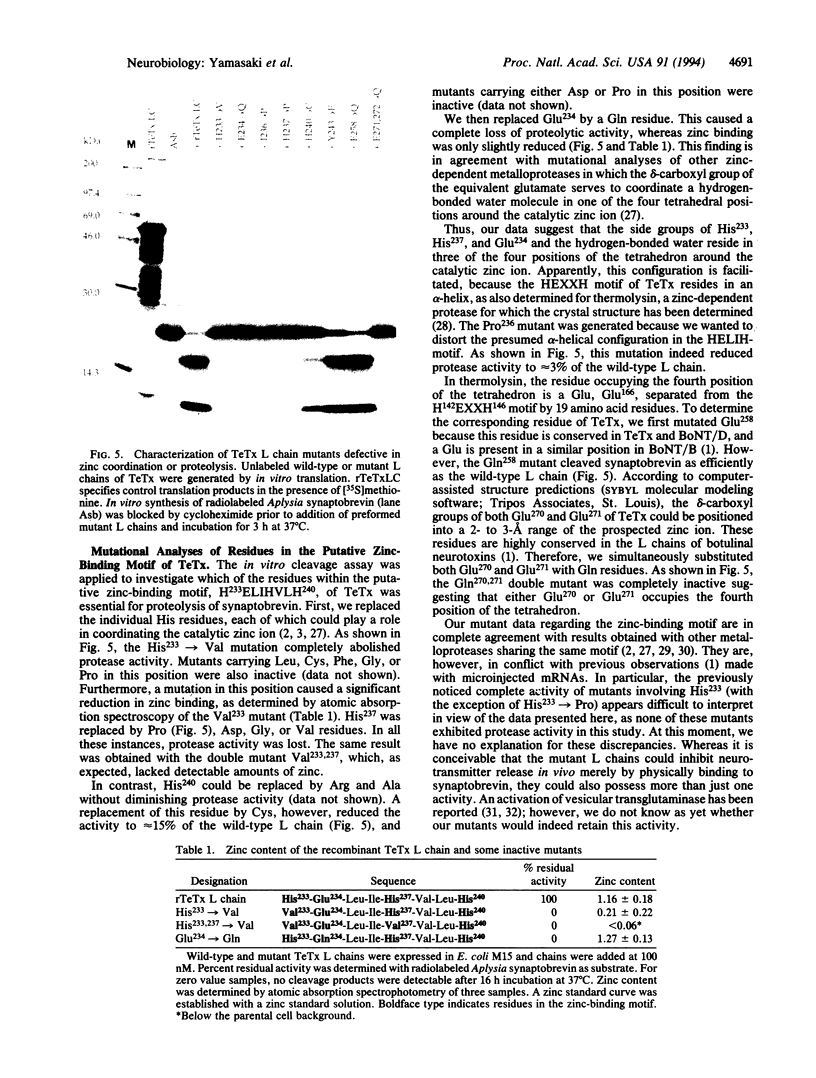
Abstract
Synaptobrevin/vesicle-associated membrane protein (VAMP) and syntaxin are potential vesicle donor and target membrane receptors of a docking complex that requires N-ethylmaleimide-sensitive factor (NSF) and soluble NSF-attachment proteins as soluble factors for vesicle fusion with target membranes. Members of this docking complex are the target of clostridial neurotoxins that act as zinc-dependent proteases. Molecular cloning of the Aplysia californica synaptobrevin cDNA revealed a 180-residue polypeptide (M(r), 19,745) with a central transmembrane region and an atypically large C-terminal intravesicular domain. This polypeptide integrates into membranes at both the co- and posttranslational level, as shown by modification of an artificially introduced N-glycosylation site. The soluble and membrane-anchored forms of synaptobrevin are cleaved by the light chains of the botulinal toxins type D and F and by tetanus toxin involving the peptide bonds Lys49-Ile50, Gln48-Lys49, and Gln66-Phe67, respectively. The active center of teh tetanus toxin light chain was identified by site-specific mutagenesis. His233, His237, Glu234, and Glu270/271 are essential to this proteolytic activity. Modification of histidine residues resulted in loss of zinc binding, whereas a replacement of Glu234 only slightly reduced the zinc content.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer B. T., 3rd, Ozçelik T., Jahn R., Francke U., Südhof T. C. Structures and chromosomal localizations of two human genes encoding synaptobrevins 1 and 2. J Biol Chem. 1990 Oct 5;265(28):17267–17273. [PubMed] [Google Scholar]
- Becker A. B., Roth R. A. An unusual active site identified in a family of zinc metalloendopeptidases. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3835–3839. doi: 10.1073/pnas.89.9.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz T., Kurazono H., Popoff M. R., Eklund M. W., Sakaguchi G., Kozaki S., Krieglstein K., Henschen A., Gill D. M., Niemann H. Nucleotide sequence of the gene encoding Clostridium botulinum neurotoxin type D. Nucleic Acids Res. 1990 Sep 25;18(18):5556–5556. doi: 10.1093/nar/18.18.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993 Sep 9;365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Yamasaki S., Binz T., Niemann H., Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993 Dec;12(12):4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Eisel U., Reynolds K., Riddick M., Zimmer A., Niemann H., Zimmer A. Tetanus toxin light chain expression in Sertoli cells of transgenic mice causes alterations of the actin cytoskeleton and disrupts spermatogenesis. EMBO J. 1993 Sep;12(9):3365–3372. doi: 10.1002/j.1460-2075.1993.tb06010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L. A., Trimble W. S., Scheller R. H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989 Jul 5;264(19):11061–11064. [PubMed] [Google Scholar]
- Facchiano F., Benfenati F., Valtorta F., Luini A. Covalent modification of synapsin I by a tetanus toxin-activated transglutaminase. J Biol Chem. 1993 Mar 5;268(7):4588–4591. [PubMed] [Google Scholar]
- Facchiano F., Luini A. Tetanus toxin potently stimulates tissue transglutaminase. A possible mechanism of neurotoxicity. J Biol Chem. 1992 Jul 5;267(19):13267–13271. [PubMed] [Google Scholar]
- Gardner D., Kandel E. R. Diphasic postsynaptic potential: a chemical synapse capable of mediating conjoint excitation and inhibition. Science. 1972 May 12;176(4035):675–678. doi: 10.1126/science.176.4035.675. [DOI] [PubMed] [Google Scholar]
- Gerst J. E., Rodgers L., Riggs M., Wigler M. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAP genes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4338–4342. doi: 10.1073/pnas.89.10.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. A., Matthews B. W. Structure of thermolysin refined at 1.6 A resolution. J Mol Biol. 1982 Oct 5;160(4):623–639. doi: 10.1016/0022-2836(82)90319-9. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Kandel E. R. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 1993 Jan;72 (Suppl):1–30. doi: 10.1016/s0092-8674(05)80025-x. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Kurazono H., Mochida S., Binz T., Eisel U., Quanz M., Grebenstein O., Wernars K., Poulain B., Tauc L., Niemann H. Minimal essential domains specifying toxicity of the light chains of tetanus toxin and botulinum neurotoxin type A. J Biol Chem. 1992 Jul 25;267(21):14721–14729. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Link E., Edelmann L., Chou J. H., Binz T., Yamasaki S., Eisel U., Baumert M., Südhof T. C., Niemann H., Jahn R. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- Mayer T., Tamura T., Falk M., Niemann H. Membrane integration and intracellular transport of the coronavirus glycoprotein E1, a class III membrane glycoprotein. J Biol Chem. 1988 Oct 15;263(29):14956–14963. doi: 10.1016/S0021-9258(18)68131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Südhof T. C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993 Jul 22;364(6435):346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Medina J. F., Wetterholm A., Rådmark O., Shapiro R., Haeggström J. Z., Vallee B. L., Samuelsson B. Leukotriene A4 hydrolase: determination of the three zinc-binding ligands by site-directed mutagenesis and zinc analysis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7620–7624. doi: 10.1073/pnas.88.17.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Iwasaki M., Sakaguchi G. Clostridium botulinum type D toxin: purification, molecular structure, and some immunological properties. Infect Immun. 1977 Aug;17(2):395–401. doi: 10.1128/iai.17.2.395-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Poulain B., Eisel U., Binz T., Kurazono H., Niemann H., Tauc L. Exogenous mRNA encoding tetanus or botulinum neurotoxins expressed in Aplysia neurons. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7844–7848. doi: 10.1073/pnas.87.20.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko A. G., Perin M. S., Davletov B. A., Ushkaryov Y. A., Geppert M., Südhof T. C. Binding of synaptotagmin to the alpha-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991 Sep 5;353(6339):65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Poulain B., Wadsworth J. D., Shone C. C., Mochida S., Lande S., Melling J., Dolly J. O., Tauc L. Multiple domains of botulinum neurotoxin contribute to its inhibition of transmitter release in Aplysia neurons. J Biol Chem. 1989 Dec 25;264(36):21928–21933. [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy V., DasGupta B. R. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J Biol Chem. 1985 Sep 5;260(19):10461–10466. [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Shone C. C., Rossetto O., Alexander F. C., Montecucco C. Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J Biol Chem. 1993 Jun 5;268(16):11516–11519. [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Baumert M., Perin M. S., Jahn R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989 May;2(5):1475–1481. doi: 10.1016/0896-6273(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]