Abstract
Background
Severe-early childhood caries (S-ECC) is one of the most common infectious diseases in children and is prevalent in lower socio-economic populations. American Indian children suffer from the highest levels of S-ECC in the United States. Members of the mutans streptococci, Streptococcus mutans, in particular, are key etiologic agents in the development of caries. Children typically acquire S. mutans from their mothers and early acquisition is often associated with higher levels of tooth decay.
Methods
We have conducted a 5-year birth cohort study with a Northern Plains Tribe to determine the temporality and fidelity of S. mutans transmission from mother to child in addition to the genotypic diversity of S. mutans in this community. Plaque samples were collected from 239 mother/child dyads at regular intervals from birth to 36 months and S. mutans were isolated and genotyped by arbitrarily primed-polymerase chain reaction (AP-PCR).
Results
Here we present preliminary findings from a subset of the cohort. The focus for this paper is on initial acquisition events in the children. We identified 17 unique genotypes in 711 S. mutans isolates in our subset of 40 children, 40 mothers and 14 primary caregivers. Twelve of these genotypes were identified in more than one individual. S. mutans colonization occurred by 16 months in 57.5% of the children and early colonization was associated with higher decayed, missing and filled surface (DMFS) scores (p=0.0007). Children colonized by S. mutans shared a common genotype with their mothers 47.8% of the time. While multiple genotypes were common in adults, only 10% of children harbored multiple genotypes.
Conclusion
These children acquire S. mutans at an earlier age than the originally described ‘window of infectivity’ and often, but not exclusively, from their mothers. Early acquisition is associated with both the caries status of the children and the mothers.
Keywords: caries, etiologic agents, American Indian, children, Streptococcus mutans, genotypic diversity, genotypes, oral microbiology, severe early childhood caries
There is growing evidence of a substantial oral health disparities problem in young children in our society (1–5). While dental caries continues to burden youth and adults at all ages, there is disparaging evidence of a devastating disease called severe-early childhood caries (S-ECC) in lower socio-economic and minority children in the United States and worldwide (6). The highest prevalence of this disease occurs in American Indian (AI) children. The most recent surveys have revealed an extremely high level of S-ECC in AI children (2, 7–9). By 5 years of age, 75% of AI children had significant caries experience with three times the severity of the national average (8). Many studies have now shown that American Indian children are at the highest risk of caries compared to any other racial/ethnic group (7, 10–12).
Many studies have shown that the mutans streptococci exhibit a number of virulence factors that can lead to the development of caries (13–17). These organisms produce copious amounts of lactic acid in the presence of dietary sucrose, which leads to demineralization of the tooth surface. Moreover, the mutans streptococci (MS) are highly aciduric, giving them the ability to survive and grow in a highly acidic environment (13, 18, 19). Coupled with their ability to form tenacious biofilms, these organisms become dominant within the supragingival plaque communities and produce a cariogenic plaque.
It has been consistently reported that the mutans streptococci are readily transmitted from mother to child (17, 20–25). Studies have revealed a window of infectivity whereby very young children are most likely to acquire these bacteria (25–28). Although it is generally accepted that vertical transmission from mother to child is the primary route of acquisition of these bacteria, there is substantial evidence that horizontal transmission amongst children also occurs frequently under the right conditions (26, 29–32).
There is evidence of extensive variation in mutans streptococci isolates (33, 34). A number of methods have been used to characterize strain diversity with DNA fingerprinting being one of the most prevalent (35). Studies have shown multiple genotypes of Streptococcus mutans in adults and children, although overall the diversity in children appears to be less. Some studies have shown different genotypes of SM that are associated with caries versus caries-free status, as well as differences in numbers and diversity of genotypes within individuals in these two subgroups, whereas others have shown opposite profiles of genotype numbers and diversity amongst caries-active individuals (33, 34, 36, 37). It is conceivable that specific SM genotypes are more readily transmitted and acquired by very young children and colonization by these specific genotypes renders these children at higher risk for caries development.
We have conducted a 5-year birth cohort study with a Northern Plains Tribe addressing the question why AI children suffer from S-ECC. We had 239 mother/child dyads enrolled in this study and we followed them from birth until the child was 36 months of age. Our focus was on transmission and acquisition of genotypes of S. mutans from mother to child. We obtained information on many other variables, including comprehensive dietary, behavioral, and environmental factors that could play a role in the establishment of a cariogenic oral microflora in these children. Our overall hypothesis was that children acquire these cariogenic bacteria at a very early age and the afore-mentioned variables act in an orchestrated manner to create a situation in which the children are at elevated risk for the development of S-ECC. This hypothesis would be in agreement with other investigators’ findings that suggest the previous ‘window of infectivity’ may be somewhat earlier than the initially proposed timeframe of 19–31 months (26, 27, 38, 39).
Here we report on some of our initial findings on transmission and acquisition of the SM in very young AI children. We also report on the diversity and commonality (14) of SM genotypes from these AI children and their mothers.
Material and methods
Study population
Our study population was from a Northern Plains American Indian Tribe. Our initial target was to have 200 mothers and newborns. However, successful recruitment of mothers who were pregnant or who had just given birth resulted in enrollment of 239 mother/child dyads in the study. Our onsite research team members were all AI and under the guidance of a study director who was a senior dental hygienist. This particular manuscript, focused on 40 families for microbiological analysis of plaque samples collected within the first 16 months of the child's life. Therefore, all data with the exception of DMFS (Decayed, Missing or Filled Surface) scores will be based on these 40 families consisting of 40 children, 40 mothers and 14 primary caregivers. DMFS score analysis is based on 39 families due to the death of one child prior to the 36-month time point but after the 12-month time point.
Samples were processed as they arrived at our laboratory and were plated for enumeration of total flora and mutans streptococci. However, the isolation of mutans streptococci and analysis of S. mutans isolates was very time consuming and all samples could not be analyzed in this way. Upon regular review of our progress and data analysis we felt it was important to describe the initial acquisition events that are presented here. Therefore, we targeted a subset of families in which substantial progress in the isolation of S. mutans had been made to the 16-month time point. We identified 40 families which were consistent with the total cohort with respect to bacterial load in mothers and children, as well as the presence/absence of mutans streptococci at each time point in the children.
Recruitment and retention
For recruitment and retention, we followed this process. Like most Northern Plains tribal communities, Indian Health Service (IHS) is the primary health care provider and most pregnant women receive their prenatal care and deliver at the local IHS hospital. Women were informed of the proposed study by the maternity support nurses and physicians during their prenatal visits. In addition, advertisements were placed in the local papers and aired on the local radio stations. Since the dental clinic staffs were very supportive of this project, and in direct contact with parents of children with ECC, they were also significantly involved in recruiting expectant mothers. If a woman was interested in participating, she was contacted by the local recruiter who explained the project, obtained informed consent and scheduled the initial screening visit. Since all births are included in the IHS patient tracking system (referred to the Resource and Patient Management System [RPMS]), we were able track each potential study participant, determine their willingness to participate, and calculate response rates in each community. To assure that recruitment plans and materials were engaging yet community appropriate, we had two focus group sessions with mothers of young children. The first focus group gathered information on the best methods for recruiting study participants (including appropriate incentives) while the second focus group helped refine the recruitment strategy, printed materials and public service announcements. Throughout the recruitment phase of the study, key informant interviews and focus groups were used to evaluate ongoing recruitment and retention strategies.
Traditional retention incentives such as birthday cards, newsletters, and small gifts would not be sufficient to maintain these high-risk families for a 2-year period. For this reason, monetary incentives were also given. Families received $90 for the first mother/child visit, and $35 for each subsequent mother/child visit for a sub-total of $245 [90+(7*35)=335]. Young families in these communities move frequently and often do not have telephones. However, many tend to stay on or near the reservation and can be located through family and friends. Using a local community member as a recruiter enabled us to maintain contact with the study participants even though they may have moved. Another important aspect of ensuring participant retention was that almost all of the study-related interactions took place in the participants’ homes. The Reservation is very rural and families often do not have access to a car (or money for gas). By traveling to the participants home, we reduced participate burden and increased long-term retention.
Inclusion/exclusion criteria for the study are as follows. Any mother with a newborn child 0–60 days old could participate in the study if she: lived in the same home as her child, lived within the boundaries of the research study area on the Indian Reservation, and anticipated living on or near the Indian Reservation for the next 3 years.
Consent
The Internal Review Board (IRB) on record was the Aberdeen Area IRB and we obtained approval. We also had to obtain approval from the University of Iowa IRB. Finally, we presented our study proposal and received approval (also a requirement) from the Tribal Research Review Board.
Clinical examination
Clinical examinations were detailed in Warren et al. (9) but will be summarized here. The caries examinations used dmfs/DMFS criteria adapted from those used by NHANES (40) and were conducted by trained and calibrated dental hygienist–examiners. These examinations were completed using the knee-to-knee method (41) for the children and with participants seated in a straight chair for the mothers. Both mothers and child examinations utilized a halogen headlight, a DenLite® illuminated mirror (Integra Miltex, York, PA) and dental explorer (Shepherd hook #23 explorer (Hu-Friedy, Chicago, IL)). The teeth were visually inspected after drying with gauze and the explorer was used only to remove debris or confirm areas of suspected decay.
Collection of plaque samples
Plaque samples were collected by a trained and calibrated dental hygienist from AI mothers and children in their homes. Samples were collected at ≤30 days (baseline), 4, 8, 12, 16, 22, 29, and 36 months (±30 days). If the child had a primary caregiver other than their mother, samples were collected from the caregiver as well. Whole mouth plaque samples were collected by swabbing all smooth surfaces of the teeth (or oral mucosa and tongues in infants without erupted teeth) with a sterile cotton swab. The swab was placed into a tube containing TSB–YE (Tryptic Soy Broth–Yeast Extract) (Difco, Sparks, MD) with 10% glycerol. Collection tubes were refrigerated until shipment to the microbiology laboratory for processing. Samples were shipped via FedEx overnight to the microbiology laboratories in the Dows Institute for Dental Research at the University of Iowa, College of Dentistry. Temperature was maintained by shipping samples in temperature controlled Saf-T-Temp™ packaging (Saf-T-Pak, Hanover, MD).
Sample processing and isolation of mutans streptococci
Swab samples were vortexed for 3 min and then placed in a sonicating water bath for 1 min to provide homogeneous suspensions of plaque bacteria. The resulting suspensions were diluted and plated on three types of media using an Autoplate® Spiral Plating System (Advanced Instruments, Inc., Norwood, MA). The samples were plated on Mitis-Salivarius-Kanamycin-Bacitracin agar (MSKB) (Difco, Sparks, MD) for determination of total mutans streptococci counts. Plates were incubated at 37°C, 5% CO2 for 96 hours.
Ten colonies displaying typical MS colony morphology were selected from the MSKB plate. Isolates were identified by fermentation profile (mannitol, raffinose, salicin, and sorbitol) and arginine decarboxylase activity. If there were fewer than 10 colonies, then all available colonies were selected. Identification was confirmed by PCR using primers specific to the S. mutans glucosyltransferase B (gtfB) gene. Confirmed S. mutans isolates were frozen down (TSB-YE, 10% glycerol) and stored at −80°C.
DNA extraction and arbitrarily primed PCR
Isolates were cultured in TSB–YE for 24 hours at 37°C, 5% CO2. DNA was extracted using the Epicentre® MasterPure™ Gram Positive DNA Purification Kit (Epicentre, Madison, WI) with the following modifications: (1) 25 ml culture resuspended in 1.8 ml TE, (2) 2 µl Ready-Lyse with 1 hour incubation, (3) 25 min Proteinase K incubation, (4) 1 hour RNase A incubation after Proteinase K incubation, (5) sample divided into three tubes for DNA precipitation steps. Genotypic diversity was examined by arbitrarily primed-polymerase chain reaction (AP-PCR) using the primer OPA-2 (5′-TGCCGAGCTG-3′). Each 50 µL PCR reaction contained 2 µL template DNA (50 ng/µL), 5 µL of 10X PCR buffer, 200 µM of dNTP, 7 mM MgCl2, 2.5 U Taq polymerase, and 4 µm of OPA-2 primer. S. mutans ATCC 25175 was used as a positive control for all reactions. Amplification was performed in a thermocycler (Eppendorf, Hauppauge, NY) programmed with the following temperature profile: initially 5 min at 94°C, followed by 45 cycles of denaturation at 94°C for 1 min, annealing at 36°C for 1 min, and elongation at 72°C for 2 min. Amplified products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide. A 100 bp DNA ladder served as a molecular size marker on the gels. Gel images were captured using a transilluminator and digital imaging system (Fotodyne, Hartland, WI). Genotypic diversity was assessed by generation of dendrograms using GelCompar ®IIv6.5 software (Applied Maths, Austin, TX). Curve based cluster analysis (1% optimization, 1% curve smoothing) using the Pearson correlation and Unweighted Pair Group Method using Arithmetic Averages (UPGMA) was used to assess strain relatedness. S. mutans isolates displaying greater than 70% similarity were considered to be the same genotype.
Statistical analysis
A total of 39 AI children were assayed for S. mutans status at 16 months using S. mutans selection media. Possible associations were evaluated between S. mutans status and caries outcomes from a surface-specific examination conducted at 36 months, as well as perinatal maternal characteristics. Bivariate evaluations utilized the Wilcoxon rank-sum test to test for differences in DMFS at 36 months, maternal DMFS, total maternal colony forming units (CFUs) for S. mutans, and overall bacterial burden. Fisher's exact test was used to test for association between gender and S. mutans status. Logistic regression was used for multivariable modeling to explore the association of maternal characteristics with the probability of S. mutans acquisition.
Results
Microbiological study
Forty mother/child dyads were analyzed during the first 16 months of the child's life, to determine acquisition of S. mutans. All of the individuals involved in the study are American Indian and live on the reservation. The 40 samples were a subset of the original 239 pairs, as previously explained. However, these 40 are representative of the entire cohort with respect to the percentage of individuals harboring S. mutans and caries. There were 711 S. mutans isolates recovered from 94 subjects (40 children, 40 mothers and 14 caregivers). 37.5% of children were colonized by 12 months of age and 57.5% were colonized by 16 months (Table 1). Preliminary analysis showed that 85% of the children were S. mutans positive by 3 years. This manuscript is focused on data from genotypic analysis up to 16 months of age in order to address S. mutans genotypic diversity at initial acquisition and fidelity of transmission from mother to child in order to determine whether vertical transmission is the primary avenue of acquisition of S. mutans in these children. In all but four of the mothers, S. mutans colonies were recovered at baseline and/or initial colonization of the child. Two of the S. mutans negative mothers would be S. mutans positive at later sampling times and two of the mothers remained S. mutans free throughout the study. One of the S. mutans negative mothers had a child that was colonized by S. mutans but that child did not share genotypes with the caregiver either.
Table 1.
Acquisition time and prevalence
| Age of child's initial colonization | Number of children with new S. mutans acquisitions | Total number of children with S. mutans |
|---|---|---|
| 4 months | 0 | 0 |
| 8 months | 2 | 2 |
| 12 months | 13 | 15 |
| 16 months | 8 | 23a |
| Not yet colonized | 17 | 17 |
New colonization represents the number of children from whom S. mutans colonies are first isolated at a given time point. The total number of children with S. mutans is the cumulative number of colonized children at each time point.
One child died between the 12 and 16 months sampling time point.
Clinical examination
Due to the death of one child, prior to 36 months but after the 16 month time point, only the surviving 39 children were included in these particular analyses. Of the 39 children (who we were able to obtain DMFS scores for), 21 were male (11 S. mutans+) and 18 were female (11 S. mutans+). Fisher's exact test did not find evidence of an association between sex and S. mutans status (p=0.7479) (data not shown). The Wilcoxon rank sum test identified significant differences in DMFS at 36 months (p=0.0007) (Fig. 1a) and maternal DMFS (p=0.0125) (Fig. 1b and Table 2), but not for maternal CFUs for S. mutans (p=0.44) or maternal levels of overall bacterial burden (p=0.42). When maternal factors were considered jointly, logistic regression identified a positive association of S. mutans status with maternal DMFS (p=0.0319), but not with either of the bacterial counts (p>0.53) (Table 3).
Fig. 1.
S. mutans (SM) status on DMFS/dmfs scores. Box plots of the distribution of (a) Children's dmfs at 36 months and (b) Maternal DMFS by the SM colonization status of the children at 16 months. The Mean (⋄), Median (Horizontal line), Interquartile range (Shaded boxes) and Max/Min values (Error bars) of DMFS scores from children at 36 months (a, p=0.0007) or mothers of children (b, p=0.0125) who were not colonized by SM early (defined as colonization by 16 months or prior) (N) and by those who were colonized by SM early (Y).
Table 2.
Descriptive statistics for maternal DMFS by S. mutans status
| S. mutans | N | Mean | Std Dev | Median | Min | Max | P |
|---|---|---|---|---|---|---|---|
| No | 17 | 16.00 | 12.00 | 12.00 | 2.00 | 40.00 | 0.0125 |
| Yes | 22 | 30.59 | 19.10 | 29.00 | 1.00 | 87.00 |
This is a summary of the comparison of maternal DMFS by S. mutans status. P-value is the significance probability associated with Wilcoxon Rank-Sum test. There is statistically significant evidence of a difference in groups.
Table 3.
Odds ratios for S. mutans acquisition
| Variable | OR | 95% CI | P | |
|---|---|---|---|---|
| S. mutans CFU | 0.958 | 0.775 | 1.185 | 0.6943 |
| Overall CFU | 1.175 | 0.705 | 1.958 | 0.5365 |
| Maternal DMFS | 1.07 | 1.006 | 1.138 | 0.0319 |
This is a summary of the logistic regression which models child S. mutans acquisition as a function of maternal factors. This multivariable model identifies that maternal disease burden is significantly associated with the probability of child S. mutans acquisition.
Genetic study
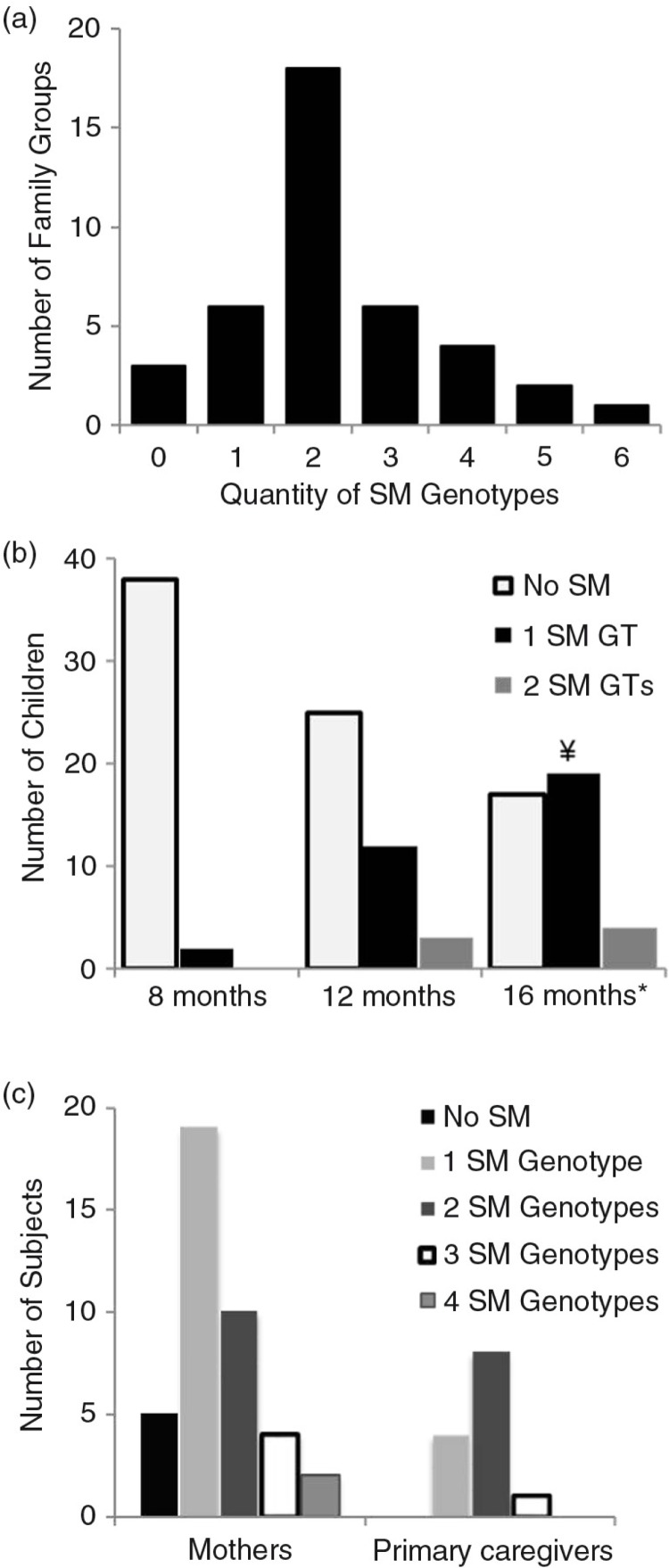
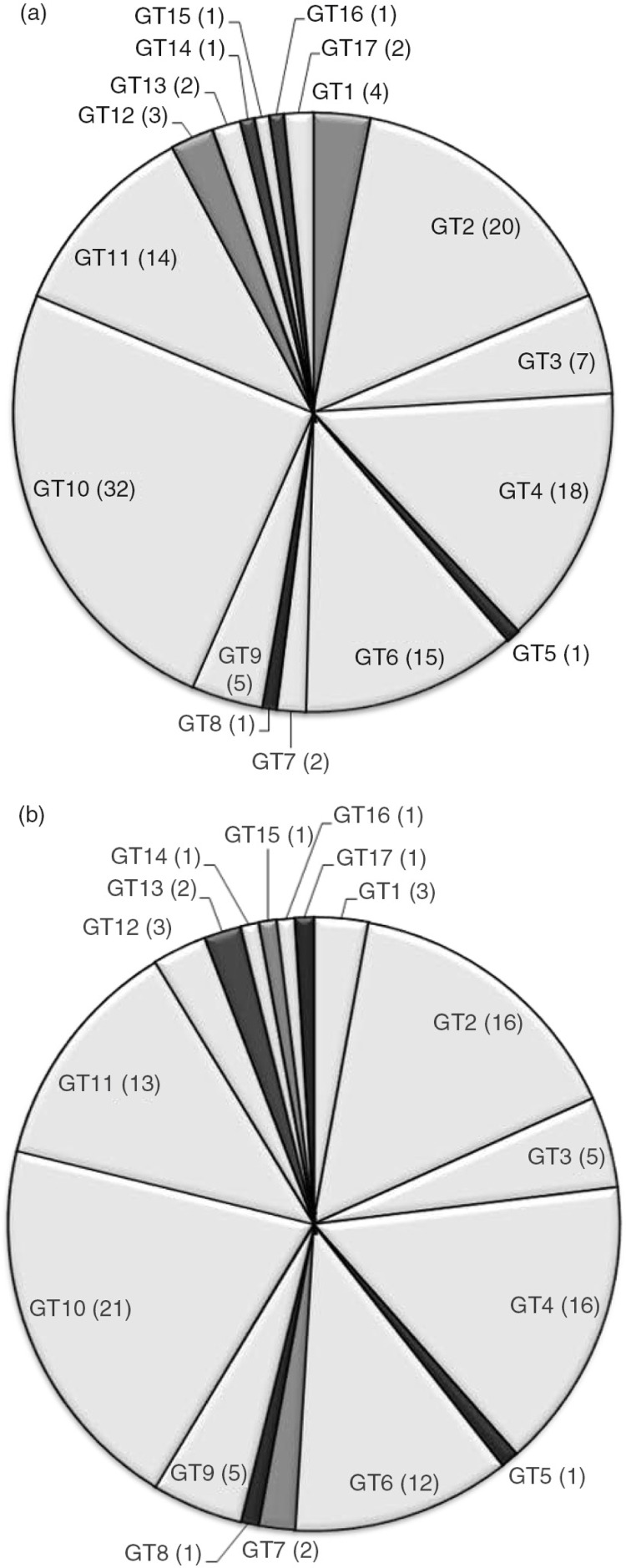
We found 17 unique S. mutans genotypes (GT) among the recovered S. mutans isolates, which formed our genotype library (Table 4). While 77.5% of families had more than one S. mutans genotype (Fig. 2a), 95.8% of families with S. mutans positive children had more than one S. mutans genotype (data not shown). The average number of genotypes among a family unit (mother [M] and child [B] or M, B and caregiver [C]) is 2.3 genotypes with the maximum number of six genotypes recovered from a given family (Fig. 2a). However, at any time point, the maximum number of genotypes recovered from a child was two genotypes (Fig. 2b). At 8 months, only one genotype was recovered from each of the two children who were colonized by S. mutans. At 12 months, 30% of children had one S. mutans genotype identified and 7.5% had two genotypes identified (mean number of genotypes/child was 1.2). At 16 months 47.5 and 10% of children had one and two genotypes identified respectively (mean number of genotypes/child was 1.19). Genotypic diversity was greater in mothers and caregivers (Fig. 2c), as multiple mothers had up to four S. mutans genotypes and one caregiver had three genotypes (mean genotypes were 1.48 and 1.77, respectively).
Table 4.
Genotype library
| Distribution of S. mutans genotypes | |||||||
|---|---|---|---|---|---|---|---|
| Children | |||||||
| Genotype | AP-PCR pattern | 8 Months | 12 Months | 16 Months | Mothers Individuals | Caregivers Individuals | Percentage of total isolates |
| GT1 |  |
1 | 1 | 2 | 2.5 | ||
| GT2 | 2 | 2 | 13 | 4 | 11.5 | ||
| GT3 | 1 | 3 | 4 | 4.8 | |||
| GT4 | 1 | 4 | 2 | 11 | 1 | 20.5 | |
| GT5 | 1 | 1.3 | |||||
| GT6 | 1 | 2 | 7 | 5 | 8.7 | ||
| GT7 | 1 | 1 | 1 | 2.4 | |||
| GT8 | 1 | 0.8 | |||||
| GT9 | 2 | 2 | 1 | 1 | 5.3 | ||
| GT10 | 6 | 7 | 18 | 5 | 26.9 | ||
| GT11 | 4 | 6 | 3 | 9.1 | |||
| GT12 | 1 | 2 | 0.6 | ||||
| GT13 | 1 | 1 | 1.5 | ||||
| GT14 | 1 | 0.7 | |||||
| GT15 | 1 | 1.4 | |||||
| GT16 | 1 | 1 | 0.3 | ||||
| GT17 | 1 | 1 | 0.8 | ||||
Genotypes identified with the number of children at 8, 12 and 16 months, as well as mothers and caregivers, which reflect the diversity and commonality of S. mutans genotypes (711 isolates from 86 individuals). The mothers and caregiver values represent genotypes identified with individuals over multiple sampling (1–3) times, while child values represent one specific sampling time point. The percentage of total isolates represents the richness of a particular genotype within the genotype library in that it is the percentage of total isolated colonies that identify with a particular genotype. Heading should read Distribution of S. mutans genotypes. Rows in bold type indicate the three most common genotypes, the incidence at each point and their richness.
Fig. 2.
Diversity of S. mutans (SM) genotypes (GTs). (a) Family groups consist of a mother/child pair or mother/child/caregiver triad. The quantity of genotypes represents the total number of SM genotypes that are identified in all members of the group. (b) The number of children with 0, 1 or 2 SM genotypes at 8, 12 and 16 months of age. No SMs were detected in children prior to 8 months. *One child died between 12 and 16 months and was only counted at 12 months. ¥One child who was colonized with SM at 12 months was only colonized with S. sobrinus at 16 months and therefore was only counted at 12 months. (Since colonization occurred with another MS species, the 12 months colonization was not considered transient.) (c) Mothers and caregivers of this population analyzed individually with respect to the number of genotypes identified in each and recorded over multiple sampling visits during a 16-month period.
The commonality of genotypes was evaluated for five sampling visits over the course of 16 months. Twelve of the 17 genotypes were shared by at least two subjects (Fig. 3a), throughout the population and 11 genotypes were shared by at least two families (Fig. 3b). These shared genotypes comprised 95.5% of all isolates. The most common genotype, genotype 10 was shared by 32 (36%) subjects and 21 (53%) families and represented 66% of all S. mutans isolates (Table 4). All children but one (96%) shared a genotype in common with another child in this group (Data not shown).
Fig. 3.
Commonality between (a) subjects and (b) families. Genotype designation (GT#) with number of (a) subjects (including children, mothers or caregivers) and (b) family groups (one member of a mother/child pair or mother/child/caregiver group) harboring each genotype in parentheses.
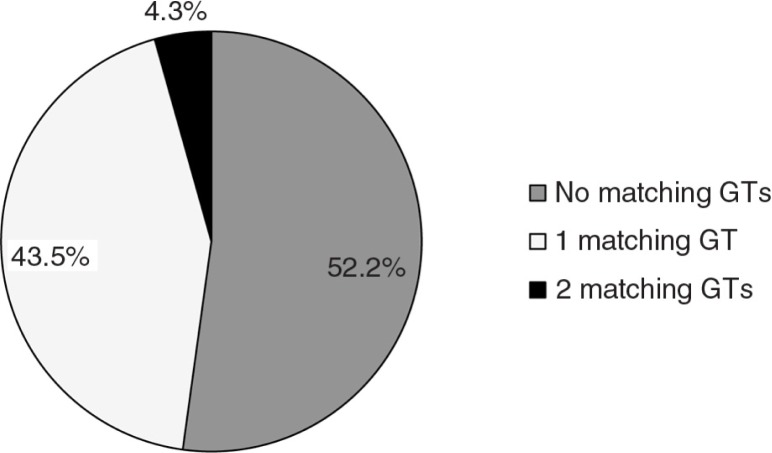
In the 23 children that had S. mutans, 47.8% exhibited at least one genotype in common with the mother (Fig. 4). Only one child had two genotypes in common with their mother. Six of the 14 caregivers sampled were associated with a S. mutans positive child. Of these six, three of the children did not share a genotype with either their mother or caregiver. Of the remaining three, one only shares a genotype with the mother and the other two share the same genotype with both the mother and caregiver. Therefore, there are no genotypes that are transmitted uniquely from caregiver to child. Members of the six mother/caregiver pairs shared at least one genotype 64.3% of the time.
Fig. 4.
Fidelity of S. mutans (SM) transmission. The percentage of 23 children who have acquired SM by 16 months who share either one or two genotypes with their mothers are represented, along with children who do not share any SM genotypes with their mothers. (Caregivers were not considered because in every instance where a child shared a GT with a caregiver, the child shared the same GT with the mother.)
When two or more genotypes are present in an individual, one genotype is dominant (over 50% of isolates) 46.4% of the time. Dominant genotypes in the mother were transmitted to children 10.4% (2 of 19 mothers) of the time. Genotype 10 was the genotype most commonly acquired by children from their mothers, representing 54.5% of transferred genotypes (6 of 11). Genotype 10 was the dominant genotype of isolated colonies at a given visit in six of the 18 mothers harboring this GT (Table 4). However, there were only two instances where Genotype 10 was the dominant GT in a mother and transferred to a child.
Discussion
A large percentage of children (37.5%) in this cohort were colonized by S. mutans by 12 months and the majority (60%) colonized by 16 months. This is earlier than the traditional ‘window of infectivity’ regarding S. mutans colonization with 19–31 months as the normal range and 26 months was the median (27). Therefore we have defined any colonization by 16 months as ‘early acquisition’. However, some low socioeconomic populations have early colonization times similar to the 16 month time point we see in this American Indian population (38, 39) and colonization instances as early as 6 months (42). These studies, along with our observations suggest that acquisition of S. mutans may occur earlier in some populations than previously thought. Part of our hypothesis was that children in this community acquire S. mutans at an earlier age than other children and that is confirmed by this initial analysis. One key factor could be that tooth eruption in this population occurs significantly earlier than in any other population (43). These tooth eruption data will be described in detail in a forthcoming manuscript.
We later observed that, by 36 months, 85% of children were colonized by S. mutans (data not shown). Here we are considering data only up to the 16-month time point of the children because we are interested in determining the diversity of S. mutans genotypes at initial acquisition and fidelity of transmission from mother to child. Since it is common, in most societies, for children to acquire S. mutans from their mothers, it is important to focus on this time point and determine if vertical transmission is the primary avenue of acquisition of S. mutans in these children.
In addition to early acquisition of S. mutans in these children, we found a strong association with the DMFS scores of the mothers at baseline (within 1 month of the child's birth). Interestingly, there was no association between maternal plaque bacteria levels and early acquisition (16 months). We observed the bacterial counts from the mothers’ plaque samples to be relatively low when compared to other studies. However, the fact that the progression of caries in the mothers correlates so strongly with early acquisition in the children, suggests that the overall bacterial burden is less important than the caries status of the mothers in predicting colonization of these children with S. mutans. Several other reports have found association between high levels of S. mutans in mothers and early acquisition in children (27, 31, 44) and it is possible that the sample set size used for this preliminary analysis did not allow for significant differences to be detected. Due to the death of one child between the 12 and 16 month time points, our caries association analysis was performed on the 39 children with recorded caries status at 36 months. Our decision to include this child in our acquisition analysis was based on the fact that it had been colonized at 12 months.
There are several key factors that have been implicated in S. mutans colonization and these have been described in Law et al. (45). Briefly, these include the following: epidemiological factors, genetic and immunological factors, salivary factors, dietary factors and behavioral factors. Our study considers epidemiological factors including: high S. mutans burden in the mothers, frequent contact with additional high MS carriers, virulent strains of S. mutans, availability of ecological sites and reduced competition, which will be described in more detail in a future manuscript. Genetic and immunological factors include HLA genes and MHC genes, salivary factors, such as, agglutinins, salivary flow and buffering capacity and immunological factors, such as immunoglobulin, lactoferrin, peroxidases and lysozyme. Dietary factors include increased frequency of sugar containing foods or drinks. Behavioral factors include a lack of oral hygiene. Dietary and behavioral factors are being analyzed as a part of our overall study and will be described in a future manuscript. In order to respect cultural sensitivities, it was agreed that our study would not collect or analyze host genetic or immunological data. While these are important factors, we must respect the wishes of our partners in the American Indian community.
Our ongoing analysis of the remaining 200 mother/child pairs could reveal additional associations. Of course, investigations that found an association between high maternal bacterial counts and early acquisition in children did not mention any analyses of maternal DMFS scores. It is possible that this could have been an even stronger association in those studies. It is reasonable to assume that many mothers with high DMFS scores would have a plaque dominated by MS and that this would play a role in early acquisitions in children. This perpetuates a viscious cycle of inheritance of cariogenic plaque and early decay.
Levels of S. mutans in children as a single variable may not be a reliable predictor of caries but rather detection of early colonization of MS may be the key for development of S-ECC. S. mutans colonization has always been associated with tooth eruption (27, 39, 46–48) but some studies have shown that S. mutans may be detected in pre-dentate children (49, 50). Nevertheless, studies have shown an association between the number of erupted teeth and S. mutans CFU (42, 51).
The genotypic diversity of this population, based on this initial subset, is quite consistent with other studies (14, 28, 38). We observed 17 distinct genotypes from 711 isolates. With regards to a link between genotype diversity and caries, it has both been reported that caries active children harbor greater numbers of S. mutans genotypes than caries-free children (37, 52, 53) and fewer genotypes (54). We will wait until we have completely analyzed this cohort over the full 3-year collection period to draw conclusions about this. Children were given a full dental examination during each sampling visit (every 3–4 months). If at any time, a white spot lesion was detected in a child, that child was treated with fluoride varnish. This could have potentially affected the colonization of S. mutans in a child and could be a confounding factor to the overall diversity of the S. mutans in plaque. This is a natural limitation based on simple ethics of treating children in a study as needed. One could argue that this could further impact later studies where we more deeply consider children's caries status. However, studies have shown that application of fluoride varnish in AI populations has less impact on the caries progress than in other children and that varnish must be applied frequently to have a measurable effect (55, 56). Therefore, we believe it is unlikely that fluoride varnish had a definitive impact on the cariogenic oral flora in these children. MSKB agar is routinely and widely used in the isolation of mutans streptococci but is sometimes criticized for its high selectivity and low recovery of S. mutans when compared to other common S. mutans selective agars (57). However, we believe that the number of potential false negatives would be quite low and the greater selectivity of the media compensates for possible lower S. mutans recovery rates.
This study also addresses the issue of genotype commonality in this community and demonstrates the possibility for horizontal transmission as well as vertical transmission (32, 58, 59). The prevailing dogma has been that S. mutans colonization initiates in a window of infectivity lasting from 19 to 31 months and remains with the individual. We have demonstrated that, in this population, colonization occurs earlier than in other studies and genotypes are shared within families and within the community. Horizontal transmission allows the possibility of transient infections by different genotypes as demonstrated by Lindquist and Emilson (31). Despite the high degree of commonality of genotypes, the assumption of horizontal transmission could not be proved by these data and is likely beyond the scope of this study. However, while an individual may be stably colonized by S. mutans after acquisition, the phenomenon of genotype switching suggests that S. mutans infections are not as stable as originally believed. Ongoing analysis of this cohort is focusing on the stability of acquired S. mutans genotypes in children over the entire 3 year period.
Genotypes 10, 2 and 4 were the most common genotypes in this cohort and were observed in children, mothers and caregivers. We did not see any associations between these genotypes and caries or earlier acquisition or fidelity of transmission compared to other, less common genotypes. It is possible that the size of this sample set was too small to show statistical significance and, therefore, we cannot rule out the possibility that our ongoing analysis of the complete cohort of 239 family groups may show these associations.
In this group, only 48% of children shared a common genotype with their mothers. Other studies have shown fidelity of transmission as high as 71% (28) and as low as 33% (60). In the latter study (where mother/child commonality was 33%) only three S. mutans colonies were selected from each sample, which increased the likelihood of not being able to isolate all genotypes that may have been present (61). The moderate level of fidelity of S. mutans transmission between mothers and their children in our study, suggests that, in this community, there is also a significant level of horizontal transmission (62). This could be due to the common practice of multiple families sharing a small house. There are many contributing factors that impact fidelity of transmission, such as the amount of time a child spends with the mother, additional caregivers, siblings and closeness of the community as well as dietary variables that are difficult to compare across studies. Therefore, differences in the level of fidelity of transmission of S. mutans most likely will vary depending on the influence of this multitude of variables. Nevertheless, our data suggest a significant level of horizontal transmission overall.
The overall dominance of genotypes within an individual did not seem to play a role in transmission of that genotype at this point in our study. Cheon et al. demonstrated that dominant genotypes may be more stable and showed an association between these genotypes and caries (14). The limited number of individuals in our initial sample subset may be too small to show statistically significant transmission and/or caries trends. Also, the early time points that are focused on acquisition do not allow for stability analysis. Ongoing analysis will seek to determine if dominant genotypes associate with greater stability of colonization within an individual, greater transmission rates or higher DMFS scores.
A very limited number of children analyzed in this sample had primary caregivers other than the biological mothers. Primary caregiver was defined as those who took care of the child for a minimum of 8 hours per day. We collected data on these caregivers as this brings up the intriguing question of whether the children acquired S. mutans from the caregivers, or the biological mothers who also lived in the same residence. In the six caregiver samples associated with SM positive children, we saw commonality with the child in three instances. In each of the three instances the common genotypes were also found in the biological mother. Therefore, determination of the source of the S. mutans genotypes could not be done as it is conceivable that the transmission occurred from either source. However, it is still noteworthy that, in this very small subset of children, half shared S. mutans genotypes with both adults.
Summary and conclusions
S-ECC is a multifactorial disease involving dietary, behavioral and environmental factors along with the plaque microbiology. Our study will consider all of these factors but here we presented our initial microbiological findings, which were compelling. These American Indian children acquired S. mutans at an earlier age than what was previously and widely believed and this early acquisition eventually associated with higher caries incidence in the children. These data also suggest that maternal vertical transmission could be a common route of initial acquisition and that mothers with high levels of caries had children who acquired S. mutans at the early time point. However, the high level of commonality for some of the genotypes suggests that other means of acquisition exist and, therefore, it's impossible to definitively state that mothers who share genotypes with their children were the source of these acquired genotypes. These initial findings provide ample evidence that early acquisition of S. mutans is a major factor in the rampant S-ECC in this community. Analysis of later time points will provide an invaluable longitudinal perspective of S. mutans colonization in these children as we link the many factors that contribute to this disease.
Acknowledgements
This study was supported by NIH grant R01-DE017736. Dr. Lynch was supported by the NIH/NIDCR Institutional Training Program in Oral Health Research T90-DE023520.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.da Fonseca MA. The effects of poverty on children's development and oral health. Pediatr Dent. 2012;34:32–8. [PubMed] [Google Scholar]
- 2.Fisher-Owens SA, Isong IA, Soobader M-J, Gansky SA, Weintraub JA, Platt LJ, et al. An examination of racial/ethnic disparities in children's oral health in the United States. J Public Health Dent. 2013;73:166–74. doi: 10.1111/j.1752-7325.2012.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JY, Divaris K. The ethical imperative of addressing oral health disparities: a unifying framework. J Dent Res. 2014;93:224–30. doi: 10.1177/0022034513511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seirawan H, Faust S, Mulligan R. The impact of oral health on the academic performance of disadvantaged children. Am J Public Health. 2012;102:1729–34. doi: 10.2105/AJPH.2011.300478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for Health S. Hyattsville, MD: National Center for Health Statistics (US); 2012. Health, United States. Health, United States, 2011: with special feature on socioeconomic status and health. [PubMed] [Google Scholar]
- 6.Congiu G, Campus G, Lugliè PF. Early childhood caries (ECC) prevalence and background factors: a review. Oral Health Prevent Dent. 2014;12:71–6. doi: 10.3290/j.ohpd.a31216. [DOI] [PubMed] [Google Scholar]
- 7.Niendorff WJ, Jones CM. Prevalence and severity of dental caries among American Indians and Alaska natives. J Public Health Dent. 2000;60:243–9. doi: 10.1111/j.1752-7325.2000.tb04069.x. [DOI] [PubMed] [Google Scholar]
- 8.Phipps KR, Ricks TL, Manz MC, Blahut P. Prevalence and severity of dental caries among American Indian and Alaska Native preschool children. J Public Health Dent. 2012;72:208–15. doi: 10.1111/j.1752-7325.2012.00331.x. [DOI] [PubMed] [Google Scholar]
- 9.Warren JJ, Kramer KWO, Phipps K, Starr D, Dawson DV, Marshall T, et al. Dental caries in a cohort of very young American Indian children. J Public Health Dent. 2012;72:265–8. doi: 10.1111/j.1752-7325.2012.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phipps KR, Ricks TL, Blahut P. Permanent first molar eruption and caries patterns in American Indian and Alaska Native children: challenging the concept of targeting second grade for school-based sealant programs. J Public Health Dent. 2013;73:175–8. doi: 10.1111/jphd.12011. [DOI] [PubMed] [Google Scholar]
- 11.Nash DA, Nagel RJ. Confronting oral health disparities among American Indian/Alaska Native children: the pediatric oral health therapist. Am J Public Health. 2005;95:1325. doi: 10.2105/AJPH.2005.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarche M, Spicer P. Poverty and health disparities for American Indian and Alaska native children. Ann N Y Acad Sci. 2008;1136:126–36. doi: 10.1196/annals.1425.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banas JA. Virulence properties of Streptococcus mutans . Front Biosci. 2004;9:1267–77. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 14.Cheon K, Moser SA, Wiener HW, Whiddon J, Momeni SS, Ruby JD, et al. Characteristics of Streptococcus mutans genotypesand dental caries in children. Eur J Oral Sci. 2013;121:148–55. doi: 10.1111/eos.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamada S, Koga T, Ooshima T. Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res. 1984;63:407–11. doi: 10.1177/00220345840630031001. [DOI] [PubMed] [Google Scholar]
- 16.Kuramitsu HK. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–76. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Yang JY, Zhi QH, Tao Y, Qiu RM, Lin HC. Factors associated with colonization of Streptococcus mutans in 8- to 32-month-old children: a cohort study. Aus Dent J. 2013;58:507–13. doi: 10.1111/adj.12113. [DOI] [PubMed] [Google Scholar]
- 18.Matsui R, Cvitkovitch D. Acid tolerance mechanisms utilized by Streptococcus mutans . Future Microbiol. 2010;5:403–17. doi: 10.2217/fmb.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita Y, Takehara T, Kuramitsu HK. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J Bacteriol. 1993;175:6220–8. doi: 10.1128/jb.175.19.6220-6228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carletto-Körber FPM, González-Ittig RE, Jiménez MG, Cornejo LS. Initial acquisition and genetic identity of Streptococcus mutans of mother-child Pairs. Pediatr Dent. 2010;32:205–11. [PubMed] [Google Scholar]
- 21.Douglass JM, Li Y, Tinanoff N. Association of mutans streptococci between caregivers and their children. Pediatr Dent. 2008;30:375–87. [PubMed] [Google Scholar]
- 22.Mitchell SC, Ruby JD, Moser S, Momeni S, Smith A, Osgood R, et al. Maternal transmission of mutans streptococci in severe-early childhood caries. Pediatr Dent. 2009;31:193. [PMC free article] [PubMed] [Google Scholar]
- 23.Teanpaisan R, Chaethong W, Piwat S, Thitasomakul S. Vertical transmission of mutans streptococci and Lactobacillus in Thai families. Pediatr Dent. 2012;34:24E–9E. [PubMed] [Google Scholar]
- 24.Zhan L, Tan S, Den Besten P, Featherstone JDB, Hoover CI. Factors related to maternal transmission of mutans streptococci in high-risk children-pilot study. Pediatr Dent. 2012;34:86E–91E. [PubMed] [Google Scholar]
- 25.Li Y, Caufield PW, Dasanayake AP, Wiener HW, Vermund SH. Mode of delivery and other maternal factors influence the acquisition of Streptococcus mutans in infants. J Dent Res. 2005;84:806–11. doi: 10.1177/154405910508400905. [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz RJ. Mutans streptococci: acquisition and transmission. Pediatr Dent. 2006;28:106–9. [PubMed] [Google Scholar]
- 27.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Caufield PW. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 1995;74:681–5. doi: 10.1177/00220345950740020901. [DOI] [PubMed] [Google Scholar]
- 29.Doméjean S, Zhan L, DenBesten PK, Stamper J, Boyce WT, Featherstone JD. Horizontal transmission of mutans streptococci in children. J Dent Res. 2010;89:51–5. doi: 10.1177/0022034509353400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alves AC, Nogueira RD, Stipp RN, Pampolini F, Moraes ABA, Gonçalves RB, et al. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J Med Microbiol. 2009;58:476–81. doi: 10.1099/jmm.0.005777-0. [DOI] [PubMed] [Google Scholar]
- 31.Lindquist B, Emilson CG. Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Res. 2004;38:95–103. doi: 10.1159/000075932. [DOI] [PubMed] [Google Scholar]
- 32.Mattos-Graner RO, Li Y, Caufield PW, Duncan M, Smith DJ. Genotypic diversity of mutans streptococci in Brazilian Nursery Children suggests horizontal transmission. J Clin Microbiol. 2001;39:2313–16. doi: 10.1128/JCM.39.6.2313-2316.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieralisi F, Rodrigues M, Segura V, Maciel S, Ferreira F, Garcia J. Genotypic diversity of Streptococcus mutans in caries-free and caries-active preschool children. Int J Dent. 2010;2010:1–5. doi: 10.1155/2010/824976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lembo FL, Longo PL, Ota-Tsuzuki C, Rodrigues CRMD, Mayer MPA. Genotypic and phenotypic analysis of Streptococcus mutans from different oral cavity sites of caries-free and caries-active children. Oral Microbiol Immunol. 2007;22:313–19. doi: 10.1111/j.1399-302X.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Caufield PW. Arbitrarily primed polymerase chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Oral Microbiol Immunol. 1998;13:17–22. doi: 10.1111/j.1399-302x.1998.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya RU, Napimoga MH, Rosa RT, Höfling JF, Gonçalves RB. Mutacin production in Streptococcus mutans genotypes isolated from caries-affected and caries-free individuals. Oral Microbiol Immunol. 2005;20:20–4. doi: 10.1111/j.1399-302X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 37.Napimoga MH, Kamiya RU, Rosa RT, Rosa EA, Höfling JF, de Oliveira Mattos-Graner R, et al. Genotypic diversity and virulence traits of Streptococcus mutans in caries-free and caries-active individuals. J Med Microbiol. 2004;53:697–703. doi: 10.1099/jmm.0.05512-0. [DOI] [PubMed] [Google Scholar]
- 38.Klein MI, Flório FM, Pereira AC, Höfling JF, Gonçalves RB. Longitudinal study of transmission, diversity, and stability of Streptococcus mutans and Streptococcus sobrinus genotypes in Brazilian nursery children. J Clin Microbiol. 2004;42:4620–6. doi: 10.1128/JCM.42.10.4620-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karn TA, O'Sullivan DM, Tinanoff N. Colonization of mutans streptococci in 8- to 15-month-old children. J Public Health Dent. 1998;58:248–9. doi: 10.1111/j.1752-7325.1998.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 40.Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G. Hyattsville, MD: U.S. Department of Health and Human Services; 2007. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Statistics. Center for Disease Control and Prevention; pp. 1–92. [PubMed] [Google Scholar]
- 41.Nowak AJ, Warren JJ. Infant oral health and oral habits. Pediatr Clin North Am. 2000;47:1043–66. doi: 10.1016/s0031-3955(05)70257-1. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara T, Sasada E, Mima N, Ooshima T. Caries prevalence and salivary mutans streptococci in 0–2-year-old children of Japan. Community Dent Oral Epidemiol. 1991;19:151–4. doi: 10.1111/j.1600-0528.1991.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 43.Dawson DV, Blanchette DR, Kramer K, Warren JJ, Phipps K, Starr D. Deciduous tooth eruption patterns in American Indian children. J Dent Res. 2014;93:1196. [Google Scholar]
- 44.Chaffee BW, Gansky SA, Weintraub JA, Featherstone JD, Ramos-Gomez FJ. Maternal oral bacterial levels predict early childhood caries development. J Dent Res. 2014;93:238–44. doi: 10.1177/0022034513517713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law V, Seow WK, Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J. 2007;52:93–100. doi: 10.1111/j.1834-7819.2007.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 46.Carlsson J, Grahnén H, Jonsson G. Lactobacilli and Streptococci in the mouth of children. Caries Res. 1975;9:333–9. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- 47.Berkowitz RJ, Jordan HV, White G. The early establishment of Streptococcus mutans in the mouths of infants. Arch Oral Biol. 1975;20:171–4. doi: 10.1016/0003-9969(75)90005-9. [DOI] [PubMed] [Google Scholar]
- 48.Berkowitz RJ, Turner J, Green P. Primary oral infection of infants with Streptococcus mutans . Arch Oral Biol. 1980;25:221–4. doi: 10.1016/0003-9969(80)90026-6. [DOI] [PubMed] [Google Scholar]
- 49.Tanner ACR, Milgrom PM, Kent R, Mokeem SA, Page RC, Riedy CA, et al. The microbiota of young children from tooth and tongue samples. J Dent Res. 2002;81:53–7. doi: 10.1177/002203450208100112. [DOI] [PubMed] [Google Scholar]
- 50.Wan AKL, Seow WK, Walsh LJ, Bird P, Tudehope DI, Purdie DM. Association of Streptococcus mutans infection and oral developmental nodules in pre-dentate infants. J Dent Res. 2001;80:1945–8. doi: 10.1177/00220345010800101601. [DOI] [PubMed] [Google Scholar]
- 51.Catalanotto FA, Shklair IL, Keene HJ. Prevalence and localization of Streptococcus mutans in infants and children. J Am Dent Assoc. 1975;91:606–9. doi: 10.14219/jada.archive.1975.0398. [DOI] [PubMed] [Google Scholar]
- 52.Hirose H, Hirose K, Isogai E, Miura H, Ueda I. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 1993;27:292–7. doi: 10.1159/000261553. [DOI] [PubMed] [Google Scholar]
- 53.Alaluusua S, Mättö J, Grönroos L, Innilä S, Torkko H, Asikainen S, et al. Oral colonization by more than one clonal type of mutans streptococcus in children with nursing-bottle dental caries. Arch Oral Biol. 1996;41:167–73. doi: 10.1016/0003-9969(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 54.Kreulen CM, de Soet HJ, Hogeveen R, Veerkamp JS. Streptococcus mutans in children using nursing bottles. ASDC J Dent Child. 1997;64:107–11. [PubMed] [Google Scholar]
- 55.Lawrence HP, Binguis D, Douglas J, McKeown L, Switzer B, Figueiredo R, et al. A 2-year community-randomized controlled trial of fluoride varnish to prevent early childhood caries in Aboriginal children. Community Dent Oral Epidemiol. 2008;36:503–16. doi: 10.1111/j.1600-0528.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 56.Holve S. An observational study of the association of fluoride varnish applied during well child visits and the prevention of early childhood caries in American Indian Children. Matern Child Health J. 2008;12:64–7. doi: 10.1007/s10995-007-0294-0. [DOI] [PubMed] [Google Scholar]
- 57.Schaeken MJM, Van der Hoeven JS, Franken HCM. Comparative recovery of Streptococcus mutans on five isolation media, including a new simple selective medium. J Dent Res. 1986;65:906–8. doi: 10.1177/00220345860650060901. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Zou J, Shang R, Zhou XD. Genotypic diversity of Streptococcus mutans in 3- to 4-year-old Chinese nursery children suggests horizontal transmission. Arch Oral Biol. 2007;52:876–81. doi: 10.1016/j.archoralbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Tedjosasongko U, Kozai K. Initial acquisition and transmission of mutans streptococci in children at day nursery. ASDC J Dent Child. 2002;69:284–88, 234–85. [PubMed] [Google Scholar]
- 60.Ersin NK, Kocabas EH, Alpoz AR, Uzel A. Transmission of Streptococcus mutans in a group of Turkish families. Oral Microbiol Immunol. 2004;19:408–10. doi: 10.1111/j.1399-302x.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 61.Cheon K, Moser SA, Whiddon J, Osgood RC, Momeni S, Ruby JD, et al. Genetic diversity of plaque mutans streptococci with rep-PCR. J Dent Res. 2011;90:331–5. doi: 10.1177/0022034510386375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berkowitz RJ. Acquisition and transmission of mutans streptococci. J Calif Dent Assoc. 2003;31:135–8. [PubMed] [Google Scholar]