Synopsis
Systemic sclerosis (SSc) is a heterogeneous disease of unknown etiology and with limited effective therapies. It is characterized by autoimmunity, vasculopathy and fibrosis and is clinically manifested by multi-organ involvement. Interstitial lung disease (ILD) is a common complication of the disease and is associated with significant morbidity and mortality. The diagnosis of ILD hinges upon careful clinical evaluation as well as pulmonary function tests (PFTs) and high resolution computed tomography (HRCT). A number of pro-inflammatory and pro-fibrotic mediators are involved in the pathogenesis of SSc-ILD, with transforming growth factor-beta (TGF-β) playing a key role in the development of fibrosis. Despite recent advances in the understanding of the mechanisms of disease initiation and progression, effective therapeutic options are still limited. A number of experimental therapies are currently in early phase clinical trials and show promise.
Keywords: Systemic Sclerosis, Interstitial Lung Disease, Fibrosis, Pathogenesis, Diagnosis, Treatment
Introduction
Systemic sclerosis (SSc) is a heterogeneous disease characterized by vasculopathy, autoimmunity and fibrosis, with multi-organ involvement and no known cure. Pulmonary complications of SSc remain one of the largest causes of morbidity and mortality in the disease. Interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH) are the most common forms of lung disease associated with SSc. This review will focus on SSc-ILD, a leading cause of mortality in SSc. Pulmonary function tests (PFTs) and chest imaging with high-resolution chest tomography (HRCT) remain important tools in the diagnosis and prognosis of SSc-ILD. Although significant advances have been made in the understanding of the pathogenesis of SSc-ILD, current treatment options have limitations in their overall effectiveness. A number of treatment modalities are currently under investigation, and novel targeted treatments that have shown promise in Idiopathic Pulmonary Fibrosis (IPF) clinical trials may ultimately be useful in SSc. This review provides a brief overview of SSc-ILD pathogenesis to date, and includes a discussion of key points in the evaluation and management of the disease, including a discussion on novel therapies.
Epidemiology
ILD is common in patients with SSc, with up to 90% of patients exhibiting evidence of interstitial changes on HRCT[1], and between 40-75% of patients having PFT abnormalities [2, 3]. Clinically significant lung fibrosis is present in approximately 25% of all SSc patients[4], but there is significant heterogeneity with regard to the incidence of pulmonary involvement based on a number of factors including the SSc subset and antibody profile. In particular, patients with diffuse cutaneous SSc (dcSSc) or Scl-70 (anti-topoisomerase) antibodies are at higher risk for the development of ILD, while patients with limited cutaneous SSc (lcSSc) or anti-centromere antibodies less commonly develop ILD. Among 3656 patients in the EULAR Scleroderma Trials and Research (EUSTAR) database, 60% of patients with positive Scl-70 antibodies had evidence of ILD, compared to 21% of patients with anti-centromere antibodies[5]. Certain clinical features, such as African American ethnicity, modified Rodnan Skin Score (mRSS), serum creatinine, creatine phosphokinase (CPK) values, and evidence of cardiac involvement have also been shown to be independent predictors of lung involvement in SSc[4]. In a recent meta-analysis looking at predictors of mortality and progression in SSc-ILD, factors including older age, lower forced vital capacity (FVC) and lower diffusing capacity of the lungs for carbon monoxide (DLCO) predicted mortality[6]. Extent of disease involvement on HRCT predicted both mortality and ILD progression.
A number of biomarkers have been studied as possible predictors of the development and progression of ILD in SSc. These markers, which are currently not available for clinical use in the United States, may play a role in prognosis and disease monitoring in the future. Specifically, the glycoproteins Krebs von den Lungen-6 (KL-6) and surfactant protein D (SP-D) have been shown to be elevated in patients with SSc-ILD and levels may correlate with ILD severity and progression[7, 8].
Pathogenesis
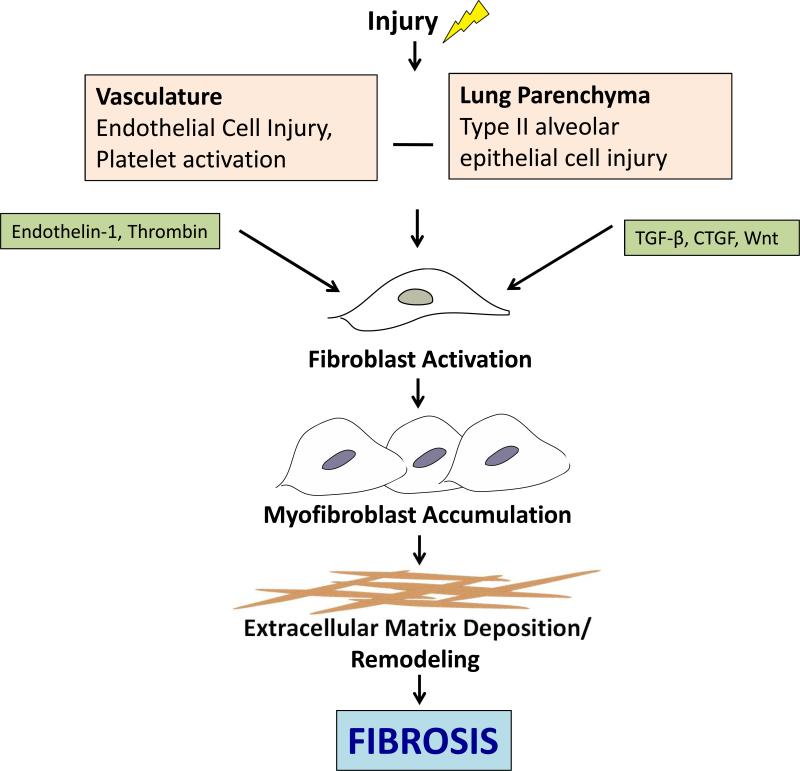
The pathogenesis of SSc-ILD is multi-factorial and incompletely understood. Endothelial cell injury with subsequent vascular damage and alveolar epithelial cell injury are key initial insults that precede fibrosis. Upon injury various mediators are released and fibroblasts are activated. Over time fibroblasts acquire features of smooth muscle cells and become myofibroblasts, resulting in dysregulated accumulation of collagen and extracellular matrix components and ultimately fibrosis (Figure 1). Some of the mediators implicated in SSc-ILD include thrombin, TGF-β, and the Wnt/ β-catenin pathway.
Figure 1. Key mediators in the pathogenesis of pulmonary fibrosis in SSc.
Pulmonary fibrosis is initiated by damage to the vasculature and lung parenchyma, resulting in endothelial and epithelial cell injury. This subsequently results in the release of a number of cytokines and growth factors which in turn activate fibroblasts, resulting in extracellular matrix deposition and ultimately fibrosis. CTGF, connective tissue growth factor; TGF-β, transforming growth factor beta.
Thrombin
Lung biopsies of SSc-ILD patients reveal evidence of endothelial and epithelial injury with interstitial edema[9, 10]. Endothelial cell injury results in thrombin production and release of endothelin-1 (ET-1) with elevated levels of thrombin detected in bronchoalveolar lavage (BAL) fluid of SSc patients compared to healthy controls[11]. Inhibition of thrombin with the oral direct thrombin inhibitor, dabigatran etexilate (Pradaxa), in the mouse bleomycin model of lung injury reduced the number of inflammatory cells in the BAL and decreased lung fibrosis[12]. Both thrombin and ET-1 also exert direct effects on fibroblasts and play a role in stimulating TGF-β production, a central mediator of SSc fibrosis.
TGF-β
TGF-β is a pleiotropic cytokine with a central role in SSc-ILD. Once activated, TGF-β binds to its receptor and leads to the phosphorylation of Smad, a group of intracellular signaling proteins[13, 14]. The Smad proteins subsequently translocate to the nucleus and act as transcription activators for a number of genes including type I collagen, fibronectin, plasminogen activator inhibitor-1 and connective tissue growth factor (CTGF)[15, 16]. In mouse models, the conditional knockout of TGF-β receptor type II and Smad3 deficient mice are protected from bleomycin-induced pulmonary fibrosis[17, 18]. Trials of small molecule blockade of Smad phosphorylation and of inhibitors of signaling molecules downstream of TGF-β are currently underway and may provide novel targets to treat SSc-ILD.
Wnt/β-catenin
The Wnt/β-catenin pathway is comprised of highly conserved growth factors, whose downstream activity leads to the accumulation of β-catenin in the cytoplasm; this then translocates to the nucleus to regulate a number of genes[19]. Wnt signaling has been shown to be important in the development of dermal fibrogenesis and the suppression of adipogenesis in a mouse model and in SSc patients[20, 21]. In addition, aberrant expression of components of the Wnt/β-catenin pathway has been implicated in the pathogenesis of IPF[22, 23]. Administration of an antibody to the downstream molecule Wnt1-inducible signaling protein-1 (WISP-1) was associated with a decrease in collagen and improved lung function in a mouse model[23], and further exploration into blockade of the Wnt/β-catenin pathway may provide additional therapeutic strategies for patients with SSc-ILD.
Clinical Manifestations
Patients with mild ILD may be asymptomatic during the early stages of the disease. As the extent of pulmonary fibrosis increases, patients often report fatigue and dyspnea on exertion, and physical exam may reveal dry “velcro” crackles at the lung bases. Of note, patients with PAH may also have exertional dyspnea and fatigue and the clinician must keep both entities in mind when evaluating an SSc patient for dyspnea. Dry cough is often present, and may correlate with DLCO and dyspnea[24]. In the Scleroderma Lung Study, cough severity improved in patients treated with cyclophosphamide, but these improvements disappeared after two years of follow up[25, 26].
Imaging findings
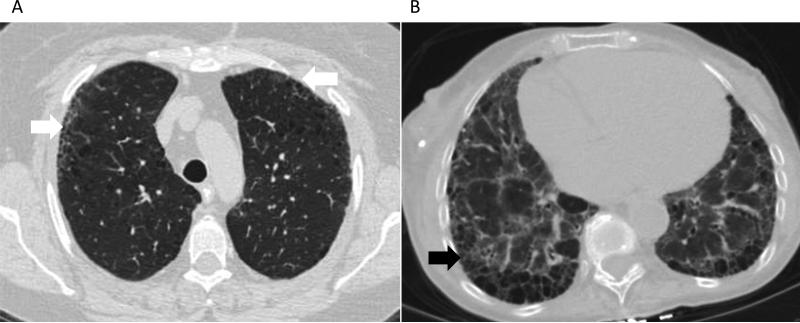
HRCT plays an important role in determining the pattern and extent of involvement of ILD in SSc patients. The most common pattern seen on HRCT is nonspecific interstitial pneumonia (NSIP), although usual interstitial pneumonia (UIP) can also be seen in 25-40% of cases[25, 27]. The NSIP pattern on HRCT is evident by ground glass opacities (GGOs) in a peripheral distribution with subpleural and basilar predominance (Figure 2A). In more severe disease, volume loss with a reticular pattern and traction bronchiectasis can also be seen. In UIP, HRCT findings include reticulonodular opacities, traction bronchiectasis and honeycomb cysts (Figure 2B). A normal chest CT at baseline is generally reassuring; in one study 85% of SSc patients who had a normal HRCT at baseline still had a normal HRCT at 5 years[28].
Figure 2. Representative radiographic findings on high-resolution computed tomography.
a) subpleural ground glass opacities (white arrows) and traction bronchiectasis consistent with nonspecific interstitial pneumonia, and b) honeycombing (black arrow), bronchiectasis and ground glass opacities suggestive of usual interstitial pneumonia.
Pulmonary Function Tests (PFTs)
PFTs are a key component in the diagnosis and long term follow up of SSc-ILD. The FVC and DLCO are both important parameters for the assessment of lung function in SSc patients, and the FVC can help stratify patients for treatment[29]. Reduced DLCO is sensitive for early ILD, but can also be an indicator of pulmonary hypertension, and needs to be interpreted in the context of the overall lung volumes. FVC and DLCO should both be >80% predicted to be considered normal. In a study of 890 SSc patients who had PFTs, 60% had no or minimal restrictive disease (FVC >75%), 27% had moderate disease (FVC 50-75%) and 14% had severe disease (FVC ≤50%)[3]. Both FVC and DLCO are prognostic factors in patients with SSc-ILD. Among 80 patients who underwent lung biopsy for SSc with fibrosing alveolitis, lower initial DLCO and FVC were associated with mortality[27].
In general, we recommend close clinical follow-up of SSc patients to monitor for a change in cardiopulmonary symptoms, usually every 3-6 months. In the absence of a clinical change, PFTs should be done annually. If there is progression of symptoms such as dyspnea or cough, PFTs should be done every 6 months, and should be followed with a HRCT if there are abnormalities on the PFTs. Others have suggested that HRCT be performed in all SSc patients as a screening exam and if mildly abnormal be followed by PFTs every 3-6 months[30]. Both HRCT and PFTs are well-accepted methods for the diagnosis of SSc-ILD, with 100% and 99% consensus among 117 SSc experts for the use of HRCT and PFTs, respectively[31]. The first five years after diagnosis of SSc are critical for the development of ILD, and patients should be monitored closely during this time. The clinician should also keep in mind the role that gastroesophageal reflux (GER) may play in worsening fibrosis and clinical symptoms[32]. Patients with SSc-ILD have been shown to have more severe GER than SSc patients without ILD[33], and this may contribute to worsening respiratory symptoms.
6-minute walk test (6MWT)
The 6MWT is an easy and noninvasive tool used for the assessment of lung function in pulmonary disease. Its utility in SSc-ILD is however limited primarily due to functional impairment of patients. In a study of 163 patients with SSc-ILD, the 6MWT did not correlate well with other parameters such as FVC, DLCO or dyspnea index scores despite it reproducibility[34]. It may play a more important role in SSc patients with PAH.
Histopathology
Histologically, SSc-ILD is characterized by early pulmonary infiltration of inflammatory cells and subsequent fibrosis of the lung parenchyma. The most common patterns seen on histologic exam are NSIP and UIP. NSIP is characterized by varying degrees of inflammation and fibrosis. In contrast, UIP is characterized by dense patchy fibrosis with “honeycombing,” primarily in a sub-pleural distribution. NSIP is the more common pattern seen in SSc-ILD and is present in 64-77% of cases[27, 35]. Lung biopsy is not necessary to confirm the histopathologic diagnosis, as the patterns of ILD are usually readily distinguishable on HRCT. Biopsy may play a role when other diagnoses, such as infection or malignancy, are suspected based on HRCT.
Treatment
Approach to treatment
Multiple treatment modalities have been used in SSc-ILD with only some having modest benefit. Drug development for ILD has expanded dramatically and a number of investigational approaches are currently in early phase clinical trials. The decision of which patients to treat must be considered carefully given the potential toxicities of many of the medications. An algorithm for determining the severity of disease has been proposed by Goh et al., and use of this algorithm may aid in treatment decisions for a patient with SSc-ILD[29]. In this algorithm, lung involvement on HRCT of >20% and FVC<70% were associated with increased mortality risk, and these parameters may help to determine which patients would benefit the most from treatment.
Current Treatment Modalities (Table 1)
Table 1.
Current Treatment Options for SSc-ILD
| Drug | Mechanism of Action | Data Supporting Use |
|---|---|---|
| Cyclopho sphamide | Alkylating agent, cross links DNA, decreasing DNA synthesis and preventing cell division | Scleroderma Lung Study (multicenter RCT), improvement in FVC, dyspnea scores, chest imaging[25] |
| Mycophenolate Mofetil | Inhibits inosine monophosphate dehydrogenase, inhibits de novo guanosine nucleotide synthesis, prevents T and B proliferation | Observational studies, stabilization or improvement in FVC or DLCO[39-41] Scleroderma Lung Study II ongoing |
| Azathioprine | Metabolites incorporated into replicating DNA, blocks the pathway for purine synthesis | Observational studies, both alone and after cyclophosphamide, stabilization or improvement in FVC and dyspnea scores[37, 43, 44] |
| Lung Transplant | N/A | Similar survival rates as non SSc ILD patients[45, 46] |
Cyclophosphamide (Cytoxan)
Cyclophosphamide (Cytoxan) has been the most rigorously studied medication in the treatment of SSc-ILD. The 2009 EULAR recommendations for the treatment of SSc suggest the use of cyclophosphamide for the treatment of SSc-ILD despite its known potential toxicities[36]. This recommendation was based on two randomized controlled trials (RCTs) comparing cyclophosphamide to placebo. In a small study of patients with SSc-ILD, monthly IV cyclophosphamide for 6 months in addition to prednisolone followed by azathioprine was compared to placebo[37]. There was a trend towards improvement in FVC, but this did not reach statistical significance, likely in part due to the small study size. In the first multi-center RCT to look at the role of cyclophosphamide in SSc-ILD, the Scleroderma Lung Study found that patients with early SSc-ILD treated with oral cyclophosphamide for one year had improvement in FVC, dyspnea index scores, chest imaging and skin scores, but not in DLCO[25]. By two years, the positive effect of cyclophosphamide on lung function had disappeared, but the improvement in dyspnea scores was still present[26]. Based on these results, cyclophosphamide should be strongly considered as first line treatment in patients with SSc-ILD. The optimal duration of therapy is not known given the risk of adverse effects; typical protocols suggest treatment up to a year followed by a switch to another agent for maintenance therapy (see below)[38].
Mycophenolate Mofetil (Cellcept)
A number of retrospective and case control studies have suggested that mycophenolate mofetil (MMF; Cellcept) may have a beneficial effect on SSc-ILD. In these studies, treatment with MMF resulted in stabilization or improvement of either FVC or DCLO during the treatment period, whereas lung function had declined in the period preceding treatment with MMF[39-41]. However, a retrospective case control study that compared patients with SSc-ILD treated with MMF versus cyclophosphamide versus control found that PFTs and clinical parameters were similar, but that radiographic findings on HRCT worsened in the group treated with MMF despite being a less sick group at onset[42]. The Scleroderma Lung Study II, which is currently ongoing, will compare one year of oral cyclophosphamide with two years of MMF for the treatment of SSc-ILD, and should provide further information on the utility of MMF in SSc-ILD (ClinicalTrials.gov Identifier: NCT00883129). MMF may provide a good option for maintenance therapy for patients with SSc-ILD after induction with cyclophosphamide[31].
Azathioprine (Imuran)
Azathioprine (Imuran) may have a role as an alternative agent to cyclophosphamide or as a maintenance medication after initial treatment with cyclophosphamide. In a retrospective study of 11 patients with SSc-ILD, treatment with azathioprine resulted in stable FVC and dyspnea index scores in 8 patients[43]. In another study of SSc-ILD patients with worsening lung function, treatment with 6 months of monthly IV cyclophosphamide followed by 18 months of azathioprine resulted in stable or improved PFTs at 6 months in 70% of patients[44].
Role of Lung Transplant
Lung transplant remains an option for patients who do not respond to conventional medical management. There have been concerns about whether involvement of other organ systems may pose a problem in the overall prognosis of SSc patients who undergo transplant, and specifically whether patients with severe GER may have recurrent aspiration events leading to increased lung damage. However, studies comparing outcomes after lung transplant in SSc-ILD patients to other ILD patients have shown similar 1 and 5 year survival rates[45, 46].
Investigational approaches (Table 2)
Table 2.
Investigational Treatments for SSc-ILD
| Drug | Mechanism of Action | Data Supporting Use |
|---|---|---|
| Rituximab | Monoclonal antibody against CD20 on B-lymphocytes | Small randomized trials, less decline or improvement in FVC[47, 48] |
| Bosentan | Endothelin-1 receptor antagonist | BUILD-2 (RCT), no difference in PFTs or 6MWT[49] |
| Imatinib | Tyrosine kinase inhibitor | Open label trials, one with improvement in FVC, one with no change in DLCO[51, 52] |
| Pirfenidone | Anti-fibrotic and anti-inflammatory effects | ASCEND (RCT in IPF), improvement in FVC and progression free survival in IPF[54] LOTUSS ongoing |
| Hematopoietic Stem Cell Transplant | Lymphocyte ablation | ASSIST, improvement in FVC[55] ASTIS, improved event-free and overall survival[56] SCOT ongoing |
Rituximab (Rituxan)
A number of trials have shown potentially promising results supporting the use of rituximab (Rituxan) in SSc-ILD. A recent study found that SSc patients treated with rituximab had a greater improvement in skin score and had less decline in FVC compared to controls[47]. A smaller study of 14 patients with SSc-ILD treated with rituximab showed similar improvements in lung function and skin scores[48]. Further multi-center randomized studies are needed to determine whether rituximab may be a reasonable alternative to cyclophosphamide as a first line treatment for SSc-ILD.
Bosentan (Tracleer)
Bosentan (Tracleer) is an endothelin-1 antagonist that is frequently used in SSc associated PAH, and that has been investigated in SSc-ILD. The BUILD-2 randomized patients with SSc-ILD to bosentan or placebo and found no difference in the 6MWT or in PFTs between the two groups at 12 months[49]. Of note, the BUILD-3 trial evaluating bosentan in IPF failed to reach its primary endpoints of time to worsening IPF or death[50].
Tyrosine Kinase Inhibitors
Studies of tyrosine kinase inhibitors such as imatinib (Gleevec) in SSc-ILD have had variable results. In one open label, phase II trial, 30 patients with diffuse SSc were treated with imatinib for 12 months[51]. Overall, there was improvement in both the mRSS and FVC, although only half of the patients had ILD at baseline. Another study treated 28 patients with morphea or diffuse SSc with 6 months of either imatinib or placebo and found no difference in the change in mRSS or DLCO between the two groups[52]. A larger randomized controlled trial of imatinib versus placebo in IPF patients did not show any effect on lung function or survival[53].
Pirfenidone (Esbriet)
Pirfenidone (Esbriet) has been studied extensively in IPF and has both anti-inflammatory and anti-fibrotic effects. A recent large multi-center RCT of pirfenidone in IPF patients showed less disease progression, as measured by FVC, improved progression free survival and reduced decline in the 6MWT in patients receiving pirfenidone[54]. Pirfenidone has been granted breakthrough therapy designation from the FDA for use in IPF. A phase II study of pirfenidone in SSc-ILD is currently underway (LOTUSS Study, ClinicalTrials.gov Identifier: NCT01933334).
Autologous Stem Cell Transplant
Autologous hematopoietic stem cell transplant (HSCT) has been studied as an attractive alternative therapeutic option given the relatively poor prognosis of patients with SSc-ILD. Three major trials, one still ongoing, have compared HSCT to cyclophosphamide in SSc patients with internal organ involvement. The ASSIST (Autologous Stem Cell Systemic Sclerosis Immune Suppression Trial) study randomized 19 patients with dcSSc and internal organ or pulmonary involvement to either non-myeloablative autologous HSCT or monthly IV cyclophosphamide for 6 months[55]. All patients in the HSCT group had improved skin scores and FVC at 12 months compared to none in the cyclophosphamide group, and 7 of 9 patients in the cyclophosphamide group switched to the HSCT group. The ASTIS (Autologous Stem cell Transplantation International Scleroderma) trial compared HSCT to 12 months of monthly IV cyclophosphamide in 156 patients with dcSSc; patients in the HSCT group had improved event-free and overall survival despite a 10% treatment related mortality in the HSCT group[56]. The SCOT (Scleroderma: Cyclophosphamide Or Transplantation) trial is currently ongoing (ClinicalTrials.gov Identifier: NCT00114530). HSCT may be an option for patients with severe disease who have been refractory to other treatment options.
Prognosis
The overall prognosis of SSc-ILD is relatively poor, but there is variability between the different pathologic subsets. Patients with NSIP tend to have better outcomes, with a median survival of 15 years compared to 3 years in patients with UIP[27]. Factors such as older age, lower FVC and lower DLCO are predictive of mortality in SSc-ILD, and extent of disease on HRCT often predicts both mortality and ILD progression[6]. Particular attention should be paid to these parameters when evaluating patients with SSc-ILD.
Conclusion
ILD is one of the most serious complications among patients with SSc, and despite advances in the understanding of the pathogenesis and treatment of the disease there is still significant morbidity and mortality. PFTs and HRCT play a central role in the diagnosis of ILD and should be monitored routinely. Although certain subgroups of SSc patients seem to be at higher risk for developing ILD, we do not have clear biomarkers for which patients will develop the disease or have disease progression. Treatment modalities that are often used with success in other autoimmune diseases have proven less beneficial in SSc-ILD. Cyclophosphamide remains the best studied agent and should be considered for any patient with progressive SSc-ILD. Newer investigational treatments such as rituximab, pirfenidone and HSCT may become options as further studies shed light on their potential benefit. Further understanding of the pathogenesis and molecular mediators will ultimately lead to novel treatment targets to modify the course of SSc-ILD.
Key Points.
Interstitial lung disease (ILD) is a significant cause of morbidity and mortality in systemic sclerosis (SSc).
Diagnostic modalities to assess ILD include pulmonary function tests (PFTs), which may show decreases in the forced vital capacity (FVC) and diffusion capacity of the lung for carbon monoxide (DLCO), as well as high resolution computed tomography (HRCT), which may show patterns consistent with nonspecific interstial pneumonia (NSIP) or usual intersitial pneumonia (UIP).
Pathogenesis revolves around an interplay of vascular injury, inflammation and subsequent fibrosis, with transcription growth factor-beta (TGF-β) playing a key role in fibrosis.
Effective treatment modalities are limited, with cyclophosphamide being the most rigorously studied treatment. Therapies that are often used in other autoimmune conditions are not as effective in SSc-ILD.
A number of alternative treatment approaches are being considered, including rituximab, bosentan, tyrosine kinase inhibitors, pirfenidone and hematopoietic stem cell transplant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Schurawitzki H, Stiglbauer R, Graninger W, et al. Interstitial lung disease in progressive systemic sclerosis: high-resolution CT versus radiography. Radiology. 1990;176(3):755–9. doi: 10.1148/radiology.176.3.2389033. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD, Owens GR, Fino GJ, et al. Pulmonary involvement in systemic sclerosis (scleroderma). Arthritis Rheum. 1985;28(7):759–67. doi: 10.1002/art.1780280706. [DOI] [PubMed] [Google Scholar]
- 3.Steen VD, Conte C, Owens GR, et al. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37(9):1283–9. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 4.McNearney TA, Reveille JD, Fischbach M, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57(2):318–26. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 5.Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66(6):754–63. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winstone TA, Assayag D, Wilcox PG, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146(2):422–36. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 7.Yanaba K, Hasegawa M, Hamaguchi Y, et al. Longitudinal analysis of serum KL-6 levels in patients with systemic sclerosis: association with the activity of pulmonary fibrosis. Clin Exp Rheumatol. 2003;21(4):429–36. [PubMed] [Google Scholar]
- 8.Yanaba K, Hasegawa M, Takehara K, et al. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J Rheumatol. 2004;31(6):1112–20. [PubMed] [Google Scholar]
- 9.Harrison NK, Myers AR, Corrin B, et al. Structural features of interstitial lung disease in systemic sclerosis. Am Rev Respir Dis. 1991;144(3 Pt 1):706–13. doi: 10.1164/ajrccm/144.3_Pt_1.706. [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Shahane A, Derk CT. Interstitial lung disease in systemic sclerosis: pathophysiology, current and new advances in therapy. Inflamm Allergy Drug Targets. 2012;11(4):266–77. doi: 10.2174/187152812800959013. [DOI] [PubMed] [Google Scholar]
- 11.Ohba T, McDonald JK, Silver RM, et al. Scleroderma bronchoalveolar lavage fluid contains thrombin, a mediator of human lung fibroblast proliferation via induction of platelet-derived growth factor alpha-receptor. Am J Respir Cell Mol Biol. 1994;10(4):405–12. doi: 10.1165/ajrcmb.10.4.7510986. [DOI] [PubMed] [Google Scholar]
- 12.Bogatkevich GS, Ludwicka-Bradley A, Nietert PJ, et al. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 2011;63(5):1416–25. doi: 10.1002/art.30255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akter T, Silver RM, Bogatkevich GS. Recent advances in understanding the pathogenesis of scleroderma interstitial lung disease. Curr Rheumatol Rep. 2014;16(4):411, 014–0411-1. doi: 10.1007/s11926-014-0411-1. [DOI] [PubMed] [Google Scholar]
- 14.Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 15.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 16.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Krishnaveni MS, Li C, et al. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121(1):277–87. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Shi W, Wang YL, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L585–93. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 19.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Wei J, Melichian D, Komura K, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63(6):1707–17. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Fang F, Lam AP, et al. Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012;64(8):2734–45. doi: 10.1002/art.34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chilosi M, Poletti V, Zamo A, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162(5):1495–502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–87. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodore AC, Tseng CH, Li N, et al. Correlation of cough with disease activity and treatment with cyclophosphamide in scleroderma interstitial lung disease: findings from the Scleroderma Lung Study. Chest. 2012;142(3):614–21. doi: 10.1378/chest.11-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 26.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176(10):1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165(12):1581–6. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 28.Launay D, Remy-Jardin M, Michon-Pasturel U, et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J Rheumatol. 2006;33(9):1789–801. [PubMed] [Google Scholar]
- 29.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 30.Solomon JJ, Olson AL, Fischer A, et al. Scleroderma lung disease. Eur Respir Rev. 2013;22(127):6–19. doi: 10.1183/09059180.00005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker KM, Pope J, participating members of the Scleroderma Clinical Trials Consortium (SCTC) et al. Treatment of systemic sclerosis complications: what to use when first-line treatment fails--a consensus of systemic sclerosis experts. Semin Arthritis Rheum. 2012;42(1):42–55. doi: 10.1016/j.semarthrit.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Christmann RB, Wells AU, Capelozzi VL, et al. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40(3):241–9. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med. 2009;179(5):408–13. doi: 10.1164/rccm.200808-1359OC. [DOI] [PubMed] [Google Scholar]
- 34.Buch MH, Denton CP, Furst DE, et al. Submaximal exercise testing in the assessment of interstitial lung disease secondary to systemic sclerosis: reproducibility and correlations of the 6-min walk test. Ann Rheum Dis. 2007;66(2):169–73. doi: 10.1136/ard.2006.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer A, Swigris JJ, Groshong SD, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134(3):601–5. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 36.Kowal-Bielecka O, Landewe R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009;68(5):620–8. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]
- 37.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54(12):3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 39.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133(2):455–60. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 40.Simeon-Aznar CP, Fonollosa-Pla V, Tolosa-Vilella C, et al. Effect of mycophenolate sodium in scleroderma-related interstitial lung disease. Clin Rheumatol. 2011;30(11):1393–8. doi: 10.1007/s10067-011-1823-1. [DOI] [PubMed] [Google Scholar]
- 41.Zamora AC, Wolters PJ, Collard HR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008;102(1):150–5. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Panopoulos ST, Bournia VK, Trakada G, et al. Mycophenolate versus cyclophosphamide for progressive interstitial lung disease associated with systemic sclerosis: a 2-year case control study. Lung. 2013;191(5):483–9. doi: 10.1007/s00408-013-9499-8. [DOI] [PubMed] [Google Scholar]
- 43.Dheda K, Lalloo UG, Cassim B, et al. Experience with azathioprine in systemic sclerosis associated with interstitial lung disease. Clin Rheumatol. 2004;23(4):306–9. doi: 10.1007/s10067-004-0906-7. [DOI] [PubMed] [Google Scholar]
- 44.Berezne A, Ranque B, Valeyre D, et al. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol. 2008;35(6):1064–72. [PubMed] [Google Scholar]
- 45.Sottile PD, Iturbe D, Katsumoto TR, et al. Outcomes in systemic sclerosis-related lung disease after lung transplantation. Transplantation. 2013;95(7):975–80. doi: 10.1097/TP.0b013e3182845f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54(12):3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 47.Jordan S, Distler JH, Maurer B, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 48.Daoussis D, Liossis SN, Tsamandas AC, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49(2):271–80. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibold JR, Denton CP, Furst DE, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum. 2010;62(7):2101–8. doi: 10.1002/art.27466. [DOI] [PubMed] [Google Scholar]
- 50.King TE, Jr, Brown KK, Raghu G, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(1):92–9. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 51.Khanna D, Saggar R, Mayes MD, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63(11):3540–6. doi: 10.1002/art.30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167(5):1138–44. doi: 10.1111/j.1365-2133.2012.11186.x. [DOI] [PubMed] [Google Scholar]
- 53.Daniels CE, Lasky JA, Limper AH, et al. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–10. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 54.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 55.Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378(9790):498–506. doi: 10.1016/S0140-6736(11)60982-3. [DOI] [PubMed] [Google Scholar]
- 56.van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311(24):2490–8. doi: 10.1001/jama.2014.6368. [DOI] [PubMed] [Google Scholar]