Abstract
Outer membrane vesicles (OMVs) from Gram-negative bacteria were first considered as artifacts and were followed with disbelief and bad reputation. Later, their existence was accepted and they became characterized as bacterial bombs, virulence bullets, and even decoys. Today, we know that OMVs also can be involved in cell–cell signaling/communication and be mediators of immune regulation and cause disease protection. Furthermore, OMVs represent a distinct bacterial secretion pathway selecting and protecting their cargo, and they can even be good Samaritans providing nutrients to the gut microbiota maintaining commensal homeostasis beneficial to the host. The versatility in functions of these nanostructures is remarkable and includes both defense and offense. The broad spectrum of usability does not stop with that, as it now seems that OMVs can be used as vaccines and adjuvants or vehicles engineered for drug treatment of emerging and new diseases not only caused by bacteria but also by virus. They may even represent new ways of selective drug treatment.
Keywords: outer membrane vesicles, offense, defense, versatility in functions
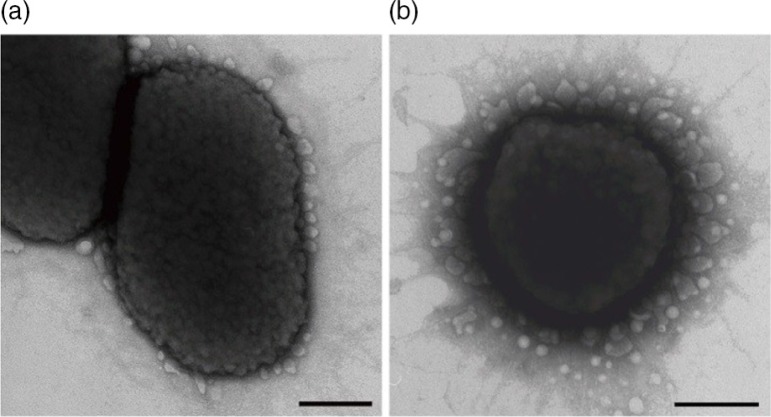
Outer membrane vesicles (OMVs) are released from Gram-negative bacterial cells, as well as from Archaea, fungi, and parasites. The production of OMVs was first reported more than 40 years ago (1) but their full biological significance was first recognized recently, particularly in Gram-negative bacteria. Membrane vesicle (MV) production in Gram-positives was long overlooked (2) but has now been demonstrated in Streptococcus pneumoniae, Streptococcus mutans, Staphylococcus aureus, and Bacillus subtilis (2–5). OMVs range in size from 20 to 300 nm in Gram-negative bacteria. MVs in Gram-positive bacteria are somewhat smaller. Due to their small size they have been characterized as nanovesicles (6, 7). The vesicle membrane in Gram-negative bacteria is somewhat different from that of the outer membrane (OM) although there are great similarities (8). It is still unclear how OMVs are generated in detail. They are formed when the OM bulges and encapsulates periplasmic components (9–11) (Fig. 1) which involve membrane remodeling (8).
Fig. 1.
OMVs observed at the outer cell membrane in P. gingivalis with transmission electron microscopy. A: Membrane-blebbing OMVs in strain ATCC 33277T (type I fimA strain). B: OMVs through the blebbing and pinching-off of the outer membrane in strain TDC 60 (type II fimA strain). Bars=200 nm.
The present review will deal mainly with OMVs from Gram-negative bacteria where they can be derived from both pathogenic and non-pathogenic species. OMVs used to have a disreputable past, first being considered as debris or artifacts (12). Today, we know that OMVs have very diverse functions. They are involved in both defense and offense. The field is quite extensive and only major functions will be dealt with. Functions mentioned for OMVs of the periodontopathogenic Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans are listed in Table 1.
Table 1.
Functions of OMVs from Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans mentioned in this review
| P. gingivalis | A. actinomycetemcomitans | |
|---|---|---|
| Ref | Ref | |
| OMVs as communication tools | ||
| OMVs as a secretion system | 17 | 16 |
| Effect on innate and adoptive immunity | 34 | |
| Ecological determinants | 42, 43, 45, 46, 47, 52, 53, 54 | 48, 51, 57 |
| OMVs as offensive weapons | ||
| Adhesion and invasion | 61, 62, 63, 64 | |
| Virulence bullets/bacterial bombs | 76, 77, 78, 81, 82, 83 | 74 |
| Sepsis | 93, 94 | |
| OMVs as possible good Samaritans | ||
| Vaccines/adjuvants | 97 | |
| Classification | 99 |
OMVs as communication tools
OMVs as secretion system
OMVs enable secretion of insoluble or hydrophobic material such as lipids, membrane proteins, and signaling molecules (13). They have a number of advantages over simple secretion systems in bacteria because the cargo is protected inside the lumen of the vesicle which can be targeted to specific destinations through receptors (14). In the case of gentamicin-containing OMVs, these OMVs may help eradicate even intracellular pathogens not reached by gentamicin in external body fluids (15). Different toxins can be transported by OMVs in different bacteria. In A. actinomycetemcomitans, biologically active cytolethal distending toxin (CDT) depended on vesicle transport into HeLa cells and human gingival fibroblasts (16). OMVs were internalized in these cells by fusion with lipid rafts in the plasma membrane and the active toxin unit, CdtB, was localized inside the nucleus of the intoxicated cells. It has been suggested that OMVs, due to their preponderance in biofilms, could be used to deliver cell toxins which would affect only the intended target (7). OMVs can also be internalized in host cells with the result that intracellular constituents can become degraded leading to cellular malfunction (17).
Many organisms use OMVs to secrete virulence factors. Examples are cytotoxin Cly from Escherichia coli and Salmonella enterica serovar Typhi, the heat-labile enterotoxin of enterotoxigenic E. coli and the vacuolating cytotoxin VacA of Helicobacter pylori (reviewed by Ref. 18). Notably, bacteria actively regulate their OMV cargo to manipulate the host-pathogen interplay.
Gentamicin has been shown to induce Pseudomonas aeruginosa to generate OMVs containing this aminoglycoside (19). The OMVs were similar to natural ones except that they contained small amounts of gentamicin. The synergistic effect of antibiotic plus autolysin in gentamicin-containing OMVs can suggest a novel strategy as how to deal with hard-to-kill pathogens (19). OMVs can also deliver enzymes and lipopolysaccharide (LPS) in high concentration to the target as a package (11) excreting membrane-perturbing substances from the cell. Dorvard et al. (20) proposed that OMVs can function as a mechanism of genetic exchange between cells because they are efficient mediators of genetic transformation. Indeed DNA in OMVs has been successfully transferred into other bacterial cells, even between cells of different species (20–22). This may represent a so far little recognized DNA delivery system for bacteria (20, 23, 24).
OMVs can contain β-lactamases which may protect bacterial species against the stress of antibiotics (25–28). This could represent a new form of drug delivery (29–31). When bacterial cells are among non-competitors their OMVs probably deliver ‘benign’ messages, whereas the cargo may change when the cells are faced with stress, competition, or prey (7). To be able to differentiate between messages from a friend or foe OMVs would probably need a barcode-like recognition system (7).
Effect on innate and adaptive immune system
Through delivery of enzymes, toxins, communication signals, and antigens, OMVs can influence the innate and adaptive immune systems (18). In bacterial OMVs, Toll-like receptor ligands such as LPS and lipoproteins stimulate maturation of and cytokine release by macrophages and dendritic cells (DCs). This may promote the pathogenesis of infections. Furthermore, peptidoglycan-containing OMVs upregulated nuclear factor-kappaB (NF-kB) and nucleotide-binding oligomerization domain (NOD)1-dependent responses in vitro (32, 33). This was suggested as a new mechanism whereby Gram-negative bacteria can deliver peptidoglycan to cytosolic NOD1 in host cells and thereby promote inflammation and pathology.
OMVs-containing antigens such as surface proteins and LPS are probably potent stimulators of the adaptive immune response and both B and T cell antigens have been identified in OMVs (18). In A. actinomycetemcomitans, OMVs after internalization into human cells, acted as a trigger of innate immunity by carrying NOD1 and NOD2-active pathogen-associated molecular patterns into host cells (34). It was suggested that OMV internalization can represent an important mechanism for intracellular exposure of antigens for A. actinomycetemcomitans. Interestingly, because A. actinomycetemcomitans triggers bone resorption mainly via NOD1, intracellular delivery of NOD1 via OMVs may induce periodontal bone loss (34).
Antigen decoys
OMVs may act as decoys in vivo meaning that they redirect the antibody response making antibodies ineffective for clearance of intact organisms (18). Thus, proteins and carbohydrates in OMVs may act as additional and significant antigen sources beyond those provided by the organism. This was seen in Moraxella that avoided direct interaction with host B cells by redirecting the adaptive humoral immune response by using superantigen-bearing OMVs as decoys resulting in the production of antibodies ineffective for elimination of intact organisms (35). Moraxella catarrhalis can actually direct the humoral immune response away from itself. In this bacterium, OMV secretion probably represents a sophisticated mechanism to modify host immune response avoiding direct contact between bacterium and host (36). Microbes can also modulate the host response to suit their lifestyle while staying inside the host, and they can modify the microbial surface to avoid immune detection. Also, OMVs can act as a decoy target for phages (37, 38). In biofilms OMVs have been suggested to serve as decoys for bacteria growing there (39). OMVs can also alleviate stress caused by peptide antibiotics by acting as decoys and transporting these molecules away from the parent cells (38).
Cross-kingdom dialogs
OMVs can mediate intercellular exchange such as cell-cell signaling. In the gut, a bacterial homolog of a eukaryotic inositol phosphate signaling enzyme (InsP6 phosphatase or MINPP) was found to mediate cross-kingdom dialog (40). It was demonstrated that MINPP from Bacteroides thetaiotaomicron (BtMinpp) was packaged inside OMVs protecting the enzyme from degradation by external bacterial proteases. Furthermore, BtMinpp-OMVs interacted with intestinal epithelial cells to promote intracellular Ca2+ signaling. In other words, a bacterial enzyme was used to mediate dialog between gut bacteria and the human host. This is a good example of how the microbiota can serve human gastrointestinal physiology and how commensal gut bacteria can use OMVs in a manner that is beneficial to the host.
Intermicrobial communication
OMVs are known to exert important functions not only in inter-kingdom communication but also in intercellular and inter-species communication (18). Intermicrobe cross talk is promoted by OMV release. Examples are P. aeruginosa that releases quorum-sensing signaling molecules pqs (Pseudomonas quinolone signal) and Haemophilus influenzae that releases DNA in OMVs (transformasomes) (18). They are effective mediators of communication even at long distances (41).
Ecological determinants
OMVs, being able to specifically concentrate the release of a large number of its virulence factors into the environment (42) could probably regulate the ecology at the site they are acting against promoting disease, for example, early onset periodontitis.
OMVs can be involved in coaggregation of bacterial cells (43–47) and in adhesion of bacteria, for example, A. actinomycetemcomitans to KB epithelial cells (subline of the ubiquitous keratin-forming tumor cell line HeLa) (48). OMVs from a biofilm-forming H. pylori strain stimulated biofilm formation in another strain (49). OMVs of P. gingivalis contained multiple complexes of adhesins which caused cellular aggregation, autoaggregation, and coaggregation with a number of oral bacteria thereby enabling formation of dental plaque and influencing its ecology (43, 45). In P. gingivalis, OMVs promoted adherence between homologous cells and also mediated attachment between non-aggregating bacterial species (43). Kamaguchi et al. (45) found that OMVs from P. gingivalis have the ability to aggregate with a wide range of Streptococcus species, Fusobacterium nucleatum, Actinomyces naeslundii, and Actinomyces viscosus. When P. gingivalis OMVs were present, S. aureus also coaggregated with Streptococcus species and the mycelial form of Candida albicans. It was suggested that strains of S. aureus, even the methicillin resistant S. aureus (MRSA) type, could adhere to subgingival plaque with P. gingivalis present. It has also been shown that P. gingivalis or its OMVs can increase attachment and invasion of Tannerella forsythia to epithelial cells (46). The mixed interaction of the two red complex bacteria P. gingivalis and T. forsythia may increase periodontitis pathogenesis through OMV action. The HGP 17 domain was found to be responsible for P. gingivalis OMV-mediated aggregation with Prevotella intermedia (47).
OMVs from A. actinomycetemcomitans promote damage to the sulcular/periodontal epithelium by transporting CDT and LPS to the subgingival area (50, 51). OMVs can therefore act as a transport system to bring virulence factors into host cells affecting the microbial ecology of these cells. OMVs from P. gingivalis mediated coaggregation and piggybacking of Treponema denticola and Lachnoanaerobaculum saburreum (52). This may be a mechanism that provides access of non-motile bacteria to new niches where they might not otherwise be able to penetrate. P. gingivalis OMVs also mediated coaggregation between Capnocytophaga ochracea and L. saburreum (43). Besides, OMVs from P. gingivalis bound to and aggregated A. viscosus and A. naeslundii cells (53). They also attached to serum-coated hydroxyapaptite (54). In the OMV-cell recognition, LPS has been suggested to play a role (7).
OMVs can also facilitate transport of material between bacteria to maintain the microbiota (36). They can transfer antibiotic-resistance plasmids among Gram-negative bacteria (20) and P. aeruginosa may deliver antibiotic-resistance enzymes to other bacteria (55). Delivery of murein hydrolase was demonstrated when P. aeruginosa OMVs fused with E. coli and associated with S. aureus (19). It has been suggested that murein hydrolases in OMVs can be an effective way of bringing enzymes to the surfaces of other cells. This could imply fusion of OMVs with foreign membranes (56, 57). Vesicles from Shigella flexneri and P. aeruginosa rapidly fused with the OM of other Gram-negative bacteria (58). Fusion may cause incorporation of vesicle components directly into the cytoplasmic membrane or the cytoplasmic lumen of host cells.
Bacteroides OMVs may have a ‘social’ function since oligosaccharides, monosaccharides, and amino acids resulting from the activity of their containing hydrolytic enzymes could be made available for other bacteria (59). OMV-packed hydrolases from this bacterium could play an important role in the microbial ecology of the gut (60). Also, digestion of polysaccharides by hydrolases present in OMVs could support the growth of bacteria that are unable to degrade polysaccharides, thereby contributing to the gut homeostasis. This could create balanced ecological units within the gut microbiota (60).
β-lactamases in M. catarrhalis OMVs were found to enhance survival of its own species and also promote infection with co-inhabiting pathogens such as H. influenzae and S. pneumoniae (25). This clearly demonstrated the role of OMVS as an ecological determinant and that can be used by bacteria to establish a colonization niche (36).
OMVs as offensive weapons
Adhesion and invasion
P. gingivalis OMVs swiftly adhered to human gingival epithelial cells in a fimbriae-dependent manner, and then entered via a lipid rafts-dependent endocytic pathway through the assembly of actin filaments (Fig. 2). The OMVs were routed to early endosomes and thereafter sorted to proteolytic lysosomes (17). Following cell entry, P. gingivalis OMV-associated gingipains degraded cellular functional molecules causing cellular impairment, which included the cellular transferrin receptor and paxillin (integrin-related signaling molecule)/focal adhesion kinase. This caused depletion of intracellular transferrin and inhibition of cellular migration (17).
Fig. 2.
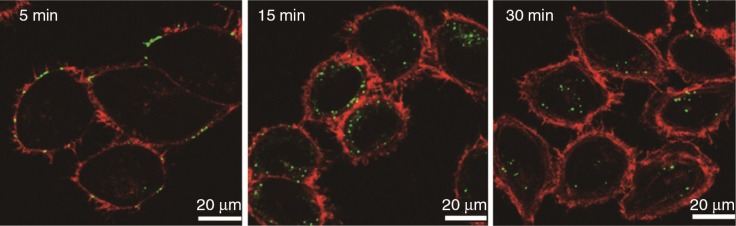
Entry of OMVs isolated from P. gingivalis ATCC 33277 into immortalized gingival epithelial cells. The cells were incubated with OMVs (30 µg/ml) for 15 min, then further incubated for the indicated times. For fluorescence microscopy, the cells were processed for staining for OMVs (green) and actin (Alexa Fluor 568-conjugated phalloidin red).
It has been shown that microspheres coated with P. gingivalis vesicles are adhesive and interact with both bacteria and host cells (61–63). They could even invade host cells and cause cell death (62, 63). Recently, it was demonstrated that minor components of long fimbriae (FimC, D and E) but not FimA were involved in the invasive activities of OMVs from P. gingivalis (64). Notably, P. gingivalis strains that lacked or had a reduced FimA expression exhibited a significant reduction in vesiculation suggesting that production and pathogenicity of P. gingivalis vesicles may depend to a large extent upon expression of the fim locus. Invasion by OMVs could be a new mechanism for P. gingivalis in periodontitis enabling their gingipain content to degrade a range of intracellular functional molecules, resulting in cellular impairment (65).
Bacterial defense
A significant task of OMVs is to remove agents that can harm the cell-surface of the parent bacterium, for example, antimicrobial peptides and T4 bacteriophages (38). When bacterial cells were treated with lytic phage OMV production increased the survival of the cells (38). The number of OMVs released from bacteria seems to be related to stress which may promote biofilm formation and biofilm-specific antibiotic tolerance and resistance (66–68). As mentioned OMVs can probably deliver antibiotics in high concentrations to the target as a package (11) and the biofilm mode of growth can protect them against antimicrobial substances (68). OMVs also have a role in antimicrobial peptide resistance (69). They protect against host antibodies and proteases thereby increasing the half-life of toxins packed inside (70, 71). Besides, they can adsorb antibiotics and complement (72).
Virulence bullets/bacterial bombs
OMVs have been designated both as virulence bullets and bacterial bombs. Because a distinction is not easy to make these terms will be used together.
OMVs can be involved in cell–cell inhibition and killing among competing bacterial cells. Thus, they can carry antimicrobials that selectively kill cells from other species (15, 73). In A. actinomycetemcomitans OMVs, a leukotoxin kills lymphocytic and monomyelocytic cell lineages which should defend the periodontal pocket against infection. OMVs from the highly leukotoxic strain JP2 were 5- to 10-fold more toxic than vesicles from the minimally toxic strain 652 (74). The vesicles of JP2 were also 4- to 5-fold more toxic than their OM preparations. Therefore, formation of OMVs in A. actinomycetemcomitans involved enrichment of leukotoxin. OMVs of P. gingivalis contain gingipains which remodel the normally symbiotic microbiota into a dysbiotic one by C5 convertase action (75). Grenier and Bélanger (76) suggested that OMVs and LPSs released by P. gingivalis could protect other bacteria from complement action in the periodontal pocket thereby favoring periodontal disease. P. gingivalis OMVs can also induce formation of murine macrophage foam cells (77) and are potent activators and aggregative factors for murine platelets, although initial adherence may be mediated by fimbriae (78).
Toxins and virulence factors in OMVs are often shipped directly to host cells (11). Actually, OMVs can deliver their toxins without environmental degradation, immune detection, or dilution of their cargo (56, 79). They can also deliver their toxins and other virulence factors at high concentrations to distant targets and mammalian cells without close contact (for review, see 34). OMVs can harbor large amounts of LPS which normally is a major component of the outer leaflet of the OM. In P. aeruginosa modulation of O-polysaccharide expression had an influence on the physical and compositional properties of OMVs suggesting a role in the differential protein sorting of different strains (10). Kadurugamuwa and Beveridge (11) found that in this species natural OMVs were strongly enriched in serotype specific antigen (B-band) in the O-antigen portion of LPS. This was in contrast to the lipid composition of the OM. Bacteria can even modify their OMV cargo according to the environment implying that the OMV components and cargo might be actively sorted by the producing cell (14). In P. gingivalis, gingipains which are major virulence factors were selectively sorted out as OMV cargo whereas other abundant OM proteins, for example, those involved in the nutrient uptake, were excluded but remained in the OM (80). Accordingly, OMV production was a result of a directed process where specific events were involved in specific exclusion and/or inclusion of protein sorting into the OM and OMVs (8).
Bacteria have different methods for recruiting cargo into their OMVs (8). The O-antigen of LPS can have a role in the selection of the protein cargo. In P. gingivalis, which produces two classes of LPS with either neutral (O-LPS) (81) or negatively charged (A-LPS) O-antigen chains (82, 83), the cargo proteins may have a domain that recognizes the long A-LPS molecules enriched in the OMVs (80). Virulence factors of ecologic importance in P. gingivalis are gingipains (RgpA/B and Kgp) which are among the favored OMV cargo (42, 80). This implies that P. gingivalis has the ability to selectively sort its C-terminal domains proteins into OMVs.
In Gram-negative bacteria OMVs can be enriched in toxins, quorum-sensing molecules, misfolded proteins, and DNA (8). Proteins of the OMVs are sorted and also glycans can be involved in the sorting. In A. actinomycetemcomitans a subpopulation of OMVs was found with slight variation in the protein composition (34). Actually, OMVs from A. actinomycetemcomitans could deliver multiple proteins simultaneously including OmpA and biologically active cytolethal toxin into HeLa cells and gingival fibroblasts (16). Also in S. pneumoniae MVs, many reported immunogenic protein antigens were found (2), including toxin Ply which is its most widely studied virulence factor. In cystic fibrosis, cystic fibrosis transmembrane conductance regulator (CTFR) is required for mucociliary clearance. P. aeruginosa promotes degradation of CTFR through the OMV-packed toxin Cif (CTFR inhibitory factor) (8). Cif is brought to host cells after OMV fusion with lipid rafts causing lysosomal degradation of CFTR (56).
OMVs from one bacterium can kill competing microbes in its vicinity (73). Killing was most efficient if the target bacteria possessed peptidoglycan similar to the OMV donor. If the peptidoglycan hydrolases were similar to those of the target strain they were unable to cleave the peptidoglycan layer (13). This may change if they fuse with cells of a different strain. In that case they can degrade the cell wall and kill the target cell (84). OMVs from bacteria can also fuse with OM of other bacteria. This may release vesicle-encapsulated autolysin to the periplasm thereby causing lysis of the target organisms. This predatory response may allow microcolonies to live in a biofilm at the expense of neighboring cells (85, 86).
Sepsis
Oral bacteria can be associated with systemic diseases (87). Bacteria frequently disseminate through the blood, particularly in periodontitis. OMVs are important sources of inflammatory stimulants both locally and systemically when entering the circulation (88). They induce a robust systemic inflammatory response causing organ damage and death in animals (89, 90). By initiating an inflammatory response, they can induce sepsis in rats even when the cells from which they were derived are absent (89). OMVs also influence the inflammatory and coagulation cascade and may thus contribute to the hypercoagulable response in sepsis (91). Important in this context is their high content of LPS which is a potent proinflammatory trigger. OMVs from different bacteria can activate the immune system in different ways (reviewed by 8) and stimulate the production of proinflammatory mediators such as IL-8, IL-6, IL-12, and TNF-α (92). P. gingivalis OMVs regulate cells that participate in immune responses (93) and even have a crucial role in mucosal immunogenicity (94).
OMVs as possible good Samaritans
Good Samaritans
OMVs can contribute to gut health via immunomodulation of host responses or by providing nutrients to members of the gut microbiota (8). Polysaccharide capsular antigen (PSA) from Bacteroides fragilis reduced inflammation in animals by inducing immune tolerance (95). It seems that OMVs delivered this PSA directly to its host through DCs (96). OMVs that are internalized by DCs induce tolerogenic DCs that generate interleukin 10 (IL-10) which in turn drives the development of IL-10 producing regulatory T-cells (TREGS). Therefore, PSA programs DCs to change into an anti-inflammatory profile that can lead to T-cell-mediated tolerance and protection from experimental disease such as colitis, inflammatory bowel disease, and Crohn's disease (96). It is likely that delivery of PSA by OMVs contributes to the probiotic properties of B. fragilis in the gut enabling communication between the microbiota and the immune system during host-bacterial mutualism (96). In this interplay PSA of B. fragilis is an archetypical symbiosis factor. Accordingly, OMVs can be important tools for modulators of the microbiota in the gut and in this sense they act as good Samaritans (12). Whether similar modification of the oral microbiota occurs is not known. However, it is likely (by extrapolation) that ‘good Samaritan’ activities might be delivered by vesicles in the mouth that are similar to those reported for the gut ecosystem. It is not clear what maintains the balance between OMVs causing destructive events intracellularly and those that induce a benefit. In the case of B. fragilis, it should be kept in mind that this bacterium belongs to the commensal microbiota of the gut and therefore probably represents no threat to Gram-negatives trying to induce a dysbiotic gut microbiota.
Vaccines/adjuvants
Application of OMVs as vaccine antigens after intranasal immunization of BALB/c mice gave high levels of P. gingivalis-specific IgA in nasal washes and saliva and in serum IgG and IgA. This suggested a potential role of P. gingivalis OMVs as non-replicating mucosal immunogens for vaccines against periodontal disease. The range of virulence factors enriched in P. gingivalis OMVs may make them particularly suited for a periodontal disease vaccine (97). Neisserial OMVs are considered a successful vaccine immunogen against bacterial meningitis (98) such as the commercially available vaccine against Neisseria meningitidis serogroup B. It is thought that OMVs are safer as antigens than whole bacterial cells and can harbor a number of cell-surface markers such as LPS and proteins to stimulate an immune response (31). OMVs have also been manipulated to act as adjuvants while displaying foreign epitopes of interest resulting in some success for producing a single vaccine against both (14).
Classification
OMVs have proved useful for classification of members of the Actinobacillus-Haemophilus-Pasteurella group (99) where several belong to the oral ecosystem.
Concluding remarks
OMVs have a number of functions as described in the current review: virulence bullets, bacterial bombs, decoys, vehicles for cell–cell signaling, mediators of immune regulation and disease protection, unique secretion systems, good Samaritans, and so on. It is amazing how these small vesicles can serve functions that are good to themselves and their parent bacteria and even to the host. However, they have also clear detrimental effects towards other bacteria and the host. There is no question; these structures are both offensive weapons and good Samaritans. Their diversity in function is remarkable. We should probably try to modify them for the benefit of the host, for example, as therapeutics against disease. A specific field of interest is new and emerging bacterial and virus infections where engineered OMVs containing proteins might be used as decoys and vaccines.
Acknowledgements
I.O. wants to acknowledge funding through the European Commission (FP7-HEALTH-306029 ‘TRIGGER’).
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors. Funding was as given under Acknowledgement.
References
- 1.Kaback HR. Transport studies in bacterial membrane vesicles. Science. 1974;186:882–92. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- 2.Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martín-Peña R, González-Reyes JA, Jiménez-Munguía, et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae . J Proteomics. 2014;106:46–60. doi: 10.1016/j.jprot.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Liao S, Klein MI, Heim KP, Fan Y, Bitoun JP, Ahn SJ, et al. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol. 2014;196:2355–66. doi: 10.1128/JB.01493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popkin TJ, Theodore TS, Cole RM. Electron microscopy during release and purification of mesosomal vesicles and protoplast membranes from Staphylococcus aureus . J Bacteriol. 1971;107:907–17. doi: 10.1128/jb.107.3.907-917.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–36. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 6.Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One. 2011;6:e27958. doi: 10.1371/journal.pone.0027958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berleman J, Auer M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ Microbiol. 2013;15:347–54. doi: 10.1111/1462-2920.12048. [DOI] [PubMed] [Google Scholar]
- 8.Haurat MF, Elhenaway W, Feldman MF. Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol Chem. 2014 doi: 10.1515/hsz-2014-0183. [DOI] [PubMed] [Google Scholar]
- 9.Mayrand D, Grenier D. Biological activities of outer membrane vesicles. Can J Microbiol. 1989;35:607–13. doi: 10.1139/m89-097. [DOI] [PubMed] [Google Scholar]
- 10.Murphy K, Park AJ, Hao Y, Brewer D, Lam JS, Khursigara CM. Influence of O polysaccharides on biofilm development and outer membrane vesicle biogenesis in Pseudomonas aeruginosa PAOI. J Bacteriol. 2014;196:1306–17. doi: 10.1128/JB.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuehn MJ. Secreted bacterial vesicles as good Samaritans. Cell Host Microbe. 2012;12:392–3. doi: 10.1016/j.chom.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni H, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology. 2014;160:2109–21. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 14.Schertzer JW, Whiteley M. Bacterial outer membrane vesicles in trafficking, communication and the host-pathogen interaction. J Mol Microbiol Biotechnol. 2013;23:118–30. doi: 10.1159/000346770. [DOI] [PubMed] [Google Scholar]
- 15.Kadurugamuwa JL, Beveridge TJ. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J Antimicrob Chemother. 1997;40:615–21. doi: 10.1093/jac/40.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans . Infect Immun. 2012;80:31–42. doi: 10.1128/IAI.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuta N, Takeuchi H, Amano A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect Immun. 2009;77:4761–70. doi: 10.1128/IAI.00841-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–57. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–74. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae . J Bacteriol. 1989;171:2499–505. doi: 10.1128/jb.171.5.2499-2505.1989. Erratum in: J Bacteriol 1989; 171: 4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn ME, Barany F, Smith HO. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci USA. 1983;80:6927–31. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microbiol. 1999;65:1843–8. doi: 10.1128/aem.65.5.1843-1848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deich RA, Hoyer LC. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J Bacteriol. 1982;152:855–64. doi: 10.1128/jb.152.2.855-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renelli M, Matias V, Lo RY, Beveridge TJ. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology. 2004;150:2161–9. doi: 10.1099/mic.0.26841-0. [DOI] [PubMed] [Google Scholar]
- 25.Schaar V, Nordström T, Mörgelin M, Riesbeck K. Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother. 2011;55:3845–53. doi: 10.1128/AAC.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaar V, Paulsson M, Mörgelin M, Riesbeck K. Outer membrane vesicles shield Moraxella catarrhalis β-lactamase from neutralization by serum IgG. J Antimicrob Chemother. 2013;68:593–600. doi: 10.1093/jac/dks444. [DOI] [PubMed] [Google Scholar]
- 27.Schaar V, Uddbäck I, Nordström T, Riesbeck K. Group A streptococci are protected from amoxicillin-mediated killing by vesicles containing β-lactamase derived from Haemophilus influenzae . J Antimicrob Chemother. 2014;69:117–20. doi: 10.1093/jac/dkt307. [DOI] [PubMed] [Google Scholar]
- 28.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–5. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald KL, Beveridge TJ. Bactericidal effect of gentamicin-induced membrane vesicles derived from Pseudomonas aeruginosa PAO1 on gram-positive bacteria. Can J Microbiol. 2002;48:810–20. doi: 10.1139/w02-077. [DOI] [PubMed] [Google Scholar]
- 30.Collins BS. Gram-negative outer membrane vesicles in vaccine development. Discov Med. 2011;12:7–15. [PubMed] [Google Scholar]
- 31.Sanders H, Feavers IM. Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines. Expert Rev Vaccines. 2011;10:323–34. doi: 10.1586/erv.11.10. [DOI] [PubMed] [Google Scholar]
- 32.Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–85. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thay B, Damm A, Kufer TA, Wai SN, Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-κB activation. Infect Immun. 2014;82:4034–46. doi: 10.1128/IAI.01980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidakovics ML, Jendholm J, Mörgelin M, Månsson A, Larsson C, Cardell LO. B cell activation by outer membrane vesicles – a novel virulence mechanism. PLoS Pathog. 2010;6:e1000724. doi: 10.1371/journal.ppat.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amano A, Takeuchi H, Furuta N. Outer membrane vesicles function as offensive weapons in host-parasite interactions. Microbes Infect. 2010;12:791–8. doi: 10.1016/j.micinf.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Loeb MR, Kilner J. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B. Biochim Biophys Acta. 1978;514:117–27. doi: 10.1016/0005-2736(78)90081-0. [DOI] [PubMed] [Google Scholar]
- 38.Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258. doi: 10.1186/1471-2180-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188:5945–57. doi: 10.1128/JB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stentz R, Osborne S, Horn N, Li AW, Hautefort I, Bongaerts R, et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014;6:646–56. doi: 10.1016/j.celrep.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang SC, Kim SR, Yoon YJ, Park KS, Kim JH, Lee J, et al. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small. 2014 doi: 10.1002/smll.201401803. [DOI] [PubMed] [Google Scholar]
- 42.Veith PD, Chen YY, Gorasia DG, Chen D, Glew MD, O'Brien-Simpson NM, et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 2014;13:2420–32. doi: 10.1021/pr401227e. [DOI] [PubMed] [Google Scholar]
- 43.Grenier D, Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis . Infect Immun. 1987;55:111–7. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 45.Kamaguchi A, Nakayama K, Ichiyama S, Nakamura R, Watanabe T, Ohta M, et al. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. 2003;47:485–91. doi: 10.1007/s00284-003-4069-6. [DOI] [PubMed] [Google Scholar]
- 46.Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia.”. Infect Immun. 2006;74:5023–8. doi: 10.1128/IAI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamaguchi A, Ohyama T, Sakai E, Nakamura R, Watanabe T, Baba H, et al. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia . Microbiology. 2003;149:1257–64. doi: 10.1099/mic.0.25997-0. [DOI] [PubMed] [Google Scholar]
- 48.Meyer DH, Fives-Taylor PM. Characteristics of adherence of Actinobacillus actinomycetemcomitans to epithelial cells. Infect Immun. 1994;62:928–35. doi: 10.1128/iai.62.3.928-935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, et al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009;9:197. doi: 10.1186/1471-2180-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damek-Poprawa M, Haris M, Volgina A, Korostoff J, DiRienzo JM. Cytolethal distending toxin damages the oral epithelium of gingival explants. J Dent Res. 2011;90:874–9. doi: 10.1177/0022034511403743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohara M, Miyauchi M, Tsuruda K, Takata T, Sugai M. Topical application of Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces cell cycle arrest in the rat gingival epithelium in vivo . J Periodontal Res. 2011;46:389–95. doi: 10.1111/j.1600-0765.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- 52.Grenier D. Porphyromonas gingivalis outer membrane vesicles mediate coaggregation and piggybacking of Treponema denticola and Lachnoanaerobaculum saburreum . Int J Dent. 2013:305476. doi: 10.1155/2013/305476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellen RP, Grove DA. Bacteroides gingivalis vesicles bind to and aggregate Actinomyces viscosus . Infect Immun. 1989;57:1618–20. doi: 10.1128/iai.57.5.1618-1620.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh U, Grenier D, McBride BC. Bacteroides gingivalis vesicles mediate attachment of streptococci to serum-coated hydroxyapatite. Oral Microbiol Immunol. 1989;4:199–203. doi: 10.1111/j.1399-302x.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 55.Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Høiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa . J Antimicrob Chemother. 2000;45:9–13. doi: 10.1093/jac/45.1.9. [DOI] [PubMed] [Google Scholar]
- 56.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5:e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demuth DR, James D, Kowashi Y, Kato S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol. 2003;5:111–21. doi: 10.1046/j.1462-5822.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 58.Kadurugamuwa JL, Beveridge TJ. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicrob Agents Chemother. 1998;42:1476–83. doi: 10.1128/aac.42.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elhenaway W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. MBio. 2014;5:e00909-14. doi: 10.1128/mBio.00909-14. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rakoff-Nahoum S, Coyne MJ, Comstock LE. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol. 2014;24:40–9. doi: 10.1016/j.cub.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duchesne P, Grenier D, Mayrand D. Demonstration of adherence properties of Porphyromonas gingivalis outer membrane vesicles using a new microassay. Oral Microbiol Immunol. 1995;10:76–80. doi: 10.1111/j.1399-302x.1995.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 62.Inaba H, Kawai S, Kato T, Nakagawa I, Amano A. Association between epithelial cell death and invasion by microspheres conjugated to Porphyromonas gingivalis vesicles with different types of fimbriae. Infect Immun. 2006;74:734–9. doi: 10.1128/IAI.74.1.734-739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuda K, Amano A, Umebayashi K, Inaba H, Nakagawa I, Nakanishi Y, et al. Molecular dissection of internalization of Porphyromonas gingivalis by cells using fluorescent beads coated with bacterial membrane vesicle. Cell Struct Funct. 2005;30:81–91. doi: 10.1247/csf.30.81. [DOI] [PubMed] [Google Scholar]
- 64.Mantri CK, Chen C, Dong X, Goodwin JS, Pratap S, Paromov V, et al. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis . Microbiologyopen. 2014 D doi: 10.1002/mbo3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amano A, Chen C, Honma K, Li C, Settem RP, Sharma A. Genetic characteristics and pathogenic mechanisms of periodontal pathogens. Adv Dent Res. 2014;26:15–22. doi: 10.1177/0022034514526237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baumgarten T, Sperling S, Seifert J, von Bergen M, Steiniger F, Wick LY, et al. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol. 2012;78:6217–24. doi: 10.1128/AEM.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl Environ Microbiol. 2014;80:3469–83. doi: 10.1128/AEM.04248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsen I. Biofilm-related antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis. 2015;34 doi: 10.1007/s10096-015-2323-z. [DOI] [PubMed] [Google Scholar]
- 69.MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012;163:607–18. doi: 10.1016/j.resmic.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aldick T, Bielaszewska M, Uhlin BE, Humpf HU, Wai SN, Karch H. Vesicular stabilization and activity augmentation of enterohaemorrhagic Escherichia coli haemolysin. Mol Microbiol. 2009;71:1496–508. doi: 10.1111/j.1365-2958.2009.06618.x. [DOI] [PubMed] [Google Scholar]
- 71.Duperthuy M, Sjöström AE, Sabharwal D, Damghani F, Uhlin BE, Wai SN. Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 2013;9:e1003620. doi: 10.1371/journal.ppat.1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan TT, Morgelin M, Forsgren A, Riesbeck K. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J Infect Dis. 2007;195:1661–70. doi: 10.1086/517611. [DOI] [PubMed] [Google Scholar]
- 73.Li Z, Clarke AJ, Beveridge TJ. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J Bacteriol. 1998;180:5478–83. doi: 10.1128/jb.180.20.5478-5483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- 75.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–25. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grenier D, Bélanger M. Protective effect of Porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun. 1991;59:3004–8. doi: 10.1128/iai.59.9.3004-3008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog. 2003;35:259–67. doi: 10.1016/j.micpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Sharma A, Novak EK, Sojar HT, Swank RT, Kuramitsu HK, Genco RJ. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol Immunol. 2000;15:393–6. doi: 10.1034/j.1399-302x.2000.150610.x. [DOI] [PubMed] [Google Scholar]
- 79.Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Microbiol Mol Microbiol. 2006;61:839–46. doi: 10.1111/j.1365-2958.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- 80.Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem. 2011;286:1269–76. doi: 10.1074/jbc.M110.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paramonov N, Bailey D, Rangarajan M, Hashim A, Kelly G, Curtis MA, et al. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur J Biochem. 2001;268:4698–707. doi: 10.1046/j.1432-1327.2001.02397.x. [DOI] [PubMed] [Google Scholar]
- 82.Rangarajan M, Aduse-Opoku J, Paramonov N, Hashim A, Bostanci N, Fraser OP, et al. Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J Bacteriol. 2008;190:2920–32. doi: 10.1128/JB.01868-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paramonov N, Rangarajan M, Hashim A, Gallagher A, Aduse-Opoku J, Slaney JM, et al. Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to Arg-gingipain glycans. Mol Microbiol. 2005;58:847–63. doi: 10.1111/j.1365-2958.2005.04871.x. [DOI] [PubMed] [Google Scholar]
- 84.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–33. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. Interactions between biofilms and the environment. FEMS Microbiol Rev. 1997;20:291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 86.Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surface of other gram-negative bacteria. Microbiology. 1999;145:2051–60. doi: 10.1099/13500872-145-8-2051. [DOI] [PubMed] [Google Scholar]
- 87.Olsen I. The periodontal-systemic connection seen from a microbiological standpoint. Acta Odontol Scand. 2015;73 doi: 10.3109/00016357.2015.1007480. in press. [DOI] [PubMed] [Google Scholar]
- 88.Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!” – epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah B, Sullivan CJ, Lonergan NE, Stanley S, Soult MC, Britt LD. Circulating bacterial membrane vesicles cause sepsis in rats. Shock. 2012;37:621–8. doi: 10.1097/SHK.0b013e318250de5d. [DOI] [PubMed] [Google Scholar]
- 90.Soult MC, Lonergan NE, Shah B, Kim WK, Britt LD, Sullivan CJ. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J Surg Res. 2013;184:458–66. doi: 10.1016/j.jss.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 91.Soult MC, Dobrydneva Y, Wahab KH, Britt LD, Sullivan CJ. Outer membrane vesicles alter inflammation and coagulation mediators. J Surg Res. 2014;192:134–42. doi: 10.1016/j.jss.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srisatjaluk R, Doyle RJ, Justus DE. Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-gamma-mediated MHC class II expression by human vascular endothelial cells. Microb Pathog. 1999;27:81–91. doi: 10.1006/mpat.1999.0287. [DOI] [PubMed] [Google Scholar]
- 94.Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, et al. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One. 2011;6:26163. doi: 10.1371/journal.pone.0026163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–20. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakao R, Takashiba S, Kosono S, Yoshida M, Watanabe H, Ohnishi M, et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. 2014;16:6–16. doi: 10.1016/j.micinf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis . Vaccine. 2009;27(Suppl 2):3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 99.Myhrvold V, Brondz I, Olsen I. Application of multivariate analyses of enzymic data to classification of members of the Actinobacillus-Haemophilus-Pasteurella group. Int J Syst Bacteriol. 1992;42:12–8. doi: 10.1099/00207713-42-1-12. [DOI] [PubMed] [Google Scholar]