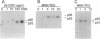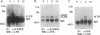Abstract
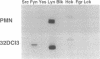
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein that critically regulates the viability, proliferation, and differentiation of granulocytic precursors and the function of neutrophils by signaling through its receptor. Cloning of the human G-CSF receptor (G-CSFR) cDNA has demonstrated sequence homology with other members of the hematopoietic/cytokine receptor superfamily. G-CSF stimulates the appearance of phosphotyrosine proteins in several types of human and murine myeloid cells. Since the receptor does not possess intrinsic tyrosine kinase activity, we hypothesized that G-CSFR interacts with and activates cytosolic protein-tyrosine kinases (PTKs). In vitro protein kinase assay of human G-CSFR immunoprecipitates demonstrated at least two tyrosine phosphoproteins, pp55 and pp70. We observed that G-CSF activated p53/p56lyn, a Src-related PTK, and p72syk, a non-Src-related PTK. Lyn and Syk were recovered in anti-G-CSFR immunoprecipitates; Lyn was detected in the absence of ligand. In addition, upon G-CSF stimulation, Lyn coimmunoprecipitated with Syk. Analysis of the G-CSFR amino acid sequence revealed a potential receptor activation motif for Syk. On the basis of immunoprecipitation and sequence analysis data, we propose that the human G-CSFR forms a three-component signaling complex with Lyn and Syk. Their sequential recruitment into the G-CSFR signaling complex demonstrates the coordinated involvement of two PTKs with a member of the hematopoietic/cytokine receptor superfamily.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal A., Salem P., Robbins K. C. Involvement of p72syk, a protein-tyrosine kinase, in Fc gamma receptor signaling. J Biol Chem. 1993 Jul 25;268(21):15900–15905. [PubMed] [Google Scholar]
- Akimaru K., Utsumi T., Sato E. F., Klostergaard J., Inoue M., Utsumi K. Role of tyrosyl phosphorylation in neutrophil priming by tumor necrosis factor-alpha and granulocyte colony stimulating factor. Arch Biochem Biophys. 1992 Nov 1;298(2):703–709. doi: 10.1016/0003-9861(92)90469-d. [DOI] [PubMed] [Google Scholar]
- Bolen J. B. Nonreceptor tyrosine protein kinases. Oncogene. 1993 Aug;8(8):2025–2031. [PubMed] [Google Scholar]
- Bolen J. B., Rowley R. B., Spana C., Tsygankov A. Y. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992 Dec;6(15):3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Turrini F., Alessi D., Ghigo D., Costamagna C., Pescarmona G., Mantovani A., Bosia A. Stimulation of the Na+/H+ exchanger in human endothelial cells activated by granulocyte- and granulocyte-macrophage-colony-stimulating factor. Evidence for a role in proliferation and migration. J Biol Chem. 1989 Nov 5;264(31):18284–18287. [PubMed] [Google Scholar]
- Cambier J. C. Signal transduction by T- and B-cell antigen receptors: converging structures and concepts. Curr Opin Immunol. 1992 Jun;4(3):257–264. doi: 10.1016/0952-7915(92)90074-o. [DOI] [PubMed] [Google Scholar]
- Corey S., Eguinoa A., Puyana-Theall K., Bolen J. B., Cantley L., Mollinedo F., Jackson T. R., Hawkins P. T., Stephens L. R. Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. EMBO J. 1993 Jul;12(7):2681–2690. doi: 10.1002/j.1460-2075.1993.tb05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G. D., Griffin J. D. Granulocyte colony-stimulating factor and its receptor. Blood. 1991 Dec 1;78(11):2791–2808. [PubMed] [Google Scholar]
- Dong F., van Buitenen C., Pouwels K., Hoefsloot L. H., Löwenberg B., Touw I. P. Distinct cytoplasmic regions of the human granulocyte colony-stimulating factor receptor involved in induction of proliferation and maturation. Mol Cell Biol. 1993 Dec;13(12):7774–7781. doi: 10.1128/mcb.13.12.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Evans J. P., Mire-Sluis A. R., Hoffbrand A. V., Wickremasinghe R. G. Binding of G-CSF, GM-CSF, tumor necrosis factor-alpha, and gamma-interferon to cell surface receptors on human myeloid leukemia cells triggers rapid tyrosine and serine phosphorylation of a 75-Kd protein. Blood. 1990 Jan 1;75(1):88–95. [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993 Sep 24;74(6):1079–1087. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Pan C. X., Seto Y., Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991 Oct;10(10):2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R., Seto Y., Mizushima S., Nagata S. Three different mRNAs encoding human granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8702–8706. doi: 10.1073/pnas.87.22.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. B lymphocyte activation is accompanied by phosphorylation of a 72-kDa protein-tyrosine kinase. J Biol Chem. 1991 Aug 15;266(23):14846–14849. [PubMed] [Google Scholar]
- Isfort R. J., Ihle J. N. Multiple hematopoietic growth factors signal through tyrosine phosphorylation. Growth Factors. 1990;2(2-3):213–220. doi: 10.3109/08977199009071507. [DOI] [PubMed] [Google Scholar]
- Larsen A., Davis T., Curtis B. M., Gimpel S., Sims J. E., Cosman D., Park L., Sorensen E., March C. J., Smith C. A. Expression cloning of a human granulocyte colony-stimulating factor receptor: a structural mosaic of hematopoietin receptor, immunoglobulin, and fibronectin domains. J Exp Med. 1990 Dec 1;172(6):1559–1570. doi: 10.1084/jem.172.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. A., Chan V. W., Datta S. K., DeFranco A. L. B-cell antigen receptor motifs have redundant signalling capabilities and bind the tyrosine kinases PTK72, Lyn and Fyn. Curr Biol. 1993 Oct 1;3(10):645–657. doi: 10.1016/0960-9822(93)90062-s. [DOI] [PubMed] [Google Scholar]
- Li Z. H., Mahajan S., Prendergast M. M., Fargnoli J., Zhu X., Klages S., Adam D., Schieven G. L., Blake J., Bolen J. B. Cross-linking of surface immunoglobulin activates src-related tyrosine kinases in WEHI 231 cells. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1536–1544. doi: 10.1016/0006-291x(92)90477-3. [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Burgess A. W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992 Jul 2;327(1):28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Evans G., Michiel D., Farrar W. L. Characterization of a 97-kDa phosphotyrosylprotein regulated by multiple cytokines. J Biol Chem. 1992 Nov 25;267(33):23993–23998. [PubMed] [Google Scholar]
- Matsuda S., Shirafuji N., Asano S. Human granulocyte colony-stimulating factor specifically binds to murine myeloblastic NFS-60 cells and activates their guanosine triphosphate binding proteins/adenylate cyclase system. Blood. 1989 Nov 15;74(7):2343–2348. [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Fukunaga R. Granulocyte colony-stimulating factor receptor and its related receptors. Growth Factors. 1993;8(2):99–107. doi: 10.3109/08977199309046930. [DOI] [PubMed] [Google Scholar]
- Pazin M. J., Williams L. T. Triggering signaling cascades by receptor tyrosine kinases. Trends Biochem Sci. 1992 Oct;17(10):374–378. doi: 10.1016/0968-0004(92)90003-r. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Griffin J. D., Simons E. R., Schafer A. I., Meshulam T., Fredette J. P., Maas A. K., Gadenne A. S., Leavitt J. L., Melnick D. A. Effects of recombinant human granulocyte and macrophage colony-stimulating factors on signal transduction pathways in human granulocytes. J Immunol. 1987 Nov 15;139(10):3422–3430. [PubMed] [Google Scholar]
- Taniguchi T., Kitagawa H., Yasue S., Yanagi S., Sakai K., Asahi M., Ohta S., Takeuchi F., Nakamura S., Yamamura H. Protein-tyrosine kinase p72syk is activated by thrombin and is negatively regulated through Ca2+ mobilization in platelets. J Biol Chem. 1993 Feb 5;268(4):2277–2279. [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Tweardy D. J., Anderson K., Cannizzaro L. A., Steinman R. A., Croce C. M., Huebner K. Molecular cloning of cDNAs for the human granulocyte colony-stimulating factor receptor from HL-60 and mapping of the gene to chromosome region 1p32-34. Blood. 1992 Mar 1;79(5):1148–1154. [PubMed] [Google Scholar]
- Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J Immunol. 1987 Jun 1;138(11):3829–3835. [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993 Apr 23;73(2):209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Yamada T., Taniguchi T., Yang C., Yasue S., Saito H., Yamamura H. Association with B-cell-antigen receptor with protein-tyrosine kinase p72syk and activation by engagement of membrane IgM. Eur J Biochem. 1993 Apr 1;213(1):455–459. doi: 10.1111/j.1432-1033.1993.tb17781.x. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T. L., Bolen J. B., Ihle J. N. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991 May;11(5):2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Bird T. A., Morella K. K., Mosley B., Gearing D. P., Baumann H. Distinct regions of the human granulocyte-colony-stimulating factor receptor cytoplasmic domain are required for proliferation and gene induction. Mol Cell Biol. 1993 Apr;13(4):2384–2390. doi: 10.1128/mcb.13.4.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]