Abstract
Heterochromatin, or condensed chromatin, has the potential to encroach into what ordinarily would be euchromatin and repress resident genes. We explore how heterochromatin is restricted to the appropriate regions of the genome, using Saccharomyces cerevisiae as a case study and emphasizing two under-appreciated aspects of silenced chromatin. First, the capacity of silenced chromatin to propagate along a chromosome is limited by the intrinsic instability of the structure. We argue that this limited potential to spread is an important factor restricting silenced chromatin to the vicinity of recruitment sites (silencers). Second, this limited capacity to spread creates the need for additional mechanisms to stabilize silenced chromatin at the required locations. Such mechanisms include the use of multiple silencers and higher-order arrangements of the chromatin fiber. Therefore, to understand how silenced chromatin is restricted to the appropriate genomic locations, researchers must take into account the mechanisms by which silenced chromatin is stabilized in appropriate locations.
Heterochromatin, or condensed chromatin, occurs in many eukaryotes in pericentromeric and subtelomeric regions of the genome, where it serves a structural role. These heterochromatic domains contain few genes and are generally repressive to transcription. Heterochromatin can also function to regulate transcription. For example, the inactivation of one X chromosome in female mammals is due to the formation of a condensed chromatin structure. One of the notable properties of heterochromatin is its capacity to propagate along a chromosome, as illustrated by the phenomenon of position effect variegation in Drosophila. In the original example described by Hermann Muller, the Drosophila white gene, which determines eye color, was translocated near pericentromeric heterochromatin, resulting in variegated expression of the white gene and hence flies with mottled white and red eyes. Thus, heterochromatin has the capacity to encroach into what ordinarily would be euchromatin and repress resident genes.
The ability of heterochromatin to propagate along a chromosome raises the important question of how such propagation is controlled, and, more generally, how heterochromatin is restricted to the appropriate regions of the genome. Recent work focuses on features external to heterochromatin, such as boundary elements and other “anti-silencing” factors, which play an important role in restricting heterochromatin. Less appreciated are intrinsic properties of heterochromatin itself that limit its capacity to spread. This review focuses on these intrinsic limitations to spreading and, as a case study, the Sir proteins from Saccharomyces cerevisiae, which generate a well-studied type of silenced chromatin.
Silenced Chromatin Is Self-Reinforcing
In the yeast S. cerevisiae, silenced chromatin forms in subtelomeric regions, such that genes placed near telomeres display variegated expression, reminiscent of position effect variegation in Drosophila. Silenced chromatin also forms at the silent mating-type loci, HMR and HML, where it constitutively represses extra copies of the genes that determine mating-type. (These extra copies enable cells to switch their mating-type.) The structural components of silenced chromatin are the Sir proteins, which have self-reinforcing properties that enable them to propagate along a chromosome. Specifically, Sir2p is a deacetylase, and Sir3p and Sir4p bind to nucleosomes that are hypoacetylated. Once the Sir complex is recruited to a silencer element, Sir2p can deacetylate neighboring nucleosomes, thereby generating binding sites for Sir3p and Sir4p, which in turn recruit additional Sir2p. If this additional molecule of Sir2p can deacetylate nucleosomes beyond those deacetylated by the original silencer-associated Sir2p molecule, the silenced chromatin will spread. Thus, the self-reinforcing properties of silenced chromatin enable the Sir complex to propagate along the chromosome by sequentially deacetylating and binding nucleosomes (Hoppe et al., 2002; Rusche et al., 2002).
Other types of heterochromatin are also self-reinforcing. In general, self-reinforcing chromatin has two important properties—a protein domain that binds to nucleosomes with particular modifications and enzymatic activities to create those same modifications. For example, heterochromatin in Drosophila is characterized by the presence of HP1 (heterochromatin protein 1) and Su(var)3–9. HP1 binds specifically to nucleosomes containing H3-K9me, and SuVar3–9 is a methyltransferase that methylates H3-K9. These self-reinforcing properties enable the propagation and inheritance of particular chromatin states but could also be dangerous to the cell if silenced chromatin spreads too far or fortuitously assembles in the wrong locations.
External Factors That Limit the Spread of Silenced Chromatin
In the case of the Sir proteins, the published literature addressing how silenced chromatin is restricted to the appropriate genomic regions has focused on external factors that limit the spread of silenced chromatin. These factors fall into two categories—boundary elements and anti-silencing modifications of nucleosomes. A well-studied boundary on the telomere-proximal side of HMR coincides with a tRNA gene, and it is thought that the assembly of the polymerase III transcription complex blocks the spreading of silenced chromatin (Donze and Kamakaka, 2001). In addition, on the telomere-proximal side of HML, the gene VBA3 diminishes silencing (Bi, 2002). However, no other boundary elements have been identified in S. cerevisiae, although there are roughly 30 other genomic locations at which a transition between silenced and active chromatin must occur. [X element combinatorial repeats, or STAR elements, which are found at many telomeres, can act as boundaries to silencing (Fourel et al., 1999). However, in their native contexts, these elements are not boundaries because they are located between subtelomeric repeats and X core sequences, both of which promote silencing.]
In the absence of discrete boundaries, it is thought that silencing terminates in a zone of competition between silenced and active chromatin. In particular, certain modifications of histones that are associated with active chromatin are proposed to reduce the affinity of the Sir complex for nucleosomes. These modifications include acetylation of H4-K16 (Kimura et al., 2002; Suka et al., 2002), methylation of H3-K4 (Santos-Rosa et al., 2004) and H3-K79 (Ng et al., 2002; van Leeuwen et al., 2002), and the histone variant H2A.Z (Meneghini et al., 2003). The model that euchromatin actively excludes Sir proteins is supported by observations that, particularly at telomeres, Sir proteins spread farther in cells lacking the enzymes that create these modifications. For example, in the absence of the acetyltransferase Sas2p, Sir proteins are detectable by chromatin IP up to 10 or 20 kb from the telomere (Kimura et al., 2002; Suka et al., 2002), as opposed to 1–2 kb in wild-type yeast. However, it should be noted that only a slight enrichment of Sir proteins is detected more than 1–2 kb from the silencer, indicating that Sir proteins spread farther in just a small fraction of cells in the population. It is also reported that Sir-dependent repression can occur over 100 kb from telomeres in cells lacking two anti-silencing factors, H2A.Z and H3-K4me (Venkatasubrahmanyam et al., 2007). However, at these locations, Sir proteins were not detectable by chromatin IP, indicating that Sir proteins were transiently associated with these sites. Thus, even in the absence of anti-silencing factors, Sir proteins are largely restricted to the vicinity of their sites of recruitment. Therefore other mechanisms must dampen the spreading of Sir-silenced chromatin.
Intrinsic Properties of Silenced Chromatin That Limit Its Extent
Compared to the external factors described above, less attention has been given to the intrinsic properties of Sir-silenced chromatin that limit its capacity to spread, although it is clear that such properties exist. For example, the enrichment of Sir proteins at telomeres, as assessed by chromatin immunoprecipitation, diminishes as the distance from the silencer increases, implying that each spreading step has a lower probability of occurring than the previous step. Moreover, this decrease in Sir-protein association is quite steep, approaching background levels within a few kilobase pairs, indicating that the capacity of Sir proteins to spread is limited. Similarly, at HMR, little association of Sir proteins is detected beyond the tRNA barrier, even when the barrier has been deleted (Oki and Kamakaka, 2005), again consistent with a limited potential to spread. Furthermore, even in the absence of anti-silencing factors, the spreading of Sir proteins is not particularly efficient, as high levels of Sir proteins are detectable only within a few kilobase pairs of recruitment sites and lower levels of Sir proteins are detectable only up to about 10 kb (Kimura et al., 2002; Suka et al., 2002; Meneghini et al., 2003).
This limited potential of Sir chromatin to spread probably results from the relatively rapid turnover of Sir proteins within silenced chromatin (Cheng and Gartenberg, 2000) combined with limiting amounts of Sir proteins in the cell. One way to understand these dynamics is to imagine a nucleosome (n), which is associated with Sir proteins. The ability of the neighboring nucleosome (n + 1) to recruit additional Sir proteins is dependent in two ways on the Sir complex associated with the first nucleosome. First, deacetylation by Sir2p associated with nucleosome (n) will increase the affinity of nucleosome (n + 1) for Sir proteins. Second, protein–protein interactions between Sir complexes associated with the two nucleosomes will stabilize the entire structure (Rudner et al., 2005). Therefore nucleosome (n + 1) is more likely to be associated with Sir proteins if its neighbor is also associated with Sir proteins. These properties enable silenced chromatin to spread. However, given the high turnover of Sir proteins within silenced chromatin, there is a probability that the Sir complex will dissociate from nucleosome (n) prior to enabling another Sir complex to bind to nucleosome (n + 1). Thus, the probability of nucleosome (n + 1) being associated with a Sir complex is lower than for nucleosome (n), and the probability for nucleosome (n + 2) is lower still. Therefore, the level of association of the Sir complex with chromatin decays as a function of distance from the site of recruitment.
Although the intrinsic instability of Sir-silenced chromatin has not been emphasized in the literature, it provides an ideal mechanism for restricting silenced chromatin to appropriate genomic locations. Because Sir-containing chromatin naturally diminishes over a distance of a few kilobase pairs, it is unable to propagate far from its site of initiation. Therefore, even without boundary elements, silenced chromatin is restricted to the vicinity of silencer elements and only modestly influences the transcription of neighboring genes. Furthermore, if Sir proteins fortuitously associate with a nucleosome that happens to be deacetylated, they will be unable to spread far from this site of accidental assembly, reducing the likelihood that an essential gene will be silenced. Perhaps, over millions of years, Sir proteins have evolved to have an appropriately modest affinity for nucleosomes such that they will not spread too far from silencers and will not assemble spontaneously in other genomic locations. In this manner, the intrinsic instability of Sir chromatin is an important mechanism for restricting silenced chromatin to appropriate genomic locations.
It should also be noted that Sir-silenced chromatin is not unique in being relatively unstable. HP1, a histone-binding protein that contributes to position effect variegation in Drosophila and many other eukaryotes, also turns over rapidly (Cheutin et al., 2003; Festenstein et al., 2003). Furthermore, the observation that euchromatic genes, such as the white gene, experience variegated expression rather than complete repression when placed near heterochromatin reveals that long-range spreading does not occur consistently in every cell and therefore that the capacity of heterochromatin to spread must be limited.
The Need for Booster Elements
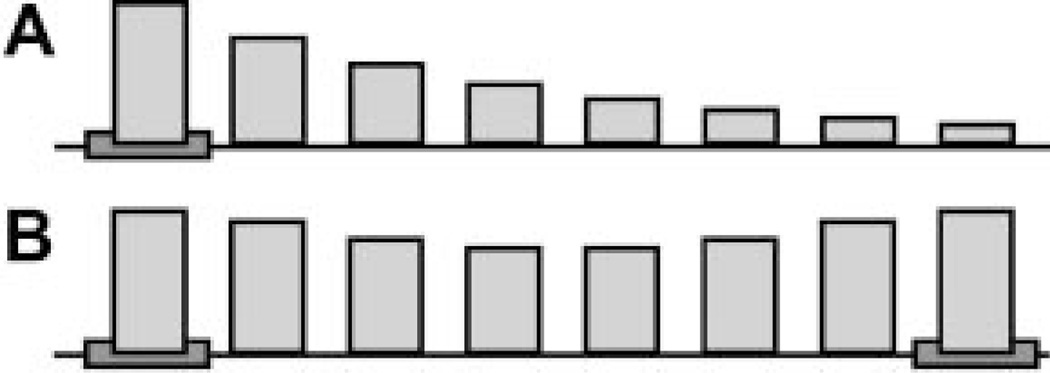
Although the intrinsic instability of Sir-silenced chromatin has the benefit of reducing the toxicity of silenced chromatin to the cell, this instability leads to a different dilemma. If the capacity of silenced chromatin to spread is low, how is effective silencing to be achieved at those locations where it is required? This problem is particularly pressing at the silent mating-type loci, HMR and HML, which must be completely silenced if cell-type identity is to be maintained. At HMR, the promoter which must be repressed is 1 kb (or six nucleosomes) away from either silencer, and at HML it is 1.3 kb from the nearest silencer. At these distances, if the Sir proteins were to spread in a linear fashion from a single silencer, they would be associated with the promoter only a fraction of the time and consequently might not achieve complete repression (Fig. 1A). This reduced association can be seen, for example, at telomere VI-R, where the enrichment of Sir proteins one kb from the telomeric repeat is only a third of that observed at the telomeric repeat itself. This reduced occupancy by Sir proteins may not be sufficient to maintain complete repression, as evidenced by the variegated expression of some reporter genes placed at this distance from a telomere (Renauld et al., 1993; Pryde and Louis, 1999; Bi, 2002). In contrast, at the mating-type loci, where absolute silencing is necessary, the cell does not rely on a single site to recruit Sir proteins. Both HMR and HML are flanked by two silencers, E and I, which reinforce one another to maintain silenced chromatin between the silencers (Fig. 1B). Furthermore, a Rap1 binding site in the promoter at HML has been reported to stabilize the association of Sir proteins and has been termed a “protosilencer.” Thus, one mechanism to overcome the intrinsic instability of Sir chromatin is the use of a combination of silencers and protosilencers to promote the association of Sir proteins within a particular region.
Fig. 1.
Silencers can cooperate to promote the assembly of silencing proteins. A: A single silencer (gray box) initiates silencing. At each position along the chromosome, the fraction of cells in which silencing proteins are associated is represented by the height of the bar. The association of silencing proteins diminishes with increasing distance from the silencer. This illustration is not meant to represent actual amounts of Sir proteins at particular distances from a silencer. B:Two cooperating silencers increase the probabilities that regions between the silencers will be associated with silencing proteins.
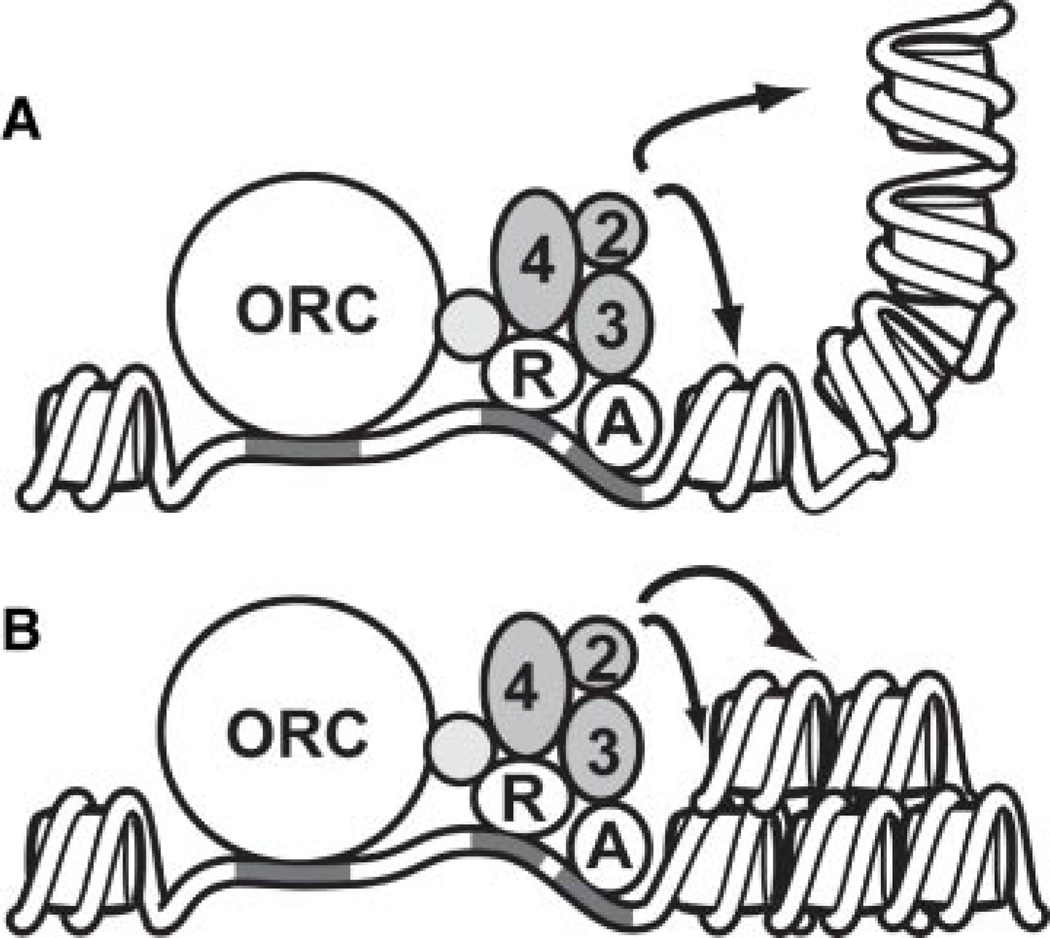
Emerging evidence suggests that silencers may also promote loops or other conformations of the chromatin fiber that strengthen the assembly of Sir chromatin by enabling it to occur in a nonlinear fashion. In particular, 3C analysis and other experimental evidence indicate that the E and I silencers flanking HMR are near one another in space and may form the base of a loop (Valenzuela et al., 2008). Such a conformation could promote the assembly of silenced chromatin by bringing multiple nucleosomes into close proximity with the silencer (Fig. 2A). The consequence of this arrangement would be that Sir2p at the silencer could deacetylate multiple nucleosomes, including some that are not immediately adjacent to the silencer. Sir3p and Sir4p would then be able to associate with these deacetylated nucleosomes. In this scenario, multiple nucleosomes throughout the region are, in essence, one spreading step away from the silencer and hence will have a higher probability of associating with Sir proteins. Other higher-order arrangements of the chromatin fiber, such as compaction, could act similarly to promote association of Sir proteins (Fig. 2B).
Fig. 2.
Higher-order arrangements of the chromatin fiber could enable the assembly of silenced chromatin. A: A loop could bring multiple nucleosomes into close proximity to the Sir complex (gray balls) at a silencer. Three silencer binding proteins, the origin recognition complex (ORC), Rap1 (R), and Abf1 (A), recruit the Sir proteins to the silencer. Once recruited, Sir2p could deacetylate any nucleosome in close proximity to the silencer (arrows). B:A compact chromatin fiber, such as the 30 nm fiber, could also result in multiple nucleosomes being close to Sir2p at the silencer.
The HMR-E silencer may even have its own built-in boosting capabilities that stabilize silenced chromatin over a distance. HMR-E has long been recognized as the most potent silencer in S. cerevisiae, and silencing mediated by HMR-E is only marginally reduced in the absence of HMR-I (Rivier et al., 1999). This lack of dependence on HMR-I is contrary to what would be predicted if Sir proteins spread in a strictly linear fashion from the E silencer in the absence of the I silencer. These observations imply that the wild-type E silencer has additional properties beyond recruiting Sir proteins that increase its capacity to establish silenced chromatin. Consistent with this conclusion, we have found that, even in the absence of the I silencer, silenced chromatin assembles significantly more rapidly at HMR than at a telomere and that insertion of HMR-E adjacent to the telomere increases the rate of assembly of silenced chromatin (Lynch and Rusche, 2009). Thus, HMR-E may enable the assembly of silenced chromatin to occur in a nonlinear fashion, resulting in strong silencing of the HMR locus, as required for mating.
Conclusion
In summary, two aspects of silenced chromatin have been under-appreciated. First, even though silenced chromatin is self-reinforcing and can propagate along a chromosome, the capacity of silenced chromatin to spread is limited. This limited potential to spread is no doubt important for reducing the toxicity of silencing proteins, which would wreak havoc on gene expression if they spontaneously assembled in the wrong locations. Second, the limited capacity of silenced chromatin to spread increases the importance of silencers and other booster elements that promote the assembly of silenced chromatin in specific locations. In particular, closely spaced silencers can act synergistically to enhance the assembly of silenced chromatin between the silencers, and some silencers may create higher-order arrangements of the chromatin fiber that enable assembly to occur in a nonlinear fashion. Without such booster elements, silencing may not be sufficiently stable in regions where it is required. Therefore, a complete picture of how silenced chromatin is restricted to the appropriate genomic locations must include the mechanisms by which silenced chromatin is stabilized in certain locations in addition to a description of barriers and anti-silencing factors.
Acknowledgments
We thank Beth Sullivan, Bayly Wheeler, and members of the Rusche lab for helpful discussions and insightful comments on this manuscript. Research in the Rusche lab is supported by a grant from the National Institutes of Health (GM073991).
Literature Cited
- Bi X. Domains of gene silencing near the left end of chromosome III in Saccharomyces cerevisiae. Genetics. 2002;160:1401–1407. doi: 10.1093/genetics/160.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T-H, Gartenberg MR. Yeast heterochromatin is a dynamic structure that requires silencers continuously. Genes Dev. 2000;14:452–463. [PMC free article] [PubMed] [Google Scholar]
- Cheutin T, McNairn AJ, Jenuwein T, Gilbert DM, Singh PB, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- Donze D, Kamakaka RT. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 2001;20:520–531. doi: 10.1093/emboj/20.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein R, Pagakis SN, Hiragami K, Lyon D, Verreault A, Sekkali B, Kioussis D. Modulation of heterochromatin protein 1 dynamics in primary mammalian cells. Science. 2003;299:719–721. doi: 10.1126/science.1078694. [DOI] [PubMed] [Google Scholar]
- Fourel G, Revardel E, Koering CE, Gilson E. Cohabitation of insulators and silencing elements in yeast subtelomeric regions. EMBO J. 1999;18:2522–2537. doi: 10.1093/emboj/18.9.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe GJ, Tanny JC, Rudner AD, Gerber SA, Danaie S, Gygi SP, Moazed D. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol. 2002;22:4167–4180. doi: 10.1128/MCB.22.12.4167-4180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Umehara T, Horikoshi M. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nat Genet. 2002;32:370–377. doi: 10.1038/ng993. [DOI] [PubMed] [Google Scholar]
- Lynch PJ, Rusche LN. A silencer promotes the assembly of silenced chromatin independently of recruitment. Mol Cell Biol. 2009;29:43–56. doi: 10.1128/MCB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini MD, Wu M, Madhani HD. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell. 2003;112:725–736. doi: 10.1016/s0092-8674(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Ng HH, Feng Q, Wang H, Erdjument-Bromage H, Tempst P, Zhang Y, Struhl K. Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev. 2002;16:1518–1527. doi: 10.1101/gad.1001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M, Kamakaka RT. Barrier function at HMR. Mol Cell. 2005;19:707–716. doi: 10.1016/j.molcel.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Pryde FE, Louis EJ. Limitations of silencing at native yeast telomeres. EMBO J. 1999;18:2538–2550. doi: 10.1093/emboj/18.9.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld H, Aparicio OM, Zierath PD, Billington BL, Chhablani SK, Gottschling DE. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 1993;7:1133–1145. doi: 10.1101/gad.7.7a.1133. [DOI] [PubMed] [Google Scholar]
- Rivier DH, Ekena JL, Rine J. HMR-I is an origin of replication and a silencer in Saccharomyces cerevisiae. Genetics. 1999;151:521–529. doi: 10.1093/genetics/151.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner AD, Hall BE, Ellenberger T, Moazed D. A nonhistone protein-protein interaction required for assembly of the SIR complex and silent chromatin. Mol Cell Biol. 2005;25:4514–4528. doi: 10.1128/MCB.25.11.4514-4528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H, Bannister AJ, Dehe PM, Geli V, Kouzarides T. Methylation ofH3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J Biol Chem. 2004;279:47506–47512. doi: 10.1074/jbc.M407949200. [DOI] [PubMed] [Google Scholar]
- Suka N, Luo K, Grunstein M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet. 2002;32:378–383. doi: 10.1038/ng1017. [DOI] [PubMed] [Google Scholar]
- Valenzuela L, Dhillon N, Dubey RN, Gartenberg MR, Kamakaka RT. Long-range communication between the silencers of HMR. Mol Cell Biol. 2008;28:1924–1935. doi: 10.1128/MCB.01647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109:745–756. doi: 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- Venkatasubrahmanyam S, Hwang WW, Meneghini MD, Tong AH, Madhani HD. Genome-wide, as opposed to local, antisilencing is mediated redundantly by the euchromatic factors Set1 and H2A.Z. Proc Natl Acad Sci USA. 2007;104:16609–16614. doi: 10.1073/pnas.0700914104. [DOI] [PMC free article] [PubMed] [Google Scholar]