Abstract
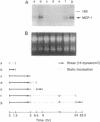
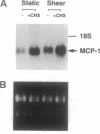
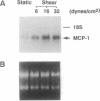
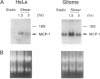
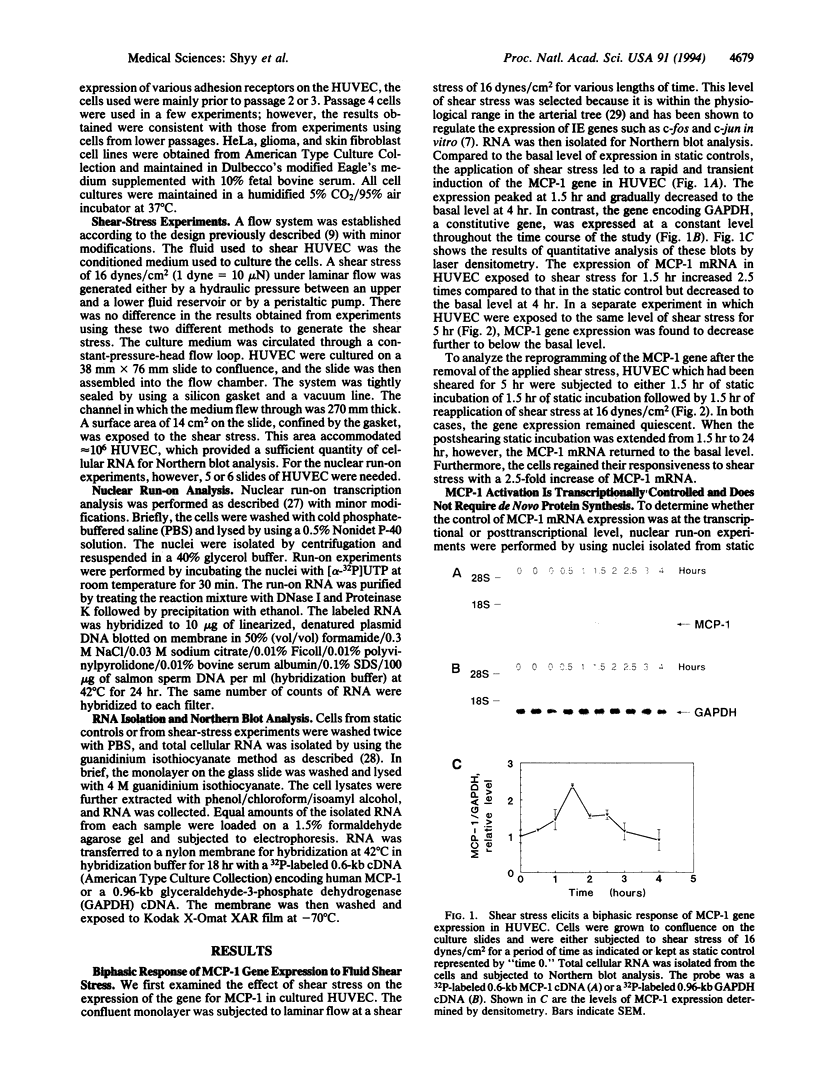
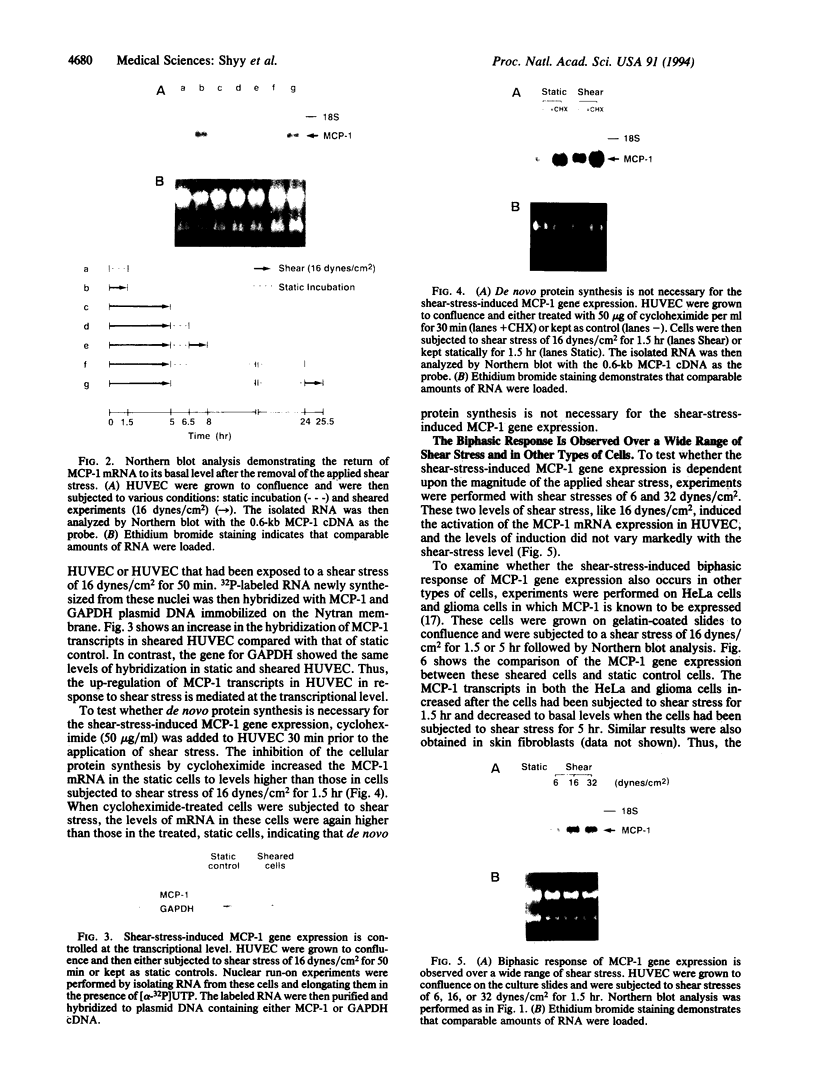
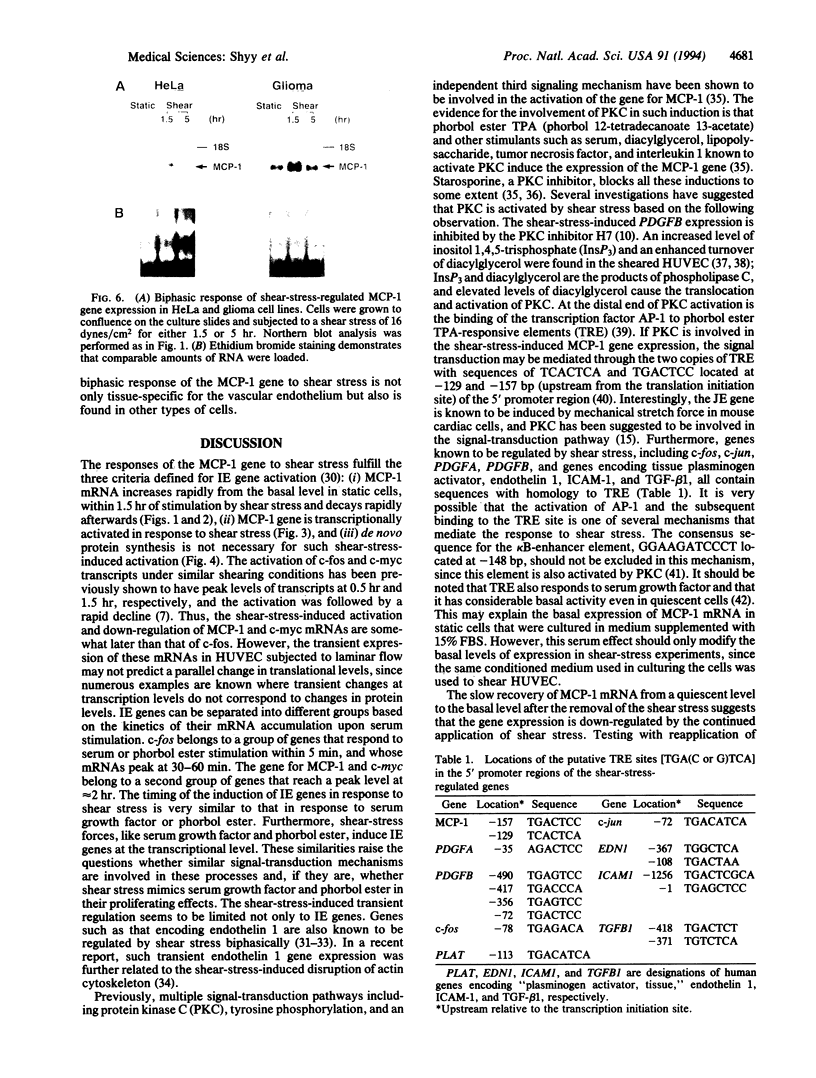
The focal distribution of atherosclerotic lesions in the arterial tree is related to the local shear stress generated by blood flow, but the molecular basis of the atherogenic response of endothelial cells in these lesion-prone areas is still unclear. We report that shear stress mediates a biphasic response of monocyte chemotactic protein 1 (MCP-1) gene expression in vascular endothelial cells (EC). Northern blot analysis indicated that the level of MCP-1 mRNA in human umbilical vein EC (HUVEC) subjected to a shear stress of 16 dynes/cm2 (1 dyne = 10 microN) for 1.5 hr increased by 2- to 3-fold when compared with static cells. The MCP-1 gene expression decreased to the basal level at 4 hr and then declined further to become completely quiescent at 5 hr after the onset of shear. Once the gene expression was fully suppressed, it remained quiescent even after static incubation for 1.5 hr and would not respond to reshearing after this static incubation. However, if the postshearing incubation extended from 1.5 to 24 hr, the MCP-1 mRNA returned to the basal level and was then able to increase after the reapplication of shear stress. Nuclear run-on experiments showed that the shear-induced increased MCP-1 mRNA in HUVEC was regulated at the transcriptional level. By using cycloheximide, it was shown that de novo protein synthesis was not necessary for the induction of MCP-1 by shear stress. The biphasic response of MCP-1 gene expression was found in experiments in which the applied shear stress was 6, 16, or 32 dynes/cm2, and it was observed not only in HUVEC but also in HeLa cells, glioma cell lines, and skin fibroblasts. This in vitro study demonstrates that the response of MCP-1 gene to shear stress represents an immediate early gene activation and suggests that this gene is probably suppressed in EC that have been exposed to a constant shear stress.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Chien S. Significance of macrorheology and microrheology in atherogenesis. Ann N Y Acad Sci. 1976;275:10–27. doi: 10.1111/j.1749-6632.1976.tb43335.x. [DOI] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond S. L., Sharefkin J. B., Dieffenbach C., Frasier-Scott K., McIntire L. V., Eskin S. G. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J Cell Physiol. 1990 May;143(2):364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Gerritsen M. E., Bloor C. M. Endothelial cell gene expression in response to injury. FASEB J. 1993 Apr 1;7(6):523–532. doi: 10.1096/fasebj.7.6.8472891. [DOI] [PubMed] [Google Scholar]
- Glagov S., Zarins C., Giddens D. P., Ku D. N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988 Oct;112(10):1018–1031. [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Physiol. 1993 Jan;154(1):143–151. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991 Feb;260(2 Pt 2):H642–H646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear-induced platelet-derived growth factor gene expression in human endothelial cells is mediated by protein kinase C. J Cell Physiol. 1992 Mar;150(3):552–558. doi: 10.1002/jcp.1041500316. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchan M. J., Frangos J. A. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol. 1993 Jan;264(1 Pt 2):H150–H156. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Liao F., Berliner J. A., Mehrabian M., Navab M., Demer L. L., Lusis A. J., Fogelman A. M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest. 1991 Jun;87(6):2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. J., Jan K. M., Chien S. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis. 1990 Sep-Oct;10(5):703–709. doi: 10.1161/01.atv.10.5.703. [DOI] [PubMed] [Google Scholar]
- Lin S. J., Jan K. M., Chien S. Temporal and spatial changes in macromolecular uptake in rat thoracic aorta and relation to [3H]thymidine uptake. Atherosclerosis. 1990 Dec;85(2-3):229–238. doi: 10.1016/0021-9150(90)90115-y. [DOI] [PubMed] [Google Scholar]
- Morita T., Kurihara H., Maemura K., Yoshizumi M., Yazaki Y. Disruption of cytoskeletal structures mediates shear stress-induced endothelin-1 gene expression in cultured porcine aortic endothelial cells. J Clin Invest. 1993 Oct;92(4):1706–1712. doi: 10.1172/JCI116757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollert M. U., Eskin S. G., McIntire L. V. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun. 1990 Jul 16;170(1):281–287. doi: 10.1016/0006-291x(90)91271-s. [DOI] [PubMed] [Google Scholar]
- Nollert M. U., Hall E. R., Eskin S. G., McIntire L. V. The effect of shear stress on the uptake and metabolism of arachidonic acid by human endothelial cells. Biochim Biophys Acta. 1989 Sep 11;1005(1):72–78. doi: 10.1016/0005-2760(89)90033-7. [DOI] [PubMed] [Google Scholar]
- Resnick N., Collins T., Atkinson W., Bonthron D. T., Dewey C. F., Jr, Gimbrone M. A., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J. JE/MCP-1: an early-response gene encodes a monocyte-specific cytokine. Cancer Cells. 1991 Dec;3(12):517–524. [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Jahn L., Takahashi T., Kulik T. J., Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992 May 25;267(15):10551–10560. [PubMed] [Google Scholar]
- Schuler G., Hambrecht R., Schlierf G., Niebauer J., Hauer K., Neumann J., Hoberg E., Drinkmann A., Bacher F., Grunze M. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation. 1992 Jul;86(1):1–11. doi: 10.1161/01.cir.86.1.1. [DOI] [PubMed] [Google Scholar]
- Sharefkin J. B., Diamond S. L., Eskin S. G., McIntire L. V., Dieffenbach C. W. Fluid flow decreases preproendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. J Vasc Surg. 1991 Jul;14(1):1–9. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Activation of MCP-1 gene expression is mediated through multiple signaling pathways. Biochem Biophys Res Commun. 1993 Apr 30;192(2):693–699. doi: 10.1006/bbrc.1993.1470. [DOI] [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Shyy Y. J., Wickham L. L., Hagan J. P., Hsieh H. J., Hu Y. L., Telian S. H., Valente A. J., Sung K. L., Chien S. Human monocyte colony-stimulating factor stimulates the gene expression of monocyte chemotactic protein-1 and increases the adhesion of monocytes to endothelial monolayers. J Clin Invest. 1993 Oct;92(4):1745–1751. doi: 10.1172/JCI116762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi M., Kurihara H., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]
- Yu X., Dluz S., Graves D. T., Zhang L., Antoniades H. N., Hollander W., Prusty S., Valente A. J., Schwartz C. J., Sonenshein G. E. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6953–6957. doi: 10.1073/pnas.89.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]