Abstract
Background
Patients with diabetes have increased risk of infections and wound complications after total knee arthroplasty (TKA). Glycemic markers identifying patients at risk for complications after TKA have not yet been elucidated.
Questions/purposes
We aimed to determine the correlations among four commonly used glycemic markers and to identify the glycemic markers most strongly associated with the occurrence of surgical site infections and postoperative wound complications in patients with diabetes mellitus after undergoing TKA.
Methods
Our retrospective study included 462 patients with diabetes, who underwent a total of 714 TKAs. Blood levels of glycemic markers, including preoperative fasting blood glucose (FBG), postprandial glucose (PPG2), glycated hemoglobin (HbA1c), and levels obtained from random glucose testing on postoperative days 2, 5, and 14, were collected on all patients as part of a medical clearance program and an established clinical pathway for patients with diabetes at our center. Complete followup was available on 93% (462 of 495) of the patients. Correlations among markers were assessed. Associations between the markers and patient development of complications were analyzed using multivariate regression analyses of relevant cutoff values. We considered any of the following as complications potentially related to diabetes, and these were considered study endpoints: surgical site infection (superficial and deep) and wound complications (drainage, hemarthrosis, skin necrosis, and dehiscence). During the period of study, there were no fixed criteria applied to what levels of glycemic control patients with diabetes needed to achieve before undergoing arthroplasty, and there were wide ranges in the levels of all glycemic markers; for example, whereas the mean HbA1c level was 7%, the range was 5% to 11.3%.
Results
There were positive correlations among the levels of the four glycemic markers; the strongest correlation was found between the preoperative HbA1c and PPG2 levels (R = 0.502, p < 0.001). After controlling for potential confounding variables using multivariate analysis, the HbA1c cutoff level of 8 (odds ratio [OR], 6.1; 95% confidence interval [CI], 1.6–23.4; p = 0.008) and FBG 200 mg/dL or higher (OR, 9.2; 95% CI, 2.2–38.2; p = 0.038) were associated with superficial surgical site infection after TKA.
Conclusions
In general, there is a positive correlation among the various available glycemic markers among patients with diabetes undergoing TKA, and patients undergoing surgery with HbA1c ≥ 8 and/or FBG ≥ 200 mg/dL were associated with superficial surgical site infection. These findings should be considered in patient selection and preoperative counseling for patients with diabetes undergoing TKA.
Level of Evidence
Level III, prognostic study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-4056-1) contains supplementary material, which is available to authorized users.
Introduction
Patients undergoing TKA often have systemic medical comorbidities. Diabetes mellitus (DM) is one of the most common and important diseases thought to be associated with an increased risk of perioperative complications among patients undergoing TKA. Previous studies have reported higher rates of infection [8, 18, 20, 21, 28], wound complications [5, 6], greater risk of deep vein thrombosis [27, 29], poorer outcomes [4, 13, 14, 22, 25], and higher mortality [2] in patients with DM undergoing TKA compared with patients without DM. Unfortunately, the prevalence of DM among patients undergoing TKA has been steadily increasing [11, 15] and orthopaedic surgeons will be faced with a growing number of surgical patients with DM. Achieving and maintaining adequate glycemic control are of paramount importance for patients with DM to reduce the risk of perioperative complications.
Various glycemic markers have been used to assess the efficacy of glycemic control in patients with DM undergoing surgery. The glycated hemoglobin (HbA1c) level has traditionally been used as the marker for perioperative glycemic control, but recent studies have called into question the adequacy of using only the HbA1c level as an index. A retrospective review of 4241 TKAs or THAs performed at a single center found that patient HbA1c levels were not reliable predictors of the risk of infection after total joint arthroplasty [7]. Furthermore, the conventional cutoff HbA1c level of less than or equal to 7 was reported to have poor predictive value for the development of postoperative wound complications and prosthetic joint infection (PJI) [1]. Other measures for assessment of patient glycemic control are preoperative fasting blood glucose (FBG) and postoperative random blood glucose levels. Patient blood glucose level fluctuates with diet and therefore represents acute glycemic control, whereas the HbA1c level generally indicates the degree of glycemic control over a 3-month period. Because the utility of HbA1c level and other glycemic markers and their appropriate cutoff values for predicting complications after TKA remain to a large degree controversial, glycemic markers that are associated with PJI and wound complications in patients with DM undergoing TKA still need to be identified.
We therefore sought to determine the correlations among four commonly used glycemic markers (preoperative FBG level, preoperative 2-hour postprandial glucose [PPG2] level, preoperative HbA1c level, and postoperative random glucose level) and to identify the glycemic markers that were most strongly associated with the occurrence of surgical site infections and postoperative wound complications in patients with DM after undergoing TKA.
Materials and Methods
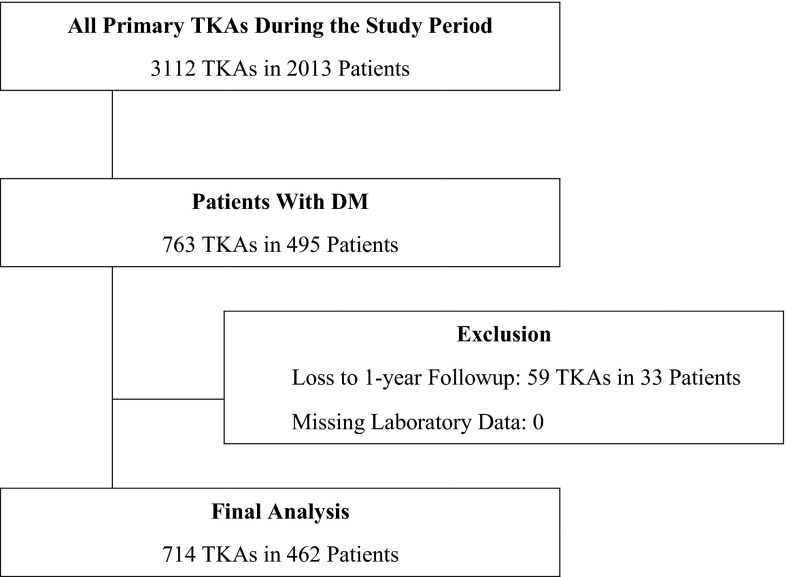
A review of hospital electronic medical records identified 3112 TKAs performed in 2013 patients at our hospital between July 2006 and June 2012. Of the 3112 TKAs, 763 procedures were completed in 495 patients with established diabetes or newly detected DM. Patients with established type 2 DM were identified by their medical history and current medications such as oral hypoglycemic agents or insulin. Patients with newly detected type 2 DM were identified during the routine medical examination performed before surgery and the DM diagnosis subsequently confirmed by endocrinologic consultation. Patients were excluded from our study if laboratory data were missing (zero patients) or the record showed less than 1 year of followup (59 TKAs in 33 patients). All patients were followed up by routine clinic visits, and those who could not visit the clinic were contacted by telephone survey. Complete followup, including data on all glycemic markers and complete chart notes at 1 year or greater, was available on 93% (462 of 495) of the patients. Consequently, 714 TKAs in 462 patients were finally included in our study (Fig. 1). Demographic information for study patients revealed mean patient age to be 70.6 ± 6.6 years (range, 49–89 years); 31 (7%) patients were men; and mean body mass index (BMI) was 27.3 ± 4.0 kg/m2 (range, 17.2–49.3 kg/m2).
Fig. 1.
A flowchart shows the patient selection process.
All surgeries were performed by the senior author (TKK). One gram of cefazolin was administered to the patient intravenously at the induction of anesthesia and then every 8 hours for 24 hours after surgery. A midline skin incision with a median parapatellar approach was used for all arthroplasties. Posterior-stabilized, cruciate-substituting implants were used for every patient, and all the components were cemented. A subcutaneous indwelling drainage catheter was routinely used. A layered closure was performed using No. 1-0 polyglactin 910 absorbable sutures in an interrupted manner for a watertight, capsular closure, followed by No. 2-0 polyglactin 910 absorbable sutures for the subcutaneous layer. Subcuticular skin closure was performed using 3-0 absorbable sutures. Skin sutures were removed 2 weeks after surgery.
The collected independent variables for our study were patient levels of glycemic markers during the perioperative period. Preoperative glycemic status was assessed using information from the electronic medical records on levels of FBG, PPG2, and HbA1c, measured within 4 weeks before surgery during routine preoperative medical examination. Postoperative glycemic status was assessed using random blood glucose levels measured on postoperative days 2, 5, and 14 after surgery. All assays for glycemic markers were performed at the central laboratory of our hospital and were collected on all patients as part of a medical clearance program and an established clinical pathway for patients with diabetes at our center.
The primary outcome variables included surgical site infection (superficial infection and deep infection) and wound complications (drainage, hemarthrosis, skin necrosis, and dehiscence), which were investigated in the electronic medical records through the last followup. Superficial infection was defined as an infection that was managed by antibiotics only or minor débridement. Two typical clinical conditions diagnosed as a superficial infection were cellulitis and infection confined to skin and subcutaneous tissue. Deep infection was defined according to the criteria of the Musculoskeletal Infection Society [19] and was managed by open débridement with insert change or two-staged exchange revision TKA. A surgical site infection, either superficial or deep, occurring within 1 year after TKA was considered to be positive for infectious complication. Drainage that persisted more than 3 days and hemarthosis that required aspiration or surgical evacuation were noted. In addition, skin necrosis or wound dehiscence that required surgical intervention was noted.
During the period of study, there were no fixed criteria applied to what level of glycemic control patients with diabetes needed to achieve before undergoing arthroplasty. We generally tried to achieve better glycemic control if a patient with diabetes had a HbA1c over 8 in preoperative evaluation, but surgery was not delayed in patients who did not respond to these efforts. There were wide ranges in the levels of all four glycemic markers (Table 1). The mean preoperative levels of FBG and PPG2 were 122 mg/dL (range, 36–398 mg/dL) and 210 mg/dL (range, 69–458 mg/dL), respectively. The mean preoperative level of HbA1c was 7 (range, 5.0–11.3). The proportion of patients with an FBG level greater than or equal to 140, 160, 180, and 200 mg/dL was 24%, 14%, 9%, and 5%, respectively. The proportions of PPG2 greater than or equal to 200, 220, 240, and 260 mg/dL were 54%, 43%, 30%, and 21%, respectively. The proportions of patients with an HbA1c level greater than or equal to 7, 8, and 9 were 41%, 12%, and 4%, respectively. The mean random glucose levels on postoperative days 2, 5, and 14 were 145 mg/dL (range, 50–326 mg/dL), 131 mg/dL (range, 24–325 mg/dL), and 139 mg/dL (range, 30–384 mg/dL), respectively.
Table 1.
Distribution of levels of glycemic markers
| Patient blood levels | N = 714 procedures |
|---|---|
| Preoperative | |
| FBG (mg/dL) | 122.3 ± 38.0 (36–398) |
| [Q1, median, Q3] | [100, 116, 134] |
| PPG2 (mg/dL) | 210.0 ± 64.3 (69–458) |
| [Q1, median, Q3] | [165, 205, 250] |
| HbA1c (%) | 6.9 ± 0.9 (5.0–11.3) |
| [Q1, median, Q3] | [6.3, 6.8, 7.4] |
| Postoperative | |
| Random glucose (day 2) (mg/dL) | 145.1 ± 44.1 (50–326) |
| [Q1, median, Q3] | [115, 136, 168] |
| Random glucose (day 5) (mg/dL) | 131.4 ± 40.4 (24–325) |
| [Q1, median, Q3] | [104, 125, 151] |
| Random glucose (day 14) (mg/dL) | 138.5 ± 50.6 (30–384) |
| [Q1, median, Q3] | [104, 128, 161] |
Data are presented as means ± SDs with range in parentheses; FBG = fasting blood glucose; PPG2 = 2-hour postprandial glucose; HbA1c = glycated hemoglobin; Q1, Q3 = first and third quartile values.
Statistical analyses were performed using SPSS for Windows (Version 20.0; SPSS, Chicago, IL, USA), and a p value < 0.05 was considered significant. Correlations among the glycemic markers were determined using the Pearson correlation coefficient. To identify glycemic markers predictive for the occurrence of surgical site infection or wound complication after TKA, multivariate logistic regression analyses were performed with the forward conditional variable selection method, adjusted by age, gender, BMI, and transfusion. The levels of each glycemic marker were dichotomized using several cutoff values for analyses, and the glycemic markers with p values < 0.1 from the exploratory univariate analyses (Supplemental Table 1 [Supplemental materials are available with the online version of CORR®.]) were entered into multivariate regression models.
Results
There were positive correlations among the values of the four glycemic markers, and the strongest correlation was found between the preoperative HbA1c level and the PPG2 level (R = 0.502, p < 0.001; Table 2). Correlations between the preoperative glycemic markers (FBG, PPG2, and HbA1c levels) were stronger than those between the postoperative markers (random glucose levels 2, 5, and 14 days after surgery) and between the preoperative markers and postoperative markers.
Table 2.
Correlations between glycemic markers using Pearson correlation coefficient
| Marker | FBG level | PPG2 level | HbA1c level | Glu2D level | Glu5D level | Glu14D level |
|---|---|---|---|---|---|---|
| FBG level | 1 | |||||
| PPG2 level | 0.403† | 1 | ||||
| HbA1c level | 0.422† | 0.502† | 1 | |||
| Glu2D level | 0.165† | 0.266† | 0.242† | 1 | ||
| Glu5D level | 0.174† | 0.208† | 0.183† | 0.261† | 1 | |
| Glu14D level | 0.083* | 0.236† | 0.227† | 0.233† | 0.243† | 1 |
* p < 0.05; †p < 0.001; FBG = fasting blood glucose; PPG2 = 2-hour postprandial glucose; HbA1c = glycated hemoglobin; Glu2D, random blood glucose level on postoperative day 2; Glu5D = random blood glucose level on postoperative day 5; Glu14D = random blood glucose level on postoperative day 14.
HbA1c ≥ 8 and FBG ≥ 200 mg/dL were identified as the markers associated with superficial surgical site infection after TKA in patients with diabetes. There were 10 superficial infections (1.4%) noted and no deep periprosthetic infections. Wound complications occurred in 47 (6.6%) patients. In multivariate analysis to account for the potential confounding variables of age, gender, BMI, and transfusion, HbA1c ≥ 8 (odds ratio [OR], 6.14; 95% confidence interval [CI], 1.62–23.36; p = 0.008) and FBG ≥ 200 mg/dL (OR, 9.17; 95% CI, 2.20–38.21; p = 0.038) were associated with an increased likelihood of a superficial surgical infection compared with patients with glycemic levels of HbA1c < 8 or FBG < 200 mg/dL (Table 3). None of the glycemic markers was found to be associated with a risk of wound complications.
Table 3.
Risk of superficial infection per perioperative glycemic marker levels*
| Model | Variable† | Adjusted OR (95% CI)* | p value |
|---|---|---|---|
| 1 | HbA1c level ≥ 8 | 6.14 (1.62–23.36) | 0.008 |
| FBG level ≥ 160 mg/dL | – | – | |
| 2 | HbA1c level ≥ 8 | 6.14 (1.62–23.36) | 0.008 |
| FBG level ≥ 180 mg/dL | – | – | |
| 3 | HbA1c level ≥ 8 | – | – |
| FBG level ≥ 200 mg/dL | 9.17 (2.20–38.21) | 0.038 | |
| 4 | HbA1c level ≥ 9 | 15.62 (3.66–66.75) | < 0.001 |
| FBG level ≥ 160 mg/dL | – | – | |
| 5 | HbA1c level ≥ 9 | 15.62 (3.66–66.75) | < 0.001 |
| FBG level ≥ 180 mg/dL | – | – | |
| 6 | HbA1c level ≥ 9 | 15.62 (3.6–66.75) | < 0.001 |
| FBG level ≥ 200 mg/dL | – | – |
* Multivariate logistic regression analyses were performed with forward conditional variable selection method, adjusted by age, gender, body mass index, and transfusion. Cells with dashes indicate that the variable was included in the multivariate model but excluded after the multivariate analysis; †comparator groups for glycemic markers in all multivariate analyses are the opposite group defined by the cutoff value. For example, the comparator group for HbA1c level ≥ 8 is HbA1c level < 8; p values are significant; OR = odds ratio; CI = confidence interval; HbA1c = glycated hemoglobin; FBG = fasting blood glucose.
Discussion
Prior studies have suggested that patients with DM undergoing TKA have a higher likelihood of wound complications and surgical site infections than those without DM [5, 6, 8, 18, 20, 21, 28]. Hence, achieving optimal glycemic control in patients with DM is important. However, evidence for which glycemic markers are most predictive of complications is less clear [1, 6, 7, 9, 16, 21]. We wanted to determine the correlations among the four commonly used glycemic markers and to identify the glycemic markers that were most strongly associated with the occurrence of surgical site infections and postoperative wound complications in patients with DM after undergoing TKA.
Our study had limitations. First, we performed a retrospective review of data from our hospital database. A retrospective study may be subject to selection bias. However, selection bias was reduced by consecutive enrollment of the study patients. Second, in this study, most (93%) knees were female and only 7% were male, which might also raise the concern of selection bias. However, we included all eligible patients with diabetes who underwent TKA during the study period, and the gender composition of all patients in our TKA database is almost identical to the gender composition of the study cohort. The gender ratio of our cohort is also similar to that reported nationally in South Korea [10, 12]. Third, our study did not enroll a cohort of patients without DM for comparison as a control group. Fourth, we did not evaluate all possible patient-level predictor variables (such as smoking) or other laboratory data (such as nutritional status), which can be confounding factors for surgical site infections and wound complications in patients after TKA, although we did consider age, sex, BMI, and transfusion as possible confounders. Thus, despite a sample size large enough to identify a HbA1c level 8 or higher as a risk factor for surgical site infection after multivariate regression analysis, our study might still be underpowered to identify others risk factors if every possibility is considered. Fifth, our definitions for complications after TKA were predefined but may not have been exhaustive and the diagnosis of complications was based on clinical judgment. For example, the distinction between superficial infection and wound complications was made on clinical judgment. Sixth, none of the patients in our cohort developed PJI (deep surgical site infection) over a 1-year followup period; therefore, we could not determine if there was an association between glycemic markers and deep infection. Finally, we used knees as the unit of measurement for data analyses. In theory, the measurement unit should be patients, not knees, because two knees in the same patient with diabetes are not independent observations considering the metabolic status of the patient. However, we intentionally performed the knee-based analyses because the outcome variable of our study was superficial infection and wound complication, which was a local complication noted for each knee rather than a systemic complication. If a patient underwent bilateral TKA and had a local complication in a single knee (ie, the “patient” had a complication, whereas one operated knee did not have a complication), it would be hard to analyze with a “patient-based” statistical measure. Furthermore, despite this theoretical concern, our recent study investigating how to handle the bilaterality issue in evaluating TKA outcomes found that knee-based analyses would not skew significantly if the number of study patients is large enough [17]. Nonetheless, we reran data analyses using patients as the unit of measurement and found that our results did not change (Supplemental Table 2 [Supplemental materials are available with the online version of CORR®.]).
We found that all the glycemic markers we evaluated showed varying degrees of positive correlation with each other; however, preoperative glycemic markers levels had stronger positive correlations with each other than with postoperative glycemic markers and with postoperative glycemic markers to each other. These findings may possibly be related to the stress of surgery and altered ambulation levels and dietary intake after surgery, which may lead to fluctuating blood glucose levels. An important finding of our study is that the marker of chronic glycemic control, the HbA1c level, was positively correlated with the acute glycemic markers, FBG and PPG2 levels. Previous studies have found similar correlations between the levels of glycemic markers [23]; however, in our study, the strength of correlation between HbA1c level and FBG/PPG2 levels was only moderate (correlation coefficients: 0.422 for FBG and 0.502 for PPG2). This finding suggests that there may be considerable disparity between the quality of chronic and acute glycemic control and that chronic and acute glycemic markers should be considered separately when assessing adequacy of glycemic control and counseling patients. For example, a patient who has notably high preoperative FBG or PPG2 but normal (or at least lower than 8) HbA1c may be advised to make more rigorous efforts at glycemic control before surgery. Conversely, a patient who has HBA1c 8 or higher but nearly normal FBG and PPG2 might be advised to postpone the scheduled surgery and maintain the current efforts for glycemic control.
We found that HbA1c ≥ 8 or FBG ≥ 200 mg was associated with superficial surgical site infections and other glycemic markers investigated did not have associations with surgical site infection or wound complications. Our study is in concordance with several previous studies. Our finding of no association of HbA1c level of 7 as the cutoff value with infection or wound complication is in concordance with previous studies reporting no increased complications after TKA in patients with uncontrolled blood glucose levels when using an HbA1c level of 7 as the cutoff [1, 7]. A previous study found an association between HbA1c levels ≥ 8 and wound complications but no association with early postoperative deep infection in patients with DM after TKA [6]. On the other hand, our finding of the only association of preoperative FBG ≥ 200 mg/dL with superficial surgical site infection is in contrast with previous studies reporting the association of preoperative FBG level ≥ 126 mg/dL [9], postoperative FBG level ≥ 126 mg/dL [21], and postoperative FBG level ≥ 200 mg/dL [16] with the occurrence of complications after total joint arthroplasty. Our findings, taken together with previous studies, suggest that surgeons should think carefully before performing this elective procedure in a patient with HbA1C ≥ 8 or FBG ≥ 200 mg. Although no patients in our study developed deep infection, which prevents us from evaluating the association of poor glycemic control with deep infection, previous studies reported that patients who develop superficial surgical site infection are at greater risk for deep periprosthetic joint infection [3, 24, 26].
We found a positive correlation among the various available glycemic markers in patients undergoing TKA, and HbA1c ≥ 8 or FBG ≥ 200 mg/dL was associated with superficial surgical site infection. Our findings should be considered in patient selection and preoperative counseling for patients with DM who will undergo TKA. We propose that HbA1c ≥ 8 or FBG ≥ 200 mg/dL be used as cutoff values for glycemic control in recommending or timing TKA for patients with diabetes.
Electronic supplementary material
Acknowledgments
We thank Kyung-Un Park, MD and Sung-Hee Choi, MD for critical review of the manuscript.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
This work was performed at the Joint Reconstruction Center, Seoul National University Bundang Hospital, Seoul, Republic of Korea.
References
- 1.Adams AL, Paxton EW, Wang JQ, Johnson ES, Bayliss EA, Ferrara A, Nakasato C, Bini SA, Namba RS. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am. 2013;95:481–487. doi: 10.2106/JBJS.L.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmont PJ, Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96:20–26. doi: 10.2106/JBJS.M.00018. [DOI] [PubMed] [Google Scholar]
- 3.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 4.Bolognesi MP, Marchant MH, Jr, Viens NA, Cook C, Pietrobon R, Vail TP. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplasty. 2008;23:92–98. doi: 10.1016/j.arth.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 5.England SP, Stern SH, Insall JN, Windsor RE. Total knee arthroplasty in diabetes mellitus. Clin Orthop Relat Res. 1990;260:130–134. [PubMed] [Google Scholar]
- 6.Han H-S, Kang S-B. Relations between long-term glycemic control and postoperative wound and infectious complications after total knee arthroplasty in type 2 diabetics. Clin Orthop Surg. 2013;5:118–123. doi: 10.4055/cios.2013.5.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(726–729):e721. doi: 10.1016/j.arth.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94:e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- 9.Jämsen E, Nevalainen P, Kalliovalkama J, Moilanen T. Preoperative hyperglycemia predicts infected total knee replacement. Eur J Intern Med. 2010;21:196–201. doi: 10.1016/j.ejim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Kim HA, Kim S, Seo YI, Choi HJ, Seong SC, Song YW, Hunter D, Zhang Y. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology (Oxford). 2008;47:88–91. doi: 10.1093/rheumatology/kem308. [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Lee JS, Lee J, Na JK, Han JH, Yoon DK, Baik SH, Choi DS, Choi KM. Prevalence of diabetes and impaired fasting glucose in Korea: Korean National Health and Nutrition Survey 2001. Diabetes Care. 2006;29:226–231. doi: 10.2337/diacare.29.02.06.dc05-0481. [DOI] [PubMed] [Google Scholar]
- 12.Koh IJ, Kim TK, Chang CB, Cho HJ, In Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin Orthop Relat Res. 2013;471:1441–1450. doi: 10.1007/s11999-012-2622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Huedo MA, Villanueva M, de Andres AL, Hernandez-Barrera V, Carrasco-Garrido P, Gil A, Martinez D, Jimenez-Garcia R. Trends 2001 to 2008 in incidence and immediate postoperative outcomes for major joint replacement among Spanish adults suffering diabetes. Eur J Orthop Surg Traumatol. 2013;23:53–59. doi: 10.1007/s00590-011-0915-6. [DOI] [PubMed] [Google Scholar]
- 14.Meding JB, Reddleman K, Keating ME, Klay A, Ritter MA, Faris PM, Berend ME. Total knee replacement in patients with diabetes mellitus. Clin Orthop Relat Res. 2003;416:208–216. doi: 10.1097/01.blo.0000093002.90435.56. [DOI] [PubMed] [Google Scholar]
- 15.Memtsoudis SG, Della Valle AG, Besculides MC, Gaber L, Laskin R. Trends in demographics, comorbidity profiles, in-hospital complications and mortality associated with primary knee arthroplasty. J Arthroplasty. 2009;24:518–527. doi: 10.1016/j.arth.2008.01.307. [DOI] [PubMed] [Google Scholar]
- 16.Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5:412–418. doi: 10.1177/193229681100500231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Na YG, Kang YG, Chang MJ, Chang CB, Kim TK. Must bilaterality be considered in statistical analyses of total knee arthroplasty? Clin Orthop Relat Res. 2013;471:1970–1981. doi: 10.1007/s11999-013-2810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95:775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Zmistowski B, Berbari E, Bauer T, Springer B. Della Valle C, Garvin K, Mont M, Wongworawat M, Zalavras C. New definition for periprosthetic joint infection: from the workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pruzansky JS, Bronson MJ, Grelsamer RP, Strauss E, Moucha CS. Prevalence of modifiable surgical site infection risk factors in hip and knee joint arthroplasty patients at an urban academic hospital. J Arthroplasty. 2014;29:272–276. doi: 10.1016/j.arth.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Reategui D, Sanchez-Etayo G, Nunez E, Tio M, Popescu D, Nunez M, Lozano L. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014 Feb 15 [Epub ahead of print]. [DOI] [PubMed]
- 22.Robertson F, Geddes J, Ridley D, McLeod G, Cheng K. Patients with Type 2 diabetes mellitus have a worse functional outcome post knee arthroplasty: a matched cohort study. Knee. 2012;19:286–289. doi: 10.1016/j.knee.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 24.Saleh K, Olson M, Resig S, Bershadsky B, Kuskowski M, Gioe T, Robinson H, Schmidt R, McElfresh E. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20:506–515. doi: 10.1016/S0736-0266(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 25.Singh JA, Lewallen DG. Diabetes: a risk factor for poor functional outcome after total knee arthroplasty. PLoS One. 2013;8:e78991. doi: 10.1371/journal.pone.0078991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surin VV, Sundholm K, Backman L. Infection after total hip replacement. With special reference to a discharge from the wound. J Bone Joint Surg Br. 1983;65:412–418. doi: 10.1302/0301-620X.65B4.6874711. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Zhao Y. Diabetes mellitus and the incidence of deep vein thrombosis after total knee arthroplasty: a retrospective study. J Arthroplasty. 2013;28:595–597. doi: 10.1016/j.arth.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Yang K, Yeo SJ, Lee BP, Lo NN. Total knee arthroplasty in diabetic patients: a study of 109 consecutive cases. J Arthroplasty. 2001;16:102–106. doi: 10.1054/arth.2001.19159. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z, Wang S, Ma W, Kong G, Zhang S, Tang Y, Zhao Y. Diabetes mellitus increases the incidence of deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg. 2014;134:79–83. doi: 10.1007/s00402-013-1894-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.