic risk and the very high treatment cost. Treatment guidelines for these patients recommend using FVIII concentrates as first choice in life-threatening bleeds and in major surgery when the inhibitor titre is <5 BU, but with caution in surgical cases and only when no other interventions are suitable in the short-term because of the risk of an anamnestic response8. In this case, although the possibility of further operations could not be excluded, it was decided to use FVIII concentrates to obtain haemostatic control at the beginning when the risk of bleeding is higher and the best possible haemostasis is necessary. Once replacement with FVIII was no longer effective, as expected, a continuous infusion of rFVIIa was started. In our hands, administration in this manner, which we have used in previous cases of major surgery4,5, has proven to be effective and has some advantages compared to bolus infusion avoiding frequent administrations (every 2 hours at the beginning) because of its short half-life and reducing the cost of this extremely expensive therapy (saving up to 40%). The total cost of this intervention, including post-operative care and physiotherapy, was around 200,000 euros which is one-third of the cost of using rFVIIa only from the beginning.
In conclusion, our management aim was a multidisciplinary approach taking into account the crucial role that walking plays in quality of life. Full extension of the leg permits easier walking than flexion and that is why we choose to realign the femur by osteotomy to enable walking without crutches. This case demonstrates that the best results, in terms of patient satisfaction as well as optimising resources, may be obtained by close cooperation between haematologists and orthopaedists.
Acknowledgements
We thank the Libera Associazione Genitori ed Emofilici del Veneto (LAGEV) and the AVIS per il Progresso Ematologico (APE). LC is a fellow of AIL Treviso.
Footnotes
Authorship contributions
GT and AR designed the study, interpreted data and drafted the manuscript; LC, FWF and PR collected data, revised the manuscript and approved its final version.
The Authors declare no conflicts of interest.
References
- 1.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–25. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 2.Hedner U, Glazer S, Pingel K, et al. Successful use of recombinant factor VIIa in patient with severe haemophilia A during synovectomy. Lancet. 1988;19:1193. doi: 10.1016/s0140-6736(88)90259-0. [DOI] [PubMed] [Google Scholar]
- 3.Rangarajan S, Austin S, Goddard NJ, et al. Consensus recommendations for the use of FEIBA® in haemophilia A patients with inhibitors undergoing elective orthopaedic and non-orthopaedic surgery. Haemophilia. 2013;19:294–303. doi: 10.1111/hae.12028. [DOI] [PubMed] [Google Scholar]
- 4.Tagariello G, De Biasi E, Gajo GB, et al. Recombinant FVIIa (NovoSeven) and total hip replacement in patients with haemophilia and high titre of inhibitors to FVIII: experience of two cases. Haemophilia. 2000;6:581–3. doi: 10.1046/j.1365-2516.2000.00400.x. [DOI] [PubMed] [Google Scholar]
- 5.Tagariello G, Bisson R, Radossi P, et al. Concurrent total hip and knee replacements in a patient with haemophilia with inhibitors using recombinant factor VIIa by continuous infusion. Haemophilia. 2003;9:738–40. doi: 10.1046/j.1351-8216.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Merchán EC. Orthopedic surgery is possible in hemophilic patients with inhibitors. Am J Orthop. 2012;41:570–4. [PubMed] [Google Scholar]
- 7.Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–44. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 8.Collins PW, Chalmers E, Hart DP, et al. UK Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia (4th edition). UK Haemophilia Centre Doctors Organization. Br J Haematol. 2013;160:153–70. doi: 10.1111/bjh.12091. [DOI] [PubMed] [Google Scholar]