Abstract
Background
Total joint arthroplasty (TJA), although considered to be highly beneficial, is associated with substantial costs to the US healthcare system. Cost utility analysis has become an increasingly important means to objectively evaluate the value of a healthcare intervention from the perspective of both extending the quantity and improving the quality of life. Relatively little is known about the overall cost utility analysis evidence base in TJA.
Questions/purposes
The goals of this review were to (1) determine the cost utility of TJA interventions; (2) critically assess the quality of published US-based cost utility analyses using the Quality of Health Economic Studies instrument; and (3) determine what characteristics were common among studies receiving a high quality score.
Methods
A systematic review of the literature using the MEDLINE database was performed to compile findings and critically appraise US-based cost utility analysis studies for total hip and knee arthroplasty. Based on review of 676 identified articles, 23 studies were included. We used the Quality of Health Economic Studies instrument to assess study quality and one-sided Fisher’s exact tests were applied to analyze the predictors of high-quality cost utility analysis.
Results
Very few studies compare the cost utility of TJA versus nonoperative intervention; however, the available evidence suggests that TJA can be cost-saving and is highly cost-effective compared with conservative management of end-stage arthritis. The majority of identified studies are focused on the cost utility of new implant technologies or comparisons among surgical alternatives. These studies suggest that the upfront costs associated with new technologies are cost-effective when there is a major reduction in a future cost. The quality of identified studies is quite high (Quality of Health Economic Studies Instrument score: mean 86.5; range, 63–100). National funding source (p = 0.095) and lifetime horizon for analysis (p = 0.07) correlate with high-quality evidence but do not reach significance.
Conclusions
Over the past 15 years, there has been a major increase in the volume of cost utility analyses published in total hip and knee arthroplasty. The quality of cost utility analyses published during that period is good. As increasing attention is paid to value in US health care, more attention should be paid to understanding the cost utility of TJA compared with nonoperative treatment modalities. Future studies may also look to incorporate patient willingness to pay.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3964-4) contains supplementary material, which is available to authorized users.
Introduction
Lower extremity arthroplasties for management of arthritis of the hip and knee have been highly successful [18]. Patients experience major improvements in function as well as relief of pain after total joint arthroplasty (TJA). As the number of elderly patients in the US population continues to increase and younger patients with degenerative joint disease expect to live more active lives, the demand for TJA is projected to continue to show substantial growth [16, 24]. TJA, although considered to be beneficial, is associated with substantial costs to the healthcare system and utilization rates have exhibited substantial regional variation [19]. Understanding the cost of care and associated value has become a focal point of healthcare policy in the United States [14, 26, 39, 45]. Some healthcare analysts have proposed that one of the reasons that the United States outpaces other industrialized nations in healthcare expenditures is a lack of attention to healthcare economic decision analysis and cost-effectiveness analysis (CEA) [34, 50]. As part of the Patient Protection and Affordable Care Act, Medicare is prohibited from considering cost when making coverage decisions; however, going forward, some economists have suggested that CEA methodology may be appropriate for resource allocation [21, 35].
CEA is a useful tool for assessing the value of an intervention by identifying the procedures that provide the greatest improvement in outcome at the lowest cost [48]. (A brief primer on CEA is provided in the Materials and Methods.) Cost utility analysis is a form of CEA that seeks to evaluate the economics of a healthcare intervention from the perspective of both extending the quantity and quality of life. As part of cost utility analysis, the benefit of an intervention is expressed as a utility, which can then be translated into a quality-adjusted life-year (QALY). In 1996, the US Panel on Cost-Effectiveness in Health and Medicine published consensus-based recommendations for performing CEA and one of the major recommendations was the use of a patient-based utility measure [64]. Patient-derived utility measures of health allow for comparison of the value of an intervention both within a specialty and across heterogeneous fields within medicine. As such, cost utility analysis is the preferred modality for reporting of medical decision analysis [48, 52].
A previous review of English language cost utility analyses in orthopaedics identified 11 cost utility analysis studies in TJA between 1975 and 2001 [9]. The study did not, however, delineate the proportion of these studies performed in the United States. A 2009 cost utility analysis in TKA suggested that the majority of prior economic analyses for TKA had been focused on non-US populations [31]. To the authors’ knowledge there is no prior systematic review of the TJA cost utility analysis literature that had a specific emphasis on the US population. There are several reasons to conduct a focused review of US-based cost utility analyses. First, the recommendations provided by governing bodies for CEA internationally compared with the United States have subtle but important differences that affect the methodological assessment of international versus US studies (for example, the British NHS encourages a payer perspective for CEA in large part to benefit the government in making funding decisions, whereas the US Panel on Cost-Effectiveness in Health and Medicine prefers the use of a societal perspective); second, health state utility preferences can change based on the population under study; thus, non-US health state preference may not reflect the preference of the US population. Finally, in European countries where CEA is at times governmentally sponsored, there may be an economic or cost bias toward reporting favorable outcome for less expensive nonoperative strategies [62].
The goals of this review were to (1) determine the cost utility of TJA interventions; (2) critically assess the quality of published US-based cost utility analyses using the Quality of Health Economic Studies instrument; and (3) determine what characteristics were common among studies receiving a high quality score.
Materials and Methods
Background on Cost Analyses
In this article we use the general term cost-effectiveness analysis to refer to the four cost analytic methods: CEA, cost utility analysis, cost identification (minimization) analysis, and cost-benefit analysis. We provide a brief primer on cost-effectiveness analysis methodology. A thorough review of value measurement and cost analyses and their application within orthopaedics has been previously published and is briefly summarized here [7, 38].
CEA refers to the cost per health unit gain. This form of analysis requires that cost is assessed against an objective health outcome such as infection or mortality. An incremental cost-effectiveness ratio can be calculated by measuring the incremental costs and incremental health benefit for an intervention compared with an alternative: (cost of intervention − cost of alternative)/(benefit of intervention − benefit of alternative). Cost utility analysis is closely related to CEA but unlike CEA, the health outcome measured is a patient-centric, subjective utility measure of health, most commonly, QALY. Similar to incremental cost-effectiveness ratio, an incremental cost utility ratio can be calculated using patient utilities. Note that because CEA and cost utility analysis have similarities, they are often jointly referred to as CEA and incremental ratios of cost and effectiveness in cost utility analysis are commonly referred to as incremental cost-effectiveness ratio.
There is debate in the literature with regard to what USD/QALY a procedure is considered cost-effective. USD 50,000/QALY gained has been the traditionally accepted threshold for cost-effectiveness. Some observers posit however that because the United States has greater economic output, USD 100,000/QALY and even USD 150,000/QALY gained is a more appropriate threshold by which to define cost-effectiveness and societal willingness to pay [11, 22, 33].
Unlike CEA and cost utility analysis, cost-benefit analysis manipulates purely financial inputs and outcomes to compare the expected monetary cost and benefit of a procedure. As part of this method, the cost of a particular intervention is often tabulated and healthcare consumers are queried to understand how much they are willing to pay for the intervention or to achieve a certain health state. Cost identification (minimization) analysis is another form of cost-effectiveness analysis that identifies the costs associated with certain interventions with the presumed goal being able to choose the least expensive option. This form of analysis, although deceptively simple to conduct, is theoretically difficult to apply because valid comparisons rely on comparator interventions having equal outcomes (ie, similar rates of fracture healing for two different methods of fixation).
Search Strategy and Criteria
The review of the literature was performed using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines with a PRISMA checklist [32]. US-based cost utility analyses published over a 15-year period between January 1999 and January 2014 were identified from the MEDLINE database using the PubMed interface. These studies were retained for further review if they satisfied the following a priori inclusion criteria: (1) index procedure(s) including THA or TKA; (2) US-based study; (3) clinical-based; and (4) performed cost utility analysis.
A starting point of 1999 was chosen to provide minimal overlap with the previous literature review on cost utility analysis in orthopaedics (studies published between 1975 and 2001) and also to allow adequate time for the recommendations from the Panel on Cost-Effectiveness in Health and Medicine to influence published research (The Panel’s recommendations were published in October of 1996.). Based on these criteria, studies were excluded if they were not relevant to TJA or if TJA was not the primary intervention under investigation, if the study was nonclinical, ie, reviews or editorials, and if the study was a non-US study.
An a priori search algorithm using PubMed Medical Subject Headings (MeSH) terms was constructed. The search function consisted of two simple search terms: “arthroplasty” and “cost”. The term “AND” was used between these two terms as a Boolean operator. The MeSH term for “arthroplasty” was inclusive of all studies reporting on any replacement procedure and thus it was unnecessary to further refine the search term by using anatomic or joint descriptors. Similarly, the MeSH term “cost” captures cost analyses and all economic terms referring to cost. An updated search was completed on December 31, 2013.
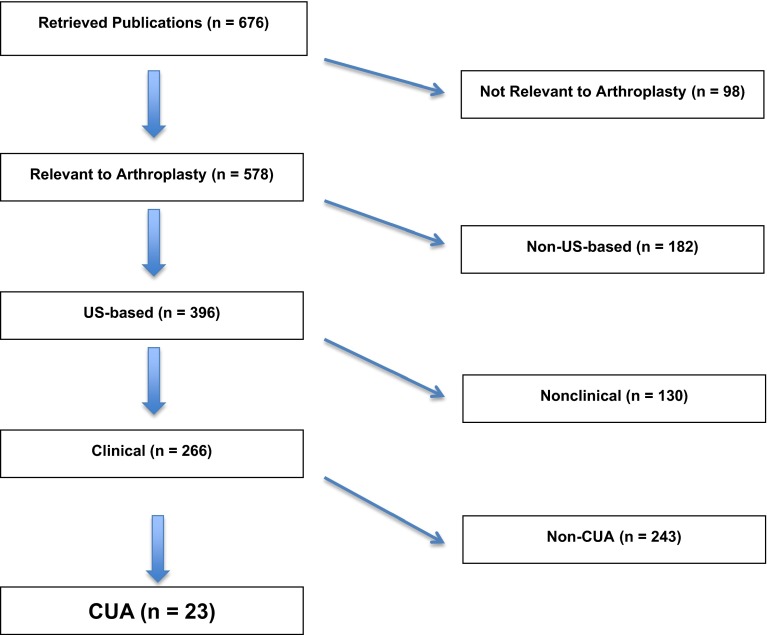
The search strategy identified 676 studies. Studies were included according to our inclusion criteria based on a review of the study titles and abstracts. In studies for which there was ambiguity regarding inclusionary status, the article was retrieved for further review. Based on review of these 676 articles, 23 studies suitable for inclusion were identified (Fig. 1). Of the 676 retrieved studies, 578 (85.5%) were relevant to the field of joint arthroplasty. Of these studies relevant to arthroplasty, 396 (68.5%) studies were US based. Two hundred sixty-six of the 396 (67%) arthroplasty-relevant studies were clinical in nature and thus eligible for more detailed review. Of these 266 studies, 23 used cost utility analysis in evaluation of THA or TKA and were thus included [2, 3, 5, 6, 12, 13, 20, 27, 28, 30, 31, 37, 40, 47, 49, 51, 54–57, 59, 61, 63].
Fig. 1.
Flowchart illustration shows study inclusion/exclusion criteria applied to studies retrieved from the MEDLINE database. CUA = cost utility analysis.
To further ensure that all appropriate studies were identified, the PubMed “related citations” search function was used for each of the identified studies and all abstracts associated with the identified studies were reviewed. The “related citations” feature is a calculated set of PubMed citations closely related to the selected article(s) retrieved using a word weight algorithm. Related articles are displayed in ranked order from most to least relevant with the “linked from” citation displayed first. This feature, although a crude search based on word association with the study title and abstract, has been shown to be useful in exhaustively reviewing the evidence base [29]. No additional studies were identified by this method. The reference list of each identified study was also reviewed for suitable studies. No additional articles met inclusion criteria by this method.
A recent study on the indexing of primary studies in orthopaedic surgery found that with appropriate search strategies, MEDLINE achieved a 90% recall rate; MEDLINE and EMBASE 91%; and MEDLINE, EMBASE, and COCHRANE 97% [53]. As such, we restricted our search to the MEDLINE database; for the purposes of our review and based on our inclusion criteria, a supplemental database search would have been unlikely to provide additional studies.
Quality Scoring
The literature was reviewed to better understand methods applied in orthopaedics for grading the quality of economic studies. We found that prior studies evaluating economic analyses in orthopaedic surgery often used subjective author-derived evaluations and qualitative methods of assessment [7–9, 15, 17, 25].
In other fields of health care, the Quality of Health Economic Studies instrument has been applied for use in assessing the quality of reporting for economic studies. The Quality of Health Economic Studies instrument is a validated questionnaire used to evaluate the quality of economic studies [10]. The Quality of Health Economic Studies instrument consists of 16 criteria framed as “yes” or “no” questions. Each question is weighted with point values ranging from 1 to 9. Responses are binary and questions answered “yes” receive the full point value, whereas questions answered “no” receive zero points. The questionnaire was derived by a panel of eight experts in health economics and the point values were derived using a random-effects general least-squares regression based on a conjoint analysis of survey results from 120 international health economists [41]. Cumulative scores for the Quality of Health Economic Studies instrument range from 0 to 100. Definitions of high-quality evidence using the Quality of Health Economic Studies instrument have varied in the literature and one early publication used a threshold of > 75 as high quality [60]. Since the introduction of the Quality of Health Economic Studies instrument, the reported mean and median scores when evaluating the quality of studies in various fields have been reported to be in the 70 to 90 range with high-quality studies scoring over 80 [23, 46, 58]. Thus, in this study, we defined a Quality of Health Economic Studies score > 85 as high quality.
Characteristics Associated With High-quality Evidence
Analysis was performed to determine predictors of scoring 85 or higher on the Quality of Health Economic Studies. Previous work has identified predictors of publication, high citation, and level of evidence in orthopaedic surgery [36, 42–44]. One prior nonorthopaedic study identified predictors of high-quality CEA using the Quality of Health Economic Studies instrument [60]. Based on these previously identified predictors, we abstracted data on certain scientific and nonscientific factors: utilization of outcome/health state data from a patient sample, analysis perspective, time horizon of analysis (ie, lifetime versus other: lifetime horizon for analysis most often allows an analysis to capture all relevant outcomes and costs), mode of economic analysis, conflict of interest statement, perceived conflict of interest, funding source, and authorship with two or more authors with advanced degrees beyond MD.
Statistical Methods
Identified studies were retrieved and independently reviewed and scored for quality by two of the authors (BUN, JLB). Both reviewers then convened to further review areas of scoring disagreement and arrive at a consensus score with preference for more conservative assessment. To determine the factors associated with high-quality evidence (Quality of Health Economic Studies ≥ 85), one-sided Fisher’s exact testing was performed to obtain a univariate odds ratio. A p value < 0.05 was considered significant. Statistical analysis was performed using Stata (Version 12.1; Statacorp; College Station, TX, USA).
Results
This review includes 23 identified studies (Table 1). A brief overview of the major findings is presented (Table 1).
Table 1.
Identified studies and major findings
| Study | Journal | Study design | Area of analysis | Perspective* | Time horizon | Major findings† |
|---|---|---|---|---|---|---|
| Odum et al. [40] (2013) | J Bone Joint Surg Am | Markov model | Knee: simultaneous versus staged bilateral TKA | Payer | 15 years | USD 590,474/QALY gained when staged bilateral TKA was chosen over simultaneous TKA in an unmatched sample; simultaneous TKA dominant over bilateral TKA in matched sample |
| Ruiz et al. [47] (2013) | J Bone Joint Surg Am | Markov model | Knee: TKA versus nonoperative | Societal | Lifetime | USD 18,930 age-weighted mean societal savings with a mean QALY gain of 2.4 when TKA is chosen over nonoperative intervention |
| Schousboe and Brown [49] (2013) | J Bone Joint Surg Am | Markov | Hip and knee: LMWH versus aspirin in THA and TKA | Payer | Lifetime | For THA, aspirin is more cost-effective than LMWH and in patients 80 years and older, aspirin is both less costly and more effective; for TKA, use of LMWH over ASA crosses above the USD 100,000/QALY gained threshold in patients 70 years and older |
| Suter et al. [61] (2013) | PLoS One | Computer simulation model | Knee: standard TKA versus innovative TKA implants | Societal | 20 years | USD < 100,000/QALY gained across all ages and comorbidities when new implants result in ≥ 50% decrease in TKA failure at ≤ 50% cost increase |
| Courville et al. [12] (2012) | Infect Control Hosp Epidemiol | Decision tree model | Hip and knee: Preoperative nasal mupirocin for TKA and THA | Societal | 1 year | Empirical treatment with nasal mupirocin preoperative to THA/TKA is dominant to “screen and treat” and “no treatment”; however, differences among all 3 for cost and QALY is modest |
| Lawless et al. [28] (2012) | CORR® | Retrospective review | Hip: THA | Healthcare system | 10 years | USD 9773/QALY gained for THA on average; no major difference in utility scores based on age or bilateral hip disease |
| Lin et al. [30] (2012) | CORR® | Retrospective review | Various: histological specimens | Payer | Lifetime (presumed) | USD 102.37 per histologic specimen collected during TJA without any observed gain in QALY |
| Slover et al. [56] (2012) | J Arthroplasty | Markov model | Knee: TKA custom cutting blocks | Societal | 20 years | Use of TKA custom cutting blocks is not cost-effective unless there are significant reductions in revision rates |
| Lavernia et al. [27] (2011) | J Arthroplasty | Longitudinal cohort | Hip: primary and revision THA | Healthcare system | 1 year | USD 5572/QWY for primary THA; USD 10, 775/QWY for revision THA |
| Watters et al. [63] (2011) | J Surg Orthop Adv | Markov model | Hip: THA versus FVFG for femoral head osteonecrosis | Healthcare system | Lifetime | USD 1026/QALY for THA versus USD 752/QALY for FVFG; FVFG is both less expensive and more effective than THA |
| Bozic et al. [6] (2010) | CORR® | Markov model | Hip: MoM HRA versus THA | Healthcare system | 30 years | ICER when MoM HRA is chosen over THA ranges from USD 28,614/QALY (men aged 55–65 years) to USD 2,483,435/QALY (women aged 65–74 years) |
| Cummins et al. [13] (2009) | J Bone Joint Surg Am | Markov model | Hip: ABC for THA | Healthcare system | Lifetime | When revision of any kind considered as a primary outcome, ABC is dominant over nonantibiotic cement; ICER of USD 37,355/QALY gained with ABC if only revision for infection considered as outcome |
| Losina et al. [31] (2009) | JAMA Intern Med | Markov model | Knee: TKA versus nonoperative | Societal | Lifetime | USD 18,300/QALY gained when TKA is chosen over nonoperative and USD 28,100/QALY gained in high-risk patients; TKA performed in low-volume centers as opposed to high volume is both more costly and less effective |
| Slover et al. [55] (2009) | J Arthroplasty | Markov model | Hip: THA versus HHA | Payer | 20 years | USD 1960/QALY gained when THA is chosen over HHA for displaced femoral neck fractures |
| Ballal et al. [2] (2008) | Curr Med Res Opin | Markov model | Knee: rFVII for TKA in hemophilia with high inhibitor titer | Payer | Lifetime | USD 50,000/QALY threshold reached if decreased bleeding events sustained beyond 10 years after original TKA with perioperative rFVII |
| Slover et al. [57] (2008) | J Bone Joint Surg Am | Markov model | Knee: computer-navigated TKA | Healthcare system | 20 years | USD 50,000/QALY threshold is maintained when centers at which 250, 150, and 25 navigated TKAs are performed annually reduce revision rate by 2%, 2.5%, and 13%, respectively |
| Sharifi et al. [51] (2008) | J Bone Joint Surg Am | Decision tree model | Hip: PAO versus THA for DDH | Societal | 30 years | For Tönnis Grade 1 coxarthrosis, PAO is dominant, whereas for Tönnis Grade 3 coxarthrosis, THA is dominant; for Tönnis Grade 2, PAO is associated with an ICER of USD 824/QALY |
| Novak et al. [37] (2007) | J Bone Joint Surg Am | Decision tree model | Knee: computer-navigated TKA | Healthcare system | 15 years | USD 45,554/QALY gained when navigated TKA is chosen over TKA with mechanical alignment guide |
| Bozic et al. [5] (2006) | J Bone Joint Surg Am | Markov model | Hip: UHMWPE versus alternate bearing surface in THA | Healthcare system | Lifetime | USD 50,000/QALY threshold is maintained when implants with incremental cost of USD 2000 result in at least 18.7% reduction in 20-year implant failure risk |
| Slover et al. [54] (2006) | J Bone Joint Surg Am | Markov model | Knee: UKA versus TKA | Payer | Lifetime | In low-demand 78-year-old patients, UKA cost USD 200 less for an additional 0.05 QALY and therefore is dominant over TKA |
| SooHoo et al. [59] (2006) | J Bone Joint Surg Am | Decision tree model | Knee: UKA versus TKA | Payer | Lifetime | USD 277/QALY gained when UKA is chosen over TKA |
| Botteman et al. [3] (2002) | Clin Ther | Decision tree model | Hip: LMWH versus Coumadin after THA | Payer | Lifetime | In the short term, USD 3733/QALY gained when LMWH is chosen over Coumadin; in the long term LMWH associated with net cost savings and QALY gains |
| Fisman et al. [20] (2001) | Clin Infect Dis | Markov model | Hip: two-stage exchange THA versus débridement | Societal | Lifetime | USD 500/QALY and USD 8200/QALY gained when débridement and retention is chosen over exchange arthroplasty in frail elderly men and women, respectively; exchange arthroplasty was the preferred strategy in 65-year-old patients, however |
* Perspective either as stated by authors or inferred from cost inputs; †base case when applicable; LMWH = low-molecular-weight heparin; FVFG = free vascularized fibular graft; MoM HRA = metal-on-metal hip surfacing arthroplasty; ABC = antibiotic-impregnated bone cement; HHA = hip hemiarthroplasty; rFVII = recombinant factor VII; PAO = periacetabular osteotomy; DDH = developmental dysplasia of the hip; UHMWPE = ultrahigh-molecular-weight polyethylene; UKA = unilateral knee arthroplasty; QALY = quality-adjusted life-year; ASA = aspirin; TJA = total joint arthroplasty; QWY = quality well year; ICER = incremental cost-effectiveness ratio.
Cost Utility of TJA
The majority of identified studies are focused on the economic impact of new implant technologies or comparisons among surgical alternatives. Very few studies compare the cost utility of joint arthroplasty when chosen over nonoperative intervention in degenerative joint disease. Losina et al. [31] and Ruiz et al. [47] quantify the cost utility of TKA versus nonoperative intervention for the knee. Losina et al. [31] assessed TKA versus nonoperative management and also evaluated the influence of patient risk and hospital volume on cost-effectiveness. The authors found that compared with a nonoperative strategy, TKA in the base case was associated with an incremental cost-effectiveness ratio of USD 18,300 per QALY. For low-risk patients and high-risk patients, the incremental cost-effectiveness ratio was USD 9700/QALY and USD 28,000/QALY, respectively. At all risk levels, performing TKA in a high-volume center was dominant to TKA performed in low-volume centers. Ruiz et al. [47] analyzed the direct and indirect costs associated with TKA versus nonoperative management and found that TKA resulted in an age-weighted mean societal cost savings of USD 18,930 with a concomitant age-weighted QALY gain of 2.4. No long-term economic utility model of arthroplasty versus nonoperative intervention was found for THA. One study however assessed the utility of THA at 1-year followup in a cohort of patients receiving THA [27]. The authors reported that from a healthcare system perspective, primary THA was associated with USD 5572 per quality of well year, whereas revision procedures were associated with USD 10,775 per quality of well year.
Quality of Identified Studies
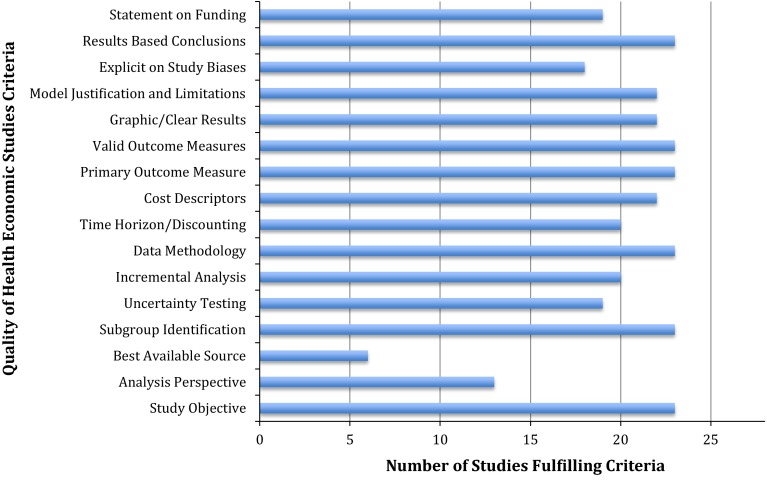
The quality of cost utility analysis in TJA is good. The mean Quality of Health Economic Studies score for all identified studies was in the good range: 86.4 (range, 63–100). The mean Quality of Health Economic Studies score for TKA studies was 89.9 and 85.0 for THA studies. The Quality of Health Economic Studies instrument and scoring methodology is included in Appendix 1 (Supplemental materials are available with the online version of CORR®.) to help readers independently judge the quality of individual studies. Certain Quality of Health Economic Studies criteria were frequently missed and contributed to lower quality scores in the 23 identified studies: statement of analysis perspective, use of best available evidence, uncertainty testing, explicit statement on study biases, and statement of funding sources (Fig. 2).
Fig. 2.
Overview is shown of Quality of Health Economic Studies criteria and the number of studies meeting criteria.
Factors Associated With Quality of Studies
We defined high quality as having a Quality of Health Economic Studies score > 85. There were no factors identified as significant predictors of a high-quality cost utility analysis. National funding sources (National Institutes of Health, American Academy of Orthopaedic Surgeons, and Orthopaedic Research Education Foundation) and a lifetime perspective for analysis approached significance (p = 0.10 and 0.07, respectively) but were not predictive of high quality with the numbers available. Patient-level data, type of economic analysis, conflict of interest statement, perceived conflict of interest, and authorship with two or more authors with advanced degrees beyond MD degree were similarly nonsignificant predictors of quality (Table 2).
Table 2.
Factors associated with Quality of Health Economic Studies Instrument score > 85
| Study characteristic | Inclusion rate | p value (one-sided) |
|---|---|---|
| Patient-level data | ||
| Yes | 26% | 0.28 |
| No | Reference | |
| Advanced degree authors | ||
| > 2 | 55% | 0.58 |
| ≤ 2 | Reference | |
| Perceived conflict of interest* | ||
| Yes | 41% | 0.56 |
| No | Reference | |
| Funding source | ||
| National | 48% | 0.095 |
| Institutional | 9% | 0.46 |
| Industry | 4% | 0.74 |
| Other | 17% | – |
| None | 22% | Reference |
| Economic analysis | ||
| Markov model | 61% | 0.16 |
| Other | 39% | Reference |
| Time horizon | ||
| Lifetime | 52% | 0.07 |
| Nonlifetime | 48% | Reference |
| Perspective | ||
| Societal | 35% | 0.67 |
| Payer | 35% | 0.67 |
| Healthcare system | 30% | Reference |
* Among those with a conflict of interest statement.
Discussion
Increased attention is being paid in the United States to understanding the value of healthcare interventions. Lower extremity arthroplasty has traditionally been a focal point for value measurement in orthopaedic surgery and as such there has been increased reporting of cost utility studies for TJA. The clinical effectiveness of TJA is well accepted; however, the cost utility of these procedures compared with conservative care is not as well established. Furthermore, relatively little is known about the overall quality and predictors of quality for cost utility studies related to joint arthroplasty. Based on the available evidence, TKA and THA both appear to be cost-effective interventions compared with conservative care; however, there is stronger evidence supporting the cost-effectiveness of TKA. The quality of available evidence is good as assessed by the Quality of Health Economic Studies instrument. Based on the included studies, there were no significant predictors of a high-quality study.
This review has a number of limitations. Like with any systematic review using keyword searches, it is possible that relevant studies were missed in the search strategy and were therefore not included. Although the Quality of Health Economic Studies instrument has substantial potential as a tool for evaluation of economic studies in orthopaedics (as a result of ease of use and time-efficiency), the assessment of factors associated with quality should also be interpreted with caution. The Quality of Health Economic Studies instrument assesses research methodology, but the primary focus of this scale is quality of reporting. Furthermore, although two well-trained individuals graded the quality of papers (BUN, JLB), the grading scheme is at risk for subjectivity bias. In addition to subjectivity biases, the instrument may suffer from a ceiling effect. As economic analyses become more common and potentially more sophisticated, there may be a role for more critical assessment tools that focus on additional criteria such as appropriate costing methodology for societal perspective (inclusion of indirect costs); generalizability/validity of measures and conclusions; appropriate selection of comparators; and implications of findings and extensiveness of sensitivity analyses. We note that there is no established numeric cutoff for high-quality cost utility analysis based on the Quality of Health Economic Studies instrument; as such, our definition of high quality might be imprecise. It should also be noted that cost utility analysis itself is subject to certain limitations. Cost utility analysis considers economics from the payer, societal, or hospital’s perspective and does not incorporate patient willingness to pay. In the United States where patients have been shown willing to pay a premium for certain healthcare services that they perceive to be of high value, patient willingness to pay offers an important perspective [4]. Cost-benefit analysis incorporates patient willingness to pay. Although not formally evaluated as part of this study, the volume of US cost-benefit analyses retrieved during the search strategy was noted to be relatively low. Future economic analyses in TJA may benefit from incorporating cost-benefit analysis. As the indications for TJA continue to expand to younger patients who place a greater premium on increased years of joint function, the willingness-to-pay perspective may be useful for payers and policymakers in evaluating elective procedures. Finally, our review may be limited by lack of inclusion of unpublished literature. There may be a bias toward the publication of cost-effective studies and as such only including published studies may predispose this review to publication bias.
There is a paucity of evidence comparing the cost utility of TJA to conservative intervention. Two studies use economic modeling and incremental analysis to assess the cost utility of TKA compared with conservative care. There is an absence of similar analysis for THA. The paucity of evidence in this area is likely the result of the fact that patients who undergo arthroplasty have already failed all attempts at nonoperative management and as such healthcare economic analysts do not seek to prove the standalone cost utility of arthroplasty procedures because their value is assumed. In the current health care-political environment, however, establishing the cost-effectiveness of very effective but yet costly procedures such as THA can be important to guide decision-making in a value-based system. As such there is a need in the literature for cost utility comparisons of THA and conservative care. The majority of identified studies are focused on the economic impact of new implant technologies or comparisons among different surgical alternatives. These types of analyses are also very useful and can help in guiding policymakers with regard to adoption of new technologies and surgical approaches. The available studies suggest that the upfront costs associated with new technologies are only cost-effective if there is a major reduction in a future cost (eg, computer navigation is worthwhile to adopt if it decreases the future costs associated with revision procedures).
In this study, we found that the quality of cost utility analyses published over the past 15 years has been good. Two previous studies by Brauer et al. [8, 9] reviewed the quality of CEA literature published across the general field of orthopaedics between 1976 and 2003. In the first study [9], Brauer and colleagues reviewed all English language cost utility analyses between 1976 and 2001. The authors found 11 studies for TJA and reported that the quality of these studies was variable and poor. In a subsequent followup review, Brauer et al. [8] updated their search to include articles published between 2001 and 2003. The authors noted in this second review that even in the short interim, there had been an increase in the volume of cost utility analysis published in orthopaedics and that there had also been a concurrent improvement in study quality. The authors hypothesized that the noted improvement in quality may have been potentially attributable to increased visibility of the recommendations from the US Panel on Cost-Effectiveness in Health and Medicine. The findings in the present review agree with Brauer et al.’s observation: over the past 15 years, there has been a dramatic increase in the volume of cost utility analyses in TJA and the quality of the published literature has been quite good. More recently, Daigle et al. [15] conducted a systematic review with an emphasis on identifying high-quality CEA in THA and TKA. The authors identified 13 studies; however, the review did not apply a validated quality assessment tool and their inclusion of international CEA studies limits generalizability of findings to the US context.
Based on the available studies, we did not identify any significant predictors of high-quality cost utility analysis. A previous study evaluating factors associated with high level of CEA evidence in 186 gastroenterology publications found the following factors associated with high-level CEA: (1) one or more authors with an advanced degree; (2) use of decision analysis; (3) federal funding; and (4) citation of the National Panel on Cost Effectiveness guidelines [60]. Although utilization of national funding sources and adoption of a lifetime perspective approached significance, they were not significant predictors of high quality. The influence of national funding sources is likely explained by the fact that national funders may select for cost utility analysis experts and projects with more rigorous methodology. Industry funding and perceived conflict of interest did not substantially impact the quality of evidence. Previous work in orthopaedics has suggested that conflict of interest relationships and industry-sponsored research are associated with a higher rate of publication, more citations, and a potentially lower level of evidence with some associated bias [1, 42–44]. The quality of economic analyses in TJA does not appear to be influenced by industry funding or conflict of interest relationships.
In this review we include the available cost utility evidence for US-based lower extremity arthroplasty. Compared with conservative measures, TKA and THA both appear to be cost-effective interventions, although there is a paucity of evidence for THA in this regard. The cost utility of new technologies in arthroplasty is often contingent on decreasing future costs. Based on scores obtained from the Quality of Health Economic Studies instrument, the overall quality of available TJA cost utility evidence is good. We did not find any significant predictors of high-quality studies. More work needs to be done to quantify the value of joint arthroplasty compared with nonoperative treatment interventions. Critical assessment tools such as the Quality of Health Economic Studies instrument may be useful in evaluating future cost utility studies, and more attention should be paid to capturing patient willingness to pay in future work.
Electronic supplementary material
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Amiri AR, Kanesalingam K, Cro S, Casey AT. Does source of funding and conflict of interest influence the outcome and quality of spinal research? Spine J. 2014;14:308–314. doi: 10.1016/j.spinee.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 2.Ballal RD, Botteman MF, Foley I, Stephens JM, Wilke CT, Joshi AV. Economic evaluation of major knee surgery with recombinant activated factor VII in hemophilia patients with high titer inhibitors and advanced knee arthropathy: exploratory results via literature-based modeling. Curr Med Res Opin. 2008;24:753–768. doi: 10.1185/030079908X273048. [DOI] [PubMed] [Google Scholar]
- 3.Botteman MF, Caprini J, Stephens JM, Nadipelli V, Bell CF, Pashos CL, Cohen AT. Results of an economic model to assess the cost-effectiveness of enoxaparin, a low-molecular-weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long-term complications in total hip replacement surgery in the United States. Clin Ther. 2002;24:1960–1986; discussion 1938. [DOI] [PubMed]
- 4.Bozic KJ, Chiu V, Slover JD, Immerman I, Kahn JG. Patient preferences and willingness to pay for arthroplasty surgery in patients with osteoarthritis of the hip. J Arthroplasty. 2012;27:503–506e.502. doi: 10.1016/j.arth.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Morshed S, Silverstein MD, Rubash HE, Kahn JG. Use of cost-effectiveness analysis to evaluate new technologies in orthopaedics. The case of alternative bearing surfaces in total hip arthroplasty. J Bone Joint Surg Am. 2006;88:706–714. doi: 10.2106/JBJS.E.00614. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Pui CM, Ludeman MJ, Vail TP, Silverstein MD. Do the potential benefits of metal-on-metal hip resurfacing justify the increased cost and risk of complications? Clin Orthop Relat Res. 2010;468:2301–2312. doi: 10.1007/s11999-010-1301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozic KJ, Saleh KJ, Rosenberg AG, Rubash HE. Economic evaluation in total hip arthroplasty: analysis and review of the literature. J Arthroplasty. 2004;19:180–189. doi: 10.1016/S0883-5403(03)00456-X. [DOI] [PubMed] [Google Scholar]
- 8.Brauer CA, Neumann PJ, Rosen AB. Trends in cost effectiveness analyses in orthopaedic surgery. Clin Orthop Relat Res. 2007;457:42–48. doi: 10.1097/BLO.0b013e31803372c9. [DOI] [PubMed] [Google Scholar]
- 9.Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87:1253–1259. doi: 10.2106/JBJS.D.02152. [DOI] [PubMed] [Google Scholar]
- 10.Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, Yu HT, Keeler EB, Henning JM, Ofman JJ. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41:32–44. doi: 10.1097/00005650-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cleemput I, Neyt M, Thiry N, De Laet C, Leys M. Using threshold values for cost per quality-adjusted life-year gained in healthcare decisions. Int J Technol Assess Health Care. 2011;27:71–76. doi: 10.1017/S0266462310001194. [DOI] [PubMed] [Google Scholar]
- 12.Courville XF, Tomek IM, Kirkland KB, Birhle M, Kantor SR, Finlayson SR. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2012;33:152–159. doi: 10.1086/663704. [DOI] [PubMed] [Google Scholar]
- 13.Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesaeter LB, Finlayson SR. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91:634–641. doi: 10.2106/JBJS.G.01029. [DOI] [PubMed] [Google Scholar]
- 14.Cutler D, Wikler E, Basch P. Reducing administrative costs and improving the health care system. N Engl J Med. 2012;367:1875–1878. doi: 10.1056/NEJMp1209711. [DOI] [PubMed] [Google Scholar]
- 15.Daigle ME, Weinstein AM, Katz JN, Losina E. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol. 2012;26:649–658. doi: 10.1016/j.berh.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19:1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 17.de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, Hutchison J, Grant A, Coyle D, Coyle K, Vale L. A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip. Health Technol Assess. 2008;12:iii–iv, ix–223. [DOI] [PubMed]
- 18.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Fisher ES, Bell JE, Tomek IM, Esty AR, Goodman DC. Trends and Regional Variation in Hip, Knee, and Shoulder Replacement. 2010. Available at: http://www.dartmouthatlas.org/downloads/reports/Joint_Replacement_0410.pdf. Accessed September 9, 2014. [PubMed]
- 20.Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419–430. doi: 10.1086/318502. [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Sofaer S, Siegelberg T. Medicare and cost-effectiveness analysis: time to ask the taxpayers. Health Aff (Millwood). 2007;26:1399–1406. doi: 10.1377/hlthaff.26.5.1399. [DOI] [PubMed] [Google Scholar]
- 22.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 23.Hussman JM, Lanctot KL, Paes B. The cost effectiveness of palivizumab in congenital heart disease: a review of the current evidence. J Med Econ. 2013;16:115–124. doi: 10.3111/13696998.2012.734886. [DOI] [PubMed] [Google Scholar]
- 24.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 25.Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21:367–375. doi: 10.1016/j.jse.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lansky D, Nwachukwu BU, Bozic KJ. Using financial incentives to improve value in orthopaedics. Clin Orthop Relat Res. 2012;470:1027–1037. doi: 10.1007/s11999-011-2127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavernia CJ, Alcerro JC. Quality of life and cost-effectiveness 1 year after total hip arthroplasty. J Arthroplasty. 2011;26:705–709. doi: 10.1016/j.arth.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Lawless BM, Greene M, Slover J, Kwon YM, Malchau H. Does age or bilateral disease influence the value of hip arthroplasty? Clin Orthop Relat Res. 2012;470:1073–1078. doi: 10.1007/s11999-011-2118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J, Wilbur WJ. PubMed related articles: a probabilistic topic-based model for content similarity. BMC Bioinformatics. 2007;8:423. doi: 10.1186/1471-2105-8-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin MM, Goldsmith JD, Resch SC, DeAngelis JP, Ramappa AJ. Histologic examinations of arthroplasty specimens are not cost-effective: a retrospective cohort study. Clin Orthop Relat Res. 2012;470:1452–1460. doi: 10.1007/s11999-011-2149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, Holt HL, Solomon DH, Yelin E, Paltiel AD, Katz JN. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113–1121; discussion 1121–1122. [DOI] [PMC free article] [PubMed]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the USD 50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 34.Neumann PJ, Palmer JA, Daniels N, Quigley K, Gold MR, Chao S. A strategic plan for integrating cost-effectiveness analysis into the US healthcare system. Am J Manag Care. 2008;14:185–188. [PubMed] [Google Scholar]
- 35.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med. 2005;353:1516–1522. doi: 10.1056/NEJMsb050564. [DOI] [PubMed] [Google Scholar]
- 36.Noordin S, Wright JG, Howard A. Relationship between declared funding support and level of evidence. J Bone Joint Surg Am. 2010;92:1647–1651. doi: 10.2106/JBJS.I.00224. [DOI] [PubMed] [Google Scholar]
- 37.Novak EJ, Silverstein MD, Bozic KJ. The cost-effectiveness of computer-assisted navigation in total knee arthroplasty. J Bone Joint Surg Am. 2007;89:2389–2397. doi: 10.2106/JBJS.F.01109. [DOI] [PubMed] [Google Scholar]
- 38.Nwachukwu BU, Hamid KS, Bozic KJ. Measuring value in orthopaedic surgery. JBJS Reviews. 2013;1\1\e.2. Available at http://reviews.jbjs.org/content/1/1/e2.full-text.pdf+html. Accessed September 9, 2014. [DOI] [PubMed]
- 39.Oberlander J. Unfinished journey—a century of health care reform in the United States. N Engl J Med. 2012;367:585–590. doi: 10.1056/NEJMp1202111. [DOI] [PubMed] [Google Scholar]
- 40.Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95:1441–1449. doi: 10.2106/JBJS.L.00373. [DOI] [PubMed] [Google Scholar]
- 41.Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, Hay JW. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9:53–61. doi: 10.18553/jmcp.2003.9.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okike K, Kocher MS, Mehlman CT, Heckman JD, Bhandari M. Nonscientific factors associated with acceptance for publication in The Journal of Bone and Joint Surgery (American Volume) J Bone Joint Surg Am. 2008;90:2432–2437. doi: 10.2106/JBJS.G.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okike K, Kocher MS, Mehlman CT, Heckman JD, Bhandari M. Publication bias in orthopaedic research: an analysis of scientific factors associated with publication in the Journal of Bone and Joint Surgery (American Volume) J Bone Joint Surg Am. 2008;90:595–601. doi: 10.2106/JBJS.G.00279. [DOI] [PubMed] [Google Scholar]
- 44.Okike K, Kocher MS, Torpey JL, Nwachukwu BU, Mehlman CT, Bhandari M. Level of evidence and conflict of interest disclosure associated with higher citation rates in orthopedics. J Clin Epidemiol. 2011;64:331–338. doi: 10.1016/j.jclinepi.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Orszag PR, Emanuel EJ. Health care reform and cost control. N Engl J Med. 2010;363:601–603. doi: 10.1056/NEJMp1006571. [DOI] [PubMed] [Google Scholar]
- 46.Peterson LE, Goodman C, Karnes EK, Chen CJ, Schwartz JA. Assessment of the quality of cost analysis literature in physical therapy. Phys Ther. 2009;89:733–755. doi: 10.2522/ptj.20080326. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz D, Jr, Koenig L, Dall TM, Gallo P, Narzikul A, Parvizi J, Tongue J. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95:1473–1480. doi: 10.2106/JBJS.L.01488. [DOI] [PubMed] [Google Scholar]
- 48.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. doi: 10.1001/jama.1996.03540140060028. [DOI] [PubMed] [Google Scholar]
- 49.Schousboe JT, Brown GA. Cost-effectiveness of low-molecular-weight heparin compared with aspirin for prophylaxis against venous thromboembolism after total joint arthroplasty. J Bone Joint Surg Am. 2013;95:1256–1264. doi: 10.2106/JBJS.L.00400. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz JA, Pearson SD. Cost consideration in the clinical guidance documents of physician specialty societies in the United States. JAMA Intern Med. 2013;173:1091–1097. doi: 10.1001/jamainternmed.2013.817. [DOI] [PubMed] [Google Scholar]
- 51.Sharifi E, Sharifi H, Morshed S, Bozic K, Diab M. Cost-effectiveness analysis of periacetabular osteotomy. J Bone Joint Surg Am. 2008;90:1447–1456. doi: 10.2106/JBJS.G.00730. [DOI] [PubMed] [Google Scholar]
- 52.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.1996.03540160061034. [DOI] [PubMed] [Google Scholar]
- 53.Slobogean GP, Verma A, Giustini D, Slobogean BL, Mulpuri K. MEDLINE, EMBASE, and Cochrane index most primary studies but not abstracts included in orthopedic meta-analyses. J Clin Epidemiol. 2009;62:1261–1267. doi: 10.1016/j.jclinepi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Slover J, Espehaug B, Havelin LI, Engesaeter LB, Furnes O, Tomek I, Tosteson A. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients. A Markov decision analysis. J Bone Joint Surg Am. 2006;88:2348–2355. doi: 10.2106/JBJS.E.01033. [DOI] [PubMed] [Google Scholar]
- 55.Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24:854–860. doi: 10.1016/j.arth.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slover JD, Rubash HE, Malchau H, Bosco JA. Cost-effectiveness analysis of custom total knee cutting blocks. J Arthroplasty. 2012;27:180–185. doi: 10.1016/j.arth.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 57.Slover JD, Tosteson AN, Bozic KJ, Rubash HE, Malchau H. Impact of hospital volume on the economic value of computer navigation for total knee replacement. J Bone Joint Surg Am. 2008;90:1492–1500. doi: 10.2106/JBJS.G.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smart KA, Lanctot KL, Paes BA. The cost effectiveness of palivizumab: a systematic review of the evidence. J Med Econ. 2010;13:453–463. doi: 10.3111/13696998.2010.499749. [DOI] [PubMed] [Google Scholar]
- 59.Soohoo NF, Sharifi H, Kominski G, Lieberman JR. Cost-effectiveness analysis of unicompartmental knee arthroplasty as an alternative to total knee arthroplasty for unicompartmental osteoarthritis. J Bone Joint Surg Am. 2006;88:1975–1982. doi: 10.2106/JBJS.E.00597. [DOI] [PubMed] [Google Scholar]
- 60.Spiegel BM, Targownik LE, Kanwal F, Derosa V, Dulai GS, Gralnek IM, Chiou CF. The quality of published health economic analyses in digestive diseases: a systematic review and quantitative appraisal. Gastroenterology. 2004;127:403–411. doi: 10.1053/j.gastro.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Suter LG, Paltiel AD, Rome BN, Solomon DH, Thornhill TS, Abrams SK, Katz JN, Losina E. Placing a price on medical device innovation: the example of total knee arthroplasty. PloS One. 2013;8:e62709. doi: 10.1371/journal.pone.0062709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toal MJ. Industry sponsored bias: NICE may be biased too. BMJ (Clin Res Ed). 2011;342:d474. doi: 10.1136/bmj.d474. [DOI] [PubMed] [Google Scholar]
- 63.Watters TS, Browne JA, Orlando LA, Wellman SS, Urbaniak JR, Bolognesi MP. Cost-effectiveness analysis of free vascularized fibular grafting for osteonecrosis of the femoral head. J Surg Orthop Adv. 2011;20:158–167. [PubMed] [Google Scholar]
- 64.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. doi: 10.1001/jama.1996.03540150055031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.