Abstract
Background and Objectives
Brucella, causative of brucellosis, has some potential virulence factors involved in Brucella replication and its strategies to circumvent the immune response. One of them is the virB gene that encodes the type IV secretion system proteins (T4SS) involved in intracellular replication of organism. Brucella virulence factor A (bvfA), and urease (ure) has also been described as being implicated in survival, and virulence in the hosts. The aim of this study was to investigate the B. melitensis virulence factor genes among Brucella isolated from aborted fetuses of sheep and goats in Fars province, southern Iran.
Materials and Methods
A total of 42 isolates of B. melitensis isolated from aborted fetuses between 2005-2011 in Fars province of Iran was used in this study. PCR assay was performed in order to detect the virB, bvfA, and ure genes using specific primers.
Results and Conclusions
The frequency of bvfA, virB, and ure genes was 78.50%, 73.80%, and 88.09% among all isolates respectively. The results of the present study showed that most Brucella isolates from this region have virulence factors genes (virB, bvfA, ure) in their genome, and most B. melitensis had ure genes that has been hypothesized to play a role in the pathogenesis of disease.
Keywords: Brucella melitensis, Virulence genes (bvfA, virB and ure), Sheep, Goat, Fars province, Iran
INTRODUCTION
Members of genus Brucella are facultative intracellular pathogens responsible for brucellosis, worldwide zoonoses (1). B.melitensis, B.abortus, and B.suis cause brucellosis in humans, leading to chronic stages of the disease that can be manifested as orchitis, spondylitis, arthritis and debililitating illness known as undulant fever (2). The pathogenesis of brucellosis is due to its ability to adapt to the environmental conditions encountered in its intracellular explicative niche including low levels of nutrients and oxygen, acidic pH and reactive oxygen intermediates (3). Smooth Brucella inhibits host cell apoptosis, favoring bacterial intracellular survival by escaping host immune surveillance, while rough Brucella mutants (B.melitensis and B.ovis are two exceptions) induce macrophages (4). Brucella uses a number of mechanisms for avoiding or suppressing bactericidal responses inside macrophages. Molecular characterization of intracellular survival process of Brucella is important as it will provide guidance for prevention and sth. control (5, 6). VirB proteins that forms the type IV secretion system (T4SS) and that are involved in intracellular replication are considered as one of Brucella ,s virulence factors (7, 1). The T4SS in Brucella is typed by the virB operon encoding 12 proteins. The mechanism of assembly of factors secreted by T4SS in Brucella is still unknown. However, the similarity with the well-studied plant pathogen Agrobacterium tumefaciens suggest that Brucella uses in it for translocation of virulence factors into mammalian cells (8-9). It is clear that VirB proteins forming the type IV secretion system are involved in Brucella virulence and intracellular replication (10-12). In addition to this secretion system, Brucella virulence factor A (bvfA) has also been described as being implicated in Brucella survival in the host. Brucella virulence factor A (bvfA) is a small 11 kDa periplasmic protein that unique to the genus Brucella (13) and suggests it may play a role in the establishment of the intracellular niche (6). Although BvfA was essential for Brucella virulence in both in vitro and in vivo, its assumed role in virulence is still unknown (13). The other Brucella virulence factor is urease (ure). The Brucella urease are interesting candidates to consider as they are important Brucella virulence factor. Urease is a virulence factor that plays a role in the resistance of Brucella to low pH conditions, both in vivo and in vitro. Brucella contains two separate urease gene clusters, ure1 and ure2. Although only ure1 codes an active urease, ure2 is also transcribed, but its contribution to Brucella biology is unknown (14). Brucella contains two urease operons, both located in chromosome I. The proposed role of Brucella urease in inhibition of phagosome acidification by ammonia release was not observed (15).
The aim of this study was to investigate Brucella virulence factor genes among B.melitensis isolates from aborted fetuses of sheep and goats, in Fars province, Iran.
MATERIALS AND METHODS
Bacterial strains
A total of 42 isolates of B. melitensis (41 isolates biovar 1, One isolate biovar 2) recovered from clinical specimens from 2005 - 2011 used for the PCR assay.
DNA preparation
A loopful of colonies of each isolate on agar plate was picked and suspended in 200 μl of distilled water. After vortexing, the suspension was boiled for 5 min, and 50 μl of the supernatant was collected after spinning at 14,000 rpm for 10 min. The DNA concentration of the boiled extracts was determined with spectrophotometer. (12).
PCR assay
PCR amplifications were performed in a final volume of 25 μL in PCR tubes. The reaction mixtures consisted of 2 μL of the DNA template, 2.5 μL 10x PCR buffer (75 mM Tris-HCl, pH 9.0, 2 mM MgCl2, 50 mM KCl, 20 mM (NH4)2SO4, 1 μL dNTPs (50 μM), 1 μL (1U Ampli Taq DNA polymerase), 1 μL (25 pmol) of forward and reverse primers, (Table 1) and the volume of the reaction mixture was completed up to 25 μL using distilled deionized water. PCR program for amplification of bvfA consisted of initial denaturation at 95°C for 4min, 32 cycles of application with denaturation at 95°C for 1min, annealing at 65°C for 1min, extension at 72°C for 1.30 min and final extension of the incompletely synthesized DNA at 72°C for 10 min in the BioRad thermal cycler (MJ Mini, BioRad, USA).This procedure was carried out for amplification of virB and ure genes with annealing temperatures described in Table 1. The PCR products were analyzed in 2.0% agarose gels containing 0.5μg /ml of ethidium bromide and subjected to electrophoresis in a 1X TAE buffer. Gels were visualized under UV light and documented using Uvitec System DOC-008.XD (EEC). A molecular weight Marker with 100 bp increments (100 bp plus ladder, Vivantis, Malaysia) was used as a DNA standard.
Table 1.
Oligonucleotide primers used in the PCR assay.
RESULTS AND DISCUSSION
DNA was successfully extracted from all 42 B.melitensis isolates. As expected the bvfA, virB and ure genes assays produced amplicons of 1282, 881 and 2100 bp respectively, (Fig 1). Of 42 B.melitensis isolates; 33 (78.5%) isolates had bvfA genes, 31(73.8%) isolates had virB genes and 37 (88.09) isolates had ure genes.
Fig 1.
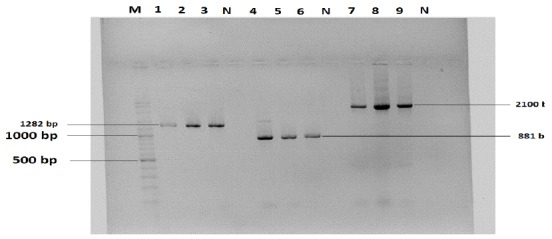
Agarose gel electrophoresis of PCR product of three brucella virulence genes. Lane M, 100 bp ladder, lanes 1- 3: PCR product of ure gene: 9-PCR product of virB gene, lanes 7: 6-product of bvfA gene, lane N, Negative control, lanes 4
The pathogenicity of Brucellae is due to its amazing ability to adapt to the environmental conditions encountered intracellularly. Brucella has evolved to avoid induction of immune system, interfere with intracellular trafficking, resist respiratory burst and adapt to oxygen-limited conditions encountered inside macrophages (6). In many regions of Iran B. abortus biovar 3 still remains the dominant biotype (16), however, B. melitensis biotype 1 is the predominant infective biotype in sheep and goats in Iran (17). The results of the present study showed that most B.melitensis isolates from Iran have virulence factors genes (virB, bvfA, ure) in their genome, and most B.melitensis isolates had ure genes that has been hypothesized to play a role in the pathogenesis of disease. Other studies show most Brucella isolates exhibit potent urease activity that has been hypothesized to play a role in the pathogenesis of disease (18). Furthermore, some isolates, such as the well-known laboratory strain B. abortus, are urease negative, while they seem to retain most of their pathogenic potential (15). Whole-genome sequencing revealed the presence of a second urease operon (ure2) with all the genes potentially active in B. suis and B. melitensis. On the other hand, B. abortus had two frameshift deletions in ureE2, and B. ovis had deletions in ureG2 and ureT (15). These data support our results that 88.09% of B.melitensis had ure gene.
Our results show that 73.8% isolates had virB genes. This is in accordance with other studies; O’Callaghan et al. (1990), described the presence of a virB region of B. suis containing 11 genes highly similar to the 11 virB genes of Agrobacterium tumefaciens and an extra ORF 12 that shares homology with an adhesion of Pseudomonas fluorescens (12). Sieria et al. (2000) suggested that putative effector molecules secreted by this T4SS determine routing of B. abortus to an endoplasmic reticulum-related replication compartment. The requirement of an active virB operon for intracellular survival of Brucellae may have two possible explanations: (i) the virB operon is essential to reach a competent intracellular replication niche or (ii) the virB operon is needed for replication once the intracellular replication niche has been established (19). Delrue et al. (2004) reported that the T4SS of Brucella encoded by the virB operon is a major virulence factor (20).
In this investigation we found that 78.5% isolates of B.melitensis had bvfA genes which is similar to other studies; Lavigne et al. (2005) reported that Brucella virulence factor A (bvfA) is a small 11 kDa periplasmic protein unique to the genus Brucella with no homologues in GenBank and no conserved domains or structural features was reported (13). It is suggested that it may play a role in the establishment of the intracellular niche (6). Although bvfA was essential for Brucella virulence in both in vitro and in vivo, its assumed role in virulence is still unknown (13). It is concluded from the study most B.melitensis isolates from Iran have virulence factors genes (virB, bvfA, ure) in their genome, and most B.melitensis isolates had ure genes that has been hypothesized to play a role in the pathogenesis of disease.
Acknowledgments
This work was supported by a Grant from the Shiraz University. The authors are grateful to Mr Shahed for excellent technical assistance.
References
- 1.Lapaque N, Forquet F, De Chastellier C, Mishal Z, Jolly G, Moreno E, Moriyon I, Heuser EJ, He HT, Gorvel PJ. Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts. Cell Microbiol. 2005;8:197–206. doi: 10.1111/j.1462-5822.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorvel JP, Moreno E. Brucella intracellular life: from invasion to intracellular replication. Vet Microbiol. 2002;90:281–297. doi: 10.1016/s0378-1135(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 3.Kohler S, Foulogene V, Ouahani - Bettache S, Bourg G, Teyssir J, Ramuz M, Liautard JP. The analysis of intrapathogenic virulence of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proceed Nation Academ Scince USA. 2002;99:15711–15716. doi: 10.1073/pnas.232454299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei J, Turse JE, Wu Q, Fitch TA. Brucella abortus rough mutants induce macrophage oncosis that requires bacterial protein synthesis and direct interaction with the macrophage. Infect Immun. 2006;74:2667–2675. doi: 10.1128/IAI.74.5.2667-2675.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapaque N, Moriyon I, Moreno E, Gorvel JP. Brucella lipopolysaccharide acts as a virulence factor. Cur Opin Microbiol. 2005;8:60–66. doi: 10.1016/j.mib.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed NS, Stephen MB, Nammalwar S. Brucella: A pathogen without classic virulence genes. Vet Microbiol. 2008;129:1–14. doi: 10.1016/j.vetmic.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Delrue RM, Deschamps C, Leonard S, Nijskens C, Danese I, Schaus JM, Bonnot S, Ferooz J, Tibor A, De Bolle X, Letesson JJ. A quorum sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell Microbiol. 2005;7:1151–1161. doi: 10.1111/j.1462-5822.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 8.Cascalase E, Chrisite PJ. The versatile bacterial type IV secretion system. Nat Rew Microbial. 2003;1:137–149. doi: 10.1038/nrmicro753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comerci DJ, Martinez-Lorenzo MJ, Sieira R, Gorvel JP, Ugalde RA. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 11.Foulongne V, Bourg G, Cazevieille C, Michaux-Charachon S, O’Callaghan D. Identification of Brucellasuis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect Immun. 2000;68:1297–1303. doi: 10.1128/iai.68.3.1297-1303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M, et al. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 13.Lavigne JP, Patey G, Sangari FJ, Bourg G, Ramuz M, O’Callaghan D, Michaux-Charachon S, et al. Identification of a new virulence factor BvfA, in Brucella suis. Infect Immun. 2005;73:5524–5529. doi: 10.1128/IAI.73.9.5524-5529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felix JS, Ana MC, Asuncion S, Juan MG. Brucella abortus ure2 region contains anacid-activated urea transporter and a nickel transport system. BMC Microbiol. 2010;10:107. doi: 10.1186/1471-2180-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangari FJ, Seoane A, Rodriguez MC, Aguero J, Garcia Lobo JM. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect Immun. 2007;75:774–780. doi: 10.1128/IAI.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zowghi E, Ebadi A, Yarahmad M. Isolation and identification of Brucella organisms in Iran. Iranian J Clin Infect Dis. 2008;3:185–18. [Google Scholar]
- 17.Saeedzadeh A, Sharifiyazdi H, Firouzi R. Molecular characterization of Brucella melitensis Rev.1 strain in aborted sheep and goats in Iran. Comp Clin Pathol. 2012:1424–7. [Google Scholar]
- 18.Smith LD, Ficht TA. Pathogenesis of Brucella. Crit Rev Microbiol. 1990;17:209–230. doi: 10.3109/10408419009105726. [DOI] [PubMed] [Google Scholar]
- 19.Sieira R, Comerci DJ, Sanchez DO, Ugalde RA. A homologue of an operon required for DNA transfer in agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J Bacteriol. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delrue RS, Lestrate L, Tibor A, Letesson JJ, Bolle XD. Brucella pathogenesis, genes identified from random large-scale screens. FEMS Microbiol Lett. 2004;231:1–12. doi: 10.1016/S0378-1097(03)00963-7. [DOI] [PubMed] [Google Scholar]


