Abstract
Background
Grade IIIB open tibia fractures are devastating injuries. Some clinicians advocate wound closure or stable muscle flap coverage within 72 hours to limit complications such as infection. Negative pressure wound therapy was approved by the FDA in 1997 and has become an adjunct for many surgeons in treating these fractures. Opinions vary regarding the extent to which negative pressure wound therapy contributes to limb salvage. Evidence-based practice guidelines are limited for use of negative pressure wound therapy in Grade IIIB tibia fractures. This systematic literature review of negative pressure wound therapy in Grade IIIB tibia fractures may substantiate current use and guide future studies.
Questions/purposes
We sought to answer the following: (1) Does the use of negative pressure would therapy compared with gauze dressings lead to fewer infections? (2) Does it allow flap procedures to be performed safely beyond 72 hours without increased infection rates? (3) Is it associated with fewer local or free flap procedures?
Methods
We conducted a systematic review of six large databases (through September 1, 2013) for studies reporting use of negative pressure wound therapy in Grade IIIB open tibia fractures, including information regarding infection rates and soft tissue reconstruction. The systematic review identified one randomized controlled trial and 12 retrospective studies: four studies compared infection rates between negative pressure wound therapy and gauze dressings, 10 addressed infection rates with extended use, and six reported on flap coverage rates in relation to negative pressure wound therapy use beyond 72 hours. None of the 13 studies was eliminated owing to lack of study quality.
Results
Negative pressure wound therapy showed a decrease in infection rates over rates for gauze dressings in two of four studies (5.4% [two of 35] versus 28% [seven of 25], and 8.4% [14 of 166] versus 20.6% [13 of 63]), an equivalent infection rate in one study (15% [eight of 53] versus 14% [five of 16]), and an increased infection rate in the fourth study (29.5% [23 of 78] versus 8% [two of 25]). In terms of the second question regarding infection rates with negative pressure wound therapy beyond 72 hours, eight of 10 studies concluded there was no increase in infection rates, whereas two of 10 reported an increase in infection rates associated with negative pressure wound therapy use beyond 72 hours. Infection rates varied from 0% to 57% in these 10 studies. Five studies reported low infection rates of 0% to 7% and five reported rates of 27% to 57%. The third question (addressed by six studies) regarded the potential decreased use of a soft tissue flap in patients treated with extended negative pressure wound therapy. Flap rates were reduced by 13% to 60% respectively compared with those of historical controls. Grade IIIB tibia fractures by definition required soft tissue procedures. The patients in these six studies had Grade IIIB tibia fractures after the first débridement. However, after extended negative pressure wound therapy, fewer patients required flaps than grading at the first débridement would have predicted.
Conclusions
There is an increasing body of data supporting negative pressure wound therapy as an adjunctive modality at all stages of treatment for Grade IIIB tibia fractures. There is an association between decreased infection rates with negative pressure wound therapy compared with gauze dressings. There is evidence to support negative pressure wound therapy beyond 72 hours without increased infection rates and to support a reduction in flap rates with negative pressure wound therapy. However, negative pressure wound therapy use for Grade IIIB tibia fractures requires extensive additional study.
Level of Evidence
Level III, therapeutic study.
Introduction
Tissue demarcation in Grade IIIB open tibia fractures often continues even after the first débridement [55]. In addition, the wound is relatively open to the hospital environment until a flap can be placed [55]. The implant used for bony stabilization may compromise regional bone blood flow and may lead to wound problems [55]. These factors and others contribute to wound-bed bacterial colonization [10, 12, 14, 27, 46, 54, 55] and progression to infection. Since Godina’s study [25], it generally has been accepted that these open fractures require early (within 72 hours) bony stabilization and soft tissue reconstruction. Unfortunately, soft tissue coverage is not always possible in the acute setting for numerous reasons. In some circumstances a flap is not placed for a week or more and a negative pressure wound therapy dressing is applied in the interim. Regardless when a flap is placed, negative pressure wound therapy has become preferred over traditional gauze dressings by some [15, 16]. The expectations of negative pressure wound therapy are for it to decrease tissue edema, enhance local blood flow, limit or prevent infection, improve flap rates, and possibly reduce the overall need for flaps. Is there clinical evidence to support replacement of gauze dressings with negative pressure wound therapy in Grade IIIB tibia fractures?
The earliest articles regarding negative pressure wound therapy and infections were published in the 1990s [21–23, 44]. In 1997, the FDA approved negative pressure wound therapy. Subsequently, animal studies [39, 41] and additional clinical reports that favored negative pressure wound therapy to reduce edema, decrease bacterial loads, increase granulation tissue formation, and other pathways to promote wound healing [2, 38] were published. The majority of studies however, regard chronic or diabetic wounds [5, 8, 18, 42, 43, 52]. The data regarding chronic and diabetic wounds might be able to be extrapolated to traumatic wounds. In Grade IIIB tibia wounds negative pressure wound therapy has the added expectation of sealing the wound from the hospital environment, acting as a temporary dermal substitute, and preventing bacterial access to the wound bed. The decrease in bacterial load [43, 54] and initiation of granulation tissue formation [2, 19, 24, 39] reported in some studies, to our knowledge, has not been reviewed or summarized for Grade IIIB tibia fractures. It also has yet to be reported whether negative pressure wound therapy is associated with favorable outcomes, such as fewer infections or flap procedures with days or weeks of therapy. Use of negative pressure wound therapy in some centers extends long beyond the acute injury phase (weeks at a time with intermittent sponge changes). These centers have found an associated decreased rate of free or local flap procedures [6, 15, 16, 29, 35, 45], and an increase in delayed primary closure or skin grafting. Whether the outcomes are as universally favorable for Grade IIIB tibia fractures needs to be determined.
We therefore performed a systematic review to look at Grade IIIB tibia fractures and negative pressure wound therapy. We asked the following questions: (1) Does the use of negative pressure wound therapy in Grade IIIB tibia fractures compared with gauze dressings lead to fewer infections? (2) Does it allow flap procedures to be performed beyond 72 hours without increased infection rates? (3) Is it associated with fewer local or free flap procedures?
Search Strategy and Criteria
We conducted a systematic literature search (through September 1, 2013) of six databases (Fig. 1). First, using the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic reviews, and the Cochrane Methodology Register, three terms for negative pressure wound therapy (negative pressure wound therapy, topical negative wound therapy, and vacuum assisted closure) were used yielding 107 randomized, controlled trials (RCTs). One hundred were excluded based on their titles most commonly because of inclusion of chronic or diabetic wounds. We reviewed the abstracts of the seven remaining studies and only one [49] included Grade IIIB tibia fractures, negative pressure wound therapy, and any of our outcome questions. A second search protocol was used with the databases of MEDLINE®, Embase, and Google Scholar (Fig. 1). The same three terms for negative pressure wound therapy (negative pressure wound therapy, topical negative wound therapy, and vacuum assisted closure) were used and yielded 1010 articles. These 1010 articles then were screened by combining exploded Medical Search Headings (MeSH® terms) and free text words using Boolean operators “OR” and “AND” in various combinations: (negative pressure wound therapy OR topical negative wound therapy OR vacuum assisted closure) AND (tibial fractures) AND (open fractures) AND (infections OR osteomyelitis) AND (muscle flap OR soft tissue reconstruction OR flap procedures) until all combinations were exhausted. The search was limited to articles published in English and involving human subjects. All levels of evidence initially were included. Secondary screening yielded 136 articles, and all but 35 were eliminated after title review, primarily owing to inclusion of chronic or diabetic wounds. Pediatric studies were not excluded. Thirteen studies remained [6, 7, 15, 16, 29, 30, 33, 35, 36, 45, 47, 49, 50] after reading the abstract and the full-length article as needed to ascertain data points. Twenty-two studies were eliminated from the final 35 articles identified because they were review articles (n = 8), articles regarding pilon fracture (n = 2), and articles with insufficient data or case reports (n = 12). All publications were from 2006 and later, likely owing to FDA approval in 1997 and several years thereafter for clinicians to use negative pressure wound therapy and several more years for patient followup. One of the 13 studies was an RCT [49], and the remaining 12 were retrospective [6, 7, 15, 16, 29, 30, 33, 35, 36, 45, 47, 50]. The RCT was not eliminated because of lack of study quality as defined by Moher et al. [37] or by Detsky et al. [17].
Fig. 1.
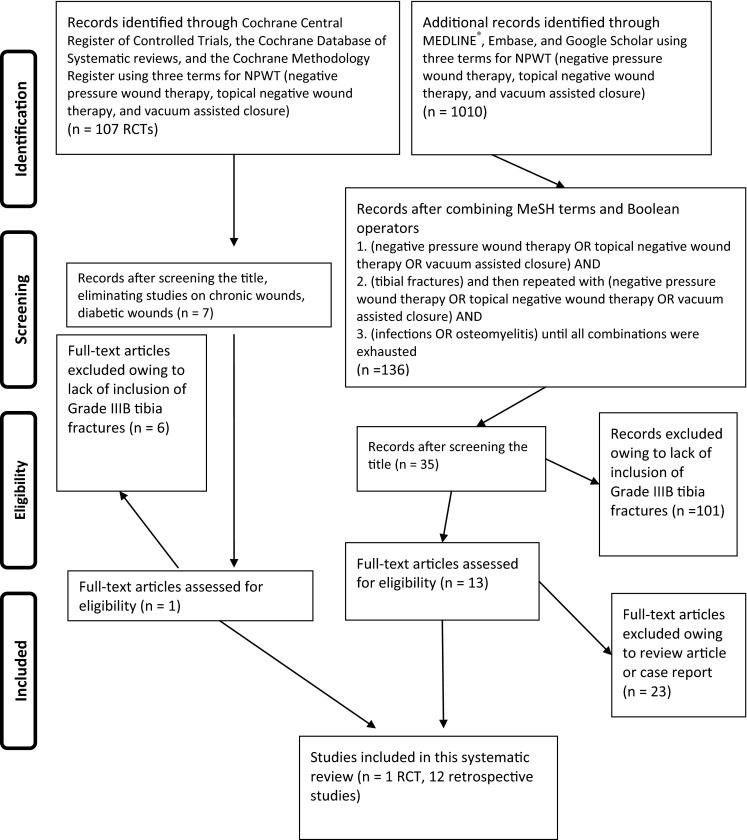
The PRISMA flowchart shows the identification process for articles in our study. RCT = randomized controlled trial; NPWT = negative pressure wound therapy.
The methodologic index for nonrandomized studies (MINORS) [48] instrument was used to assess the 12 retrospective studies in our review. We used all 12 criteria in the MINORS assessment method [48]. Five criteria were found to be most relevant to our three questions: (1) a clearly stated study aim, (2) inclusion of consecutive patients, (3) end points appropriate to the aim of the study, (4) followup appropriate to the aim of the study, and (5) loss to followup less than 5%. The 12 retrospective articles were assessed for but not eliminated for: (1) unbiased assessment of the study end point because infection determination, for example, could not be blinded from the treating authors, (2) prospective data collection, or (3) prospective calculation of the study size because this would have eliminated all the retrospective studies. Three of the final four criteria for the MINORS method [48] of evaluation of nonrandomized studies assessed the control groups. All 12 retrospective studies were limited either by lack of a control group beyond historic information or small control group size; thus, these criteria would have eliminated all 12 studies. These criteria were not strictly applied. The final criterion, statistical analysis, was included and because the end points, such as infection rates, were straightforward, no bias was noted toward any of the study conclusions. No retrospective studies were eliminated after using the MINORS method.
Each of the 13 papers was read and reviewed to determine which of our three questions were addressed in each of the studies. Some studies addressed more than one question. Only one article [35] reported data for all three questions (Table 1). To answer our first question regarding infection risk, we found four of the 13 articles [7, 35, 45, 49] compared gauze dressings with negative pressure wound therapy (Table 1). Ten [6, 15, 16, 29, 30, 33, 35, 36, 47, 50] of 13 studies evaluated infection rates when flap coverage and negative pressure wound therapy use extended beyond 72 hours. Six studies [6, 15, 16, 29, 35, 45] reported data on flap procedure rates when negative pressure wound therapy extended beyond 72 hours. For the purposes of this review, any patient treated with antibiotics alone or antibiotics and surgical incision and drainage or other procedure was considered to have an infection. Seventy-two hours generally has become the cutoff point [25] for defining early versus late wound closure. Subacute closure is defined as closure between 3 and 7 days. Data for late wound coverage, defined as closure after 7 days, also were reported [6, 15, 16, 30, 35, 39]. For the purposes of this review, we considered early closure as before 72 hours.
Table 1.
Summary of the three literature search questions. Six of 13 studies addressed two of 3 search questions, one of 13 studies addressed three of 3 search questions
| Study | NPWT versus gauze dressings, fewer infections in NPWT? | NPWT extends time until wound coverage without rate increase? | NPWT decreases flap rate? |
|---|---|---|---|
| Bhattacharyya et al. [6] | N/A | No | Yes |
| Blum et al. [7] | Yes 8.4% (14/166) vs 20.6% (13/63) |
N/A | N/A |
| Dedmond et al. [15] | N/A | Yes | Yes |
| Dedmond et al. [16] | N/A | Yes | Yes |
| Hou et al. [29] | N/A | No | Yes |
| Karanas et al. [30] | N/A | Yes | N/A |
| Li et al. [33] | N/A | Yes | N/A |
| Liu et al. [36] | N/A | Yes | N/A |
| Liu et al. [35] | No* 29.5% (23/78) vs 8.0% (2/25) |
Yes | Yes |
| Parrett et al. [45] | Equivalent 15% (8/53) vs 14% (5/35) |
N/A | Yes |
| Rinker et al. [47] | N/A | Yes | N/A |
| Stannard et al. [49] | Yes 5.4% (2/35) vs 28% (7/25) |
N/A | N/A |
| Steiert et al. [50] | N/A | Yes | N/A |
| Total | Four of 13 | 10 of 13 | Six of 13 |
| # of studies supporting NPWT | two of four** | eight of 10 | six of 6 |
NPWT = negative pressure wound therapy; N/A = not available; *a fourfold higher rate of exposed hardware occurred in the NPWT group versus the gauze group, which may correlate with the increased infection rates in the NPWT group; **one of 4 studies reported equivalent data.
Results
Negative Pressure Wound Therapy and Infection Reduction in Grade IIIB Tibial Fractures
Negative pressure wound therapy versus gauze dressings showed a decrease in infection rates for negative pressure wound therapy in two [7, 49] of four studies [7, 35, 45, 49] (5.4% [two of 35] versus 28% [seven of 25], and 8.4% [14 of 166] versus 20.6% [13 of 63]), an equivalent infection rate in one study [45] (15% [eight of 53] versus 14% [five of 16]), and an increased infection rate in one study [35] (29.5% [23 of 78] versus 8% [two of 25]). Infection rates ranged from 5.4% to 29.0% for negative pressure wound therapy dressings and from 8% to 28% for gauze dressings in all four studies [7, 35, 45, 49]. The RCT [49] reported patients receiving dressings were one-fifth as likely to have an infection develop compared with patients receiving gauze dressings. Parrett et al. [45] looked at a group of patients with only gauze dressings (before negative pressure wound therapy, 1992–1995). They then compared this group with a group of patients treated with negative pressure wound therapy between 2000 and 2003. This second period was chosen specifically because it bypassed their earliest clinical experiences with negative pressure wound therapy. That period was 1997 to 2000 which abuts the 1997 FDA approval of negative pressure wound therapy. The infection rates were equivalent at 14% for gauze dressings and 15% for negative pressure wound therapy in their study [45]. The patients in the two studies [7, 49] with decreased and the one [45] with equivalent infection rates and negative pressure wound therapy dressings all had wound closure within 7 days. Liu et al., in a retrospective study [35], reported increased infection rates with negative pressure wound therapy. Their study had a subgroup of patients who had wound closure after 7 days. The most common causative infectious organisms reported were methicillin-sensitive Staphylococcus aureus, methicillin-resistant S aureus, Pseudomonas, and Enterococcus. Only Stannard et al. [49] reported a specific antibiotic regimen, which included a broad-spectrum cephalosporin or aminoglycoside plus a first-generation cephalosporin. Penicillin was added when wounds were severely contaminated. The other studies briefly stated that all patients received antibiotics at the time of initial presentation and for a minimum of 24 hours (range, 24–72 hours) after wound closure.
Does Negative Pressure Wound Therapy Facilitate Late Flap Procedures
Ten retrospective studies [6, 15, 16, 29, 30, 33, 35, 36, 47, 50] reported data on extended use (beyond 72 hours) of negative pressure wound therapy in Grade IIIB tibia fractures. The combined infection rates ranged from 0% to 57%. Two subgroups emerged. Five studies [30, 33, 36, 47, 50] reported infection rates of 0% to 7%, and five [6, 15, 16, 29, 35] reported rates of 27% to 57%. Only two [6, 29] of the 10 studies however, reported an increase in infection rates associated with extended negative pressure wound therapy use. Hou et al. [29] reported an infection rate with negative pressure wound therapy of 45% (10 of 22) versus 10% (one of 10) with gauze dressings. Bhattacharyya et al. [6] reported an infection rate with negative pressure wound therapy of 57% (eight of 14) versus 12.5% (three of 24) with gauze dressings. In both of these studies with higher negative pressure wound therapy infection rates the cutoff for early and extended negative pressure wound therapy use was 7 days rather than 72 hours. The most common infective organism(s) were similar to those for question one in the preceding paragraph. Eight [6, 15, 16, 29, 30, 33, 35, 47] of 10 studies reported incision and drainage procedures were performed in the operating room every 24 to 48 hours. One of the 10 studies [50] reported the negative pressure wound therapy dressing was changed less frequently (every 3 to 9 days), and in another of the 10 studies [36], the negative pressure wound therapy was left in place unchanged after the initial incision and drainage. The negative pressure wound therapy dressing continued for 7 to 10 days and was removed only at wound closure [36]. Total time for use of negative pressure wound therapy ranged from 7 to 53 days across all 10 studies [6, 15, 16, 29, 30, 33, 35, 36, 47, 50]. One study [30] with 14 patients reported a mean time of 27 days (range, 8–53 days) to flap procedure and a zero infection rate. The method of bony stabilization was variable across studies. Some authors reported temporary external fixation was followed by a plate or an intramedullary device. Some reported external fixation was maintained until the time of union. No study reported the duration of temporary external fixation or the exact timing of either external or internal fixation in relation to the timing of wound closure. For example, Li et al. [33] had limited fixation information, stating only: “External fixation or simple limited internal fixation was selected to reconstruct bone.” Karanas et al. [30] reported no information regarding the bony stabilization method. In eight studies [6, 15, 16, 29, 35, 36, 47, 50], external fixation was used in 5% to 100% of patients, an intramedullary rod was used in 0% to 66%, and open reduction and internal fixation was used in 0% to 42%.
Is the Use of Negative Pressure Wound Therapy Associated with Less-frequent Use of Flap Procedures?
Six [6, 15, 16, 29, 35, 45] of the 13 studies used extended negative pressure wound therapy and had flap rate data to report. In a longitudinal study [45], Parrett et al. reported free flap rates of 42% before the advent of negative pressure wound therapy. During a 4-year period (2000 through 2003) starting 3 years after FDA approval of negative pressure wound therapy in 1997, their free flap rate decreased to a mean of 11%. Their local flap rate however, stayed constant at 38% to 40%. Their local wound care increased from 22% to 49% with the advent of negative pressure wound therapy [45]. This comprised skin grafts and/or delayed primary or secondary wound closure [45]. Five other studies [6, 15, 16, 29, 35] using extended negative pressure wound therapy until wound closure showed 13% to 60% decreases in their flap rates. These authors calculated their flap rate changes either by using previous flap rates at their institutions as a reference or with the amount of exposed bone after the first débridement, which means limbs without adequate bony coverage were Grade IIB fractures with the full expectation of needing a flap. This flap expectation was after the first débridement and before starting negative pressure wound therapy. Many of these leg wounds were closed by means other than a flap despite an initial Grade IIIB assignment. Dedmond et al. [15, 16] reported a decrease in the rate of flap use relative to the wound classification at the first débridement. Seven [7, 30, 33, 36, 47, 49, 50] of 13 studies in our systemic review had no flap data to report. In two studies [7, 49] no data were available because all wounds were closed and all flaps were placed by 72 hours. Five studies [30, 33, 36, 47, 50] reported flap placement after 72 hours but there was no variability in wound dressings or coverage methods. All patients in these five studies [30, 33, 36, 47, 50] had either a local or free flap procedure and no skin grafting or secondary closures.
Discussion
Negative pressure wound therapy is preferred by some for treatment of Grade IIIB tibia fractures because negative pressure wound therapy has been reported to increase granulation tissue formation, enhance local blood flow, and decrease bacterial burden and infection rates, among other metrics. Limb salvage in Grade IIIB tibia fractures is particularly challenging. The evidence for efficacy of negative pressure wound therapy varies in this setting. Some studies [6, 7, 15, 16, 29, 30, 33, 35, 36, 45, 47, 49, 50] address a decrease of Grade IIIB tibia infections with negative pressure wound therapy and/or whether open tibial fractures otherwise considered Grade IIIB (need for muscle flap coverage) can be treated successfully with simpler soft tissue coverage techniques. The purpose of our study was to summarize the evidence regarding negative pressure wound therapy and Grade IIIB tibia fractures after a systematic review of the current literature. We specifically investigated Grade IIIB tibia fractures and infection rates associated with short- and long-term use of negative pressure wound therapy and flap rates. In 1986, Godina [25], recommended stabilizing Grade IIIB tibia fractures and complete soft tissue reconstruction within 72 hours. This time is not always feasible. Furthermore, the wound bed (particularly a highly contaminated wound) is still demarcating at Day 3 and beyond and requires more time and more débridements. Since its introduction in 1997, numerous surgeons have used negative pressure wound therapy successfully beyond 72 hours as a dynamic wound dressing for Grade IIIB tibia fractures [15, 16, 30, 33, 36, 47, 50]. Some clinicians have noted improvement with extended negative pressure wound therapy to the point whereby local wound care and not a flap procedure completed bony coverage [6, 15, 16, 29, 45]. Negative pressure wound therapy dressings may extend the time until wound closure or stable muscle flap coverage beyond 3 days. They also may provide an advantage in terms of infection rates versus traditional gauze dressings in Grade IIIB tibia fractures.
We searched the literature for evidence regarding negative pressure wound therapy use in Grade IIIB tibia fractures. Specifically, we asked: (1) Does the use of negative pressure wound therapy in Grade IIIB tibial fractures compared with gauze dressings lead to fewer infections? (2) Does it allow flap procedures to be performed beyond 72 hours without increased infection rates? (3) Is it associated with fewer local or free flap procedures?
Our literature search was limited in several ways. The first limitation was that nearly all of the more than 1000 articles regarding negative pressure wound therapy were either basic science and nonclinical or pertained to other wound types. We identified only 13 articles [6, 7, 15, 16, 29, 30, 33, 35, 36, 45, 47, 49, 50] using the strict search algorithm described above. Four [7, 35, 45, 49] of the 13 articles were germane to our first question, 10 [6, 15, 16, 29, 30, 33, 35, 36, 47, 50] to our second question, and six [6, 15, 16, 29, 35, 45] to our third question. Because the numbers of articles found per question were small, conclusive statements regarding negative pressure wound therapy are limited. Another major limitation was that numerous studies reported limited and nonspecific information regarding their methods of bony stabilization. No study provided information regarding fixation in terms of length of time for external fixation, or when internal fixation was performed. No study provided detailed information regarding other factors, including hardware used (stainless steel versus titanium, locking constructs, plate length), or dimensions of a bony defect if it existed. The success or failure of negative pressure wound therapy versus gauze dressings and use of extended negative pressure wound therapy could be influenced by the fixation method. In other words, infection rates were reported but a secondary breakdown by method of bony stabilization whether temporary with external fixation, definitive with external fixation, or was external to internal fixation, was not detailed well in most of the 13 studies. There was no way to ascertain if the method and sequence of bony stabilization had any association with the overall infection rates. Therefore, our ability is limited to make conclusive statements regarding the influence, role, or associations bony stabilization methods had on infection rates and flap rates. Negative pressure wound therapy is one of many potential factors when it comes to an infection occurrence. In lieu of these limitations there were trends in the use of negative pressure wound therapy for Grade IIIB tibia fractures.
Overall, our systematic review suggests that negative pressure wound therapy is associated with fewer infections when compared with gauze dressings. The RCT [49] in our literature search reported patients treated with negative pressure wound therapy were one-fifth as likely to have an infection compared with patients treated with gauze dressings. Blum et al. [7] included a multivariate analysis for Gustilo fracture Types I to IIIC, concluding negative pressure wound therapy reduced deep infection risk by nearly 80%. One study [45] had data regarding dressing use and outcomes before the advent of negative pressure wound therapy and after its introduction in 1997. There was no reported difference in infection rates between these periods; however, there was a reduction in free flaps from 42% of patients before negative pressure wound therapy to 11% after negative pressure wound therapy [45]. The fourth study [35] looking at negative pressure wound therapy as a primary dressing showed a preflap infection rate for negative pressure wound therapy of 29.5% versus only 8% for gauze dressings. However, in that study [35], the exposed hardware rate was 50% in the negative pressure wound therapy group versus only 12% in the gauze group. This is statistically significant and complicates any conclusive statements regarding dressing protocols and infection rates [35]. Furthermore, it was not clear in any of the 13 studies how much bone was exposed, the wound dimensions, the extent of wound contamination, or the need for bone grafting. Some authors acknowledged the need for additional details by stating a lack of overall patient population size prohibited subgroup analysis [6, 7, 29, 30, 35, 47]. These are variables requiring further study.
Some studies reported safe flap procedures without an increase in infection rates when negative pressure wound therapy was used beyond 72 hours [15, 16, 30, 33, 35, 36, 47, 49]. The question was whether use of negative pressure wound therapy beyond 72 hours would result in increased infection rates compared with historic controls [1, 11, 13, 20, 26, 28]. Reported infection rates are highly variable for Grade IIIB tibia wounds with the upper end at 67% [10]. Eight [15, 16, 30, 33, 35, 36, 47, 50] of the 10 studies with data regarding extended use of negative pressure wound therapy reported infection rates comparable to historic rates or lower. One study [6] did not favor extended use beyond 1 week (they defined early versus late coverage as 1 week) because the infection rate in the extended group was 57%. The infection rate in the early group was 12.5%. Another [30] of the 10 studies concluded that negative pressure wound therapy may help reduce flap size and/or reduce the need for a flap. However, Hou et al. [29] reported that prolonged periods of negative pressure wound therapy resulted in higher infection and amputation rates. The infection rate in their early group was 40% (four of 10), and in the extended negative pressure wound therapy group it was 45% (10 of 22). The amputation rates in both infected groups were 50% (two of four), and 70% (seven of 10) respectively. The amputation rates reported in the other 12 studies [6, 7, 15, 16, 30, 33, 35, 36, 45, 47, 49, 50] ranged from 0% to 16.7%. The study by Hou et al. [29], in concluding that use of negative pressure wound therapy was detrimental after 7 days, was an outlier regarding amputation rates in the acute and extended groups of patients with negative pressure wound therapy. Their study may not represent negative pressure wound therapy failures but the challenge in identifying appropriate cases for limb salvage. Furthermore, their small series of 32 patients limits any subgroup analysis to possibly link other variables such as fixation method, defect management, and débridement protocol to their high infection and amputation rates. This does not mean their protocols or conclusions are incorrect but does reaffirm that negative pressure wound therapy is one variable among many.
Six of 13 studies from our systematic review addressed our final question regarding flap rates with extended use of negative pressure wound therapy. Negative pressure wound therapy was associated with a decreased rate of flap procedures. All six studies [6, 15, 16, 29, 35, 45] with data regarding flap rates and extended negative pressure wound therapy use reported a decrease in flap procedures with extended negative pressure wound therapy. Two [15, 16] of the studies reported infection complications similar to those of historic controls [1, 11, 13, 20, 26, 28]. Dedmond et al. [15, 16] concluded the avoidance of flap procedures, particularly in the pediatric population, in the end outweighed the equivalent infection rates. They concluded that negative pressure wound therapy was beneficial because the infections were treated, the limbs were salvaged, and there were less flaps (and their associated morbidity) for 41.6% (10 of 24) and 50% (three of six) of their patients [15, 16]. In a longitudinal study, Parrett et al. [45] reported free flap rates of 42% before the advent of negative pressure wound therapy. In a 4-year period (2000 through 2003) starting 3 years after FDA approval in 1997, their free flap rate decreased to a mean of 11%, however their local flap rate stayed constant at 38% to 40%. Collectively in six studies [6, 15, 16, 29, 35, 45], the flap rates decreased between 13% and 60% at the respective institutions. No study center reported an increase in flap procedures owing to use of extended negative pressure wound therapy. In these studies, the alternative to flaps was local wound care consisting of skin grafts, delayed primary closure, or delayed secondary closure. Limited information was available regarding the long-term durability of the nonflap closure protocols. Long-term data (5 years or more) were not reported whether the alternative wound closure methods failed, broke down, and/or needed revision closure for any reason long term.
Corroborations from Basic Science and Other Disciplines
Aside from the previous evidence, Level 4 evidence has been published for six patients with high-energy open fractures where the need for muscle flap coverage and infection were avoided [51, 53]. Morykwas et al. [41], in histologic studies regarding burns in a porcine model, described preservation of tissue with negative pressure wound therapy otherwise destined for necrosis (controls) [40]. Implied is the cascade of molecular events that occur after a thermal wound characterized by edema, impaired microcirculation, and heightened capillary afterload (reperfusion injury) with secondary necrosis and additional tissue loss [40, 51, 53]. A study using a rat cremaster model supports the contention that this phenomenon is real and is remedied at the microscopic level by early use of negative pressure wound therapy by enhancing the resolution of interstitial edema and clearance of albumin from the third space [31]. Relevant to these mechanisms, animal studies have shown successful use of negative pressure wound therapy in favorably preserving tissue in the setting of myocardial infarction or brain tissue loss after closed head trauma [3, 4, 34]. Given the evidence provided in these settings, one may attribute some of the diminution in infection and improvements in tissue healing attributable to negative pressure wound therapy to avoidance of secondary necrosis. The avoidance of secondary necrosis by negative pressure wound therapy may account for the favorable outcomes with no need for flaps and no infections in the 88 high-energy wounds sustained by 77 patients in the Iraq war reported by Leininger et al. [32].
It is secondary necrosis which years before widespread adoption of negative pressure wound therapy prompted the recommendation to perform a second-look débridement of the high-energy Grade III open fracture at 36 to 48 hours [9]. This recommendation was prompted by the “knowledge that crushed tissue demarcates during a 72-hour course, and any change in wound status is an indication for surgical exploration and redébridement” [9]. Negative pressure wound therapy has been shown to minimize secondary tissue necrosis when used after a high-energy wound as indicated by basic studies and Level IV clinical studies cited above.
Conclusions
It was not convincingly clear in any of the studies we reviewed that the use of negative pressure wound therapy led to more infections, nor was it clear that negative pressure wound therapy was the primary factor leading to other complications or the end point of an amputation. Some evidence [7, 49] suggests that in the acute phase, negative pressure wound therapy dressings result in fewer infections compared with gauze dressings. There was evidence [15, 16, 30, 33, 35, 36, 47, 50] to support extended use of negative pressure wound therapy until soft tissue reconstruction can be completed without patient compromise. Six studies [6, 15, 16, 29, 35, 45] show a decreased rate of local and free flap procedures when extended negative pressure wound therapy was an adjunct in management. The importance of emergent, thorough, and aggressive débridements and irrigations was emphasized in all 13 studies we reviewed. No authors concluded greater success of a local flap versus a free flap. Surgeons with free flap success emphasized vascular anastomosis outside the zone of injury [29]. Dedmond et al. [15], in a study including pediatric patients, had no conclusions regarding acute use of negative pressure wound therapy versus gauze dressings but reported a decrease in flap procedures with extended use. Their flap rates decreased considerably with extended negative pressure wound therapy [15, 16]. Their infection rates remained comparable to historic infection rates.
Decreases in flap procedure rates and infections should be the focus of future studies. Before concluding negative pressure wound therapy has failed or succeeded, one should look at the end goal. The easiest way to define failure of negative pressure wound therapy would be by the occurrence of more infections or the need for multiple additional surgeries. However, since the overall goal is limb salvage with the least amount of patient morbidity, an increase in amputations would best define negative pressure wound therapy failure. Furthermore, if negative pressure wound therapy buys time until soft tissue coverage can be performed, more limbs may be salvaged. Even if there is no decrease in infection rates, this may be acceptable if the limbs are salvaged. If negative pressure wound therapy decreases patient morbidity through a decrease in flap procedure rates despite constant infection rates, is this success or failure? If limb salvage rates improve and patient morbidity improves, these may be the more important factors to consider with negative pressure wound therapy. We investigated infection and flap rates because these are often-asked questions and assumed to factor in the end point of an amputation. The data in the 13 studies we reviewed [6, 7, 15, 16, 29, 30, 33, 35, 36, 45, 47, 49, 50] indicate that infection rates alone are not predictive of limb salvage and patient morbidity. This systematic review could be repeated asking the question: Does negative pressure wound therapy lead to more amputations? Twelve of the 13 studies we reviewed would conclude that negative pressure wound therapy does not lead to more amputations.
Since the study by Godina [25] in 1986, the recommendation for Grade IIIB tibia fractures has been early (within 72 hours) bony stabilization and soft tissue reconstruction. This time is challenging to achieve and may be extended with negative pressure wound therapy. There are numerous factors associated with successful treatment of these complex injuries. The studies we reviewed were highly variable in methods and 12 of 13 were retrospective. More high-level studies are needed to delineate the role of negative pressure wound therapy in high-energy open tibia fractures.
Footnotes
Each author certifies that he, nor a member of his immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
This work was performed at Atlanta Medical Center, Atlanta, GA, USA.
References
- 1.Alberts KA, Loohagen G, Einarsdottir H. Open tibial fractures: faster union after unreamed nailing than external fixation. Injury. 1999;30:519–523. doi: 10.1016/S0020-1383(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 2.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576. doi: 10.1097/00000637-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Argenta LC, Morykwas MJ, Mays JJ, Thompson EA, Hammon JW, Jordan JE. Reduction of myocardial ischemia-reperfusion injury by mechanical tissue resuscitation using sub-atmospheric pressure. J Card Surg. 2010;25:247–252. doi: 10.1111/j.1540-8191.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 4.Argenta LC, Zheng Z, Bryant A, Tatter SB, Morykwas MJ. A new method for modulating traumatic brain injury with mechanical tissue resuscitation. Neurosurgery. 2012;70:1281–1295. doi: 10.1227/NEU.0b013e3182446760. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DG, Lavery LA, Diabetic Foot Study Consortium Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya T, Mehta P, Smith M, Pomahac B. Routine use of wound vacuum-assisted closure does not allow coverage delay for open tibia fractures. Plast Reconst Surg. 2008;121:1263–1266. doi: 10.1097/01.prs.0000305536.09242.a6. [DOI] [PubMed] [Google Scholar]
- 7.Blum ML, Esser M, Richardson M, Paul E, Rosenfeldt FL. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. J Orthop Trauma. 2012;26:499–505. doi: 10.1097/BOT.0b013e31824133e3. [DOI] [PubMed] [Google Scholar]
- 8.Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390–397. doi: 10.1097/01.prs.0000227675.63744.af. [DOI] [PubMed] [Google Scholar]
- 9.Brumback RJ. Wound Debridement. In: Yaremchuk MJ, Burgess AR, Brumback RJ, editors. Lower Extremity Salvage and Reconstruction: Orthopaedic and Plastic Surgical Management. Amsterdam, Netherlands: Elsevier; 1989. pp. 71–80. [Google Scholar]
- 10.Carsenti-Etesse H, Doyon F, Desplaces N, Gagey O, Tancrede C, Pradier C, Dunais B, Dellamonica P. Epidemiology of bacterial infection during management of open leg fractures. Eur J Clin Microbiol Infect Dis. 1999;18:315–323. doi: 10.1007/PL00015012. [DOI] [PubMed] [Google Scholar]
- 11.Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM, LEAP Study Group Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19:151–157. doi: 10.1097/00005131-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Caudle RJ, Stern PJ. Severe open fractures of the tibia. J Bone Joint Surg Am. 1987;69:801–807. [PubMed] [Google Scholar]
- 13.Charalambous CP, Siddique I, Zenios M, Roberts S, Samarji R, Paul A, Hirst P. Early versus delayed surgical treatment of open tibial fractures: effect on the rates of infection and need of secondary surgical procedures to promote bone union. Injury. 2005;36:656–661. doi: 10.1016/j.injury.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Chester DL, Waters R. Adverse alteration of wound flora with topical negative-pressure therapy: a case report. Br J Plast Surg. 2002;55:510–511. doi: 10.1054/bjps.2002.3890. [DOI] [PubMed] [Google Scholar]
- 15.Dedmond BT, Kortesis B, Punger K, Simpson J, Argenta J, Kulp B, Morykwas M, Webb LX. Subatmospheric pressure dressings in the temporary treatment of soft tissue injuries associated with type III open tibial shaft fractures in children. J Pediatr Orthop. 2006;26:728–732. doi: 10.1097/01.bpo.0000242434.58316.ad. [DOI] [PubMed] [Google Scholar]
- 16.Dedmond BT, Kortesis B, Punger K, Simpson J, Argenta J, Kulp B, Morykwas M, Webb LX. The use of negative-pressure wound therapy (NPWT) in the temporary treatment of soft-tissue injuries associated with high-energy open tibial shaft fractures. J Orthop Trauma. 2007;21:11–17. doi: 10.1097/BOT.0b013e31802cbc54. [DOI] [PubMed] [Google Scholar]
- 17.Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45:255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- 18.Evans D, Land L. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2001:CD001898. [DOI] [PubMed]
- 19.Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, Summitt JB, Merryman JI, Schaeffer TD, Sargent LA, Burns RP. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg. 2000;66:1136–1143. [PubMed] [Google Scholar]
- 20.Finkemeier CG, Schmidt AH, Kyle RF, Templeman DC, Varecka TF. A prospective, randomized study of intramedullary nails inserted with and without reaming for the treatment of open and closed fractures of the tibial shaft. J Orthop Trauma. 2000;14:187–193. doi: 10.1097/00005131-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Fleischmann W, Lang E, Russ M. [Treatment of infection by vacuum sealing][in German] Unfallchirurg. 1997;100:301–304. doi: 10.1007/s001130050123. [DOI] [PubMed] [Google Scholar]
- 22.Fleischmann W, Russ M, Westhauser A, Stampehl M. [Vacuum sealing as carrier system for controlled local drug administration in wound infection][in German] Unfallchirurg. 1998;101:649–654. doi: 10.1007/s001130050318. [DOI] [PubMed] [Google Scholar]
- 23.Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures][in German] Unfallchirurg. 1993;96:488–492. [PubMed] [Google Scholar]
- 24.Genecov DG, Schneider AM, Morykwas MJ, Parker D, White WL, Argenta LC. A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40:219–225. doi: 10.1097/00000637-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–292. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82:959–966. doi: 10.1302/0301-620X.82B7.10482. [DOI] [PubMed] [Google Scholar]
- 27.Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990;72:299–304. [PubMed] [Google Scholar]
- 28.Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12:1–7. doi: 10.1097/00005131-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hou Z, Irgit K, Strohecker KA, Matzko ME, Wingert NC, DeSantis JG, Smith WR. Delayed flap reconstruction with vacuum-assisted closure management of the open IIIB tibial fracture. J Trauma. 2011;71:1705–1708. doi: 10.1097/TA.0b013e31822e2823. [DOI] [PubMed] [Google Scholar]
- 30.Karanas YL, Nigriny J, Chang J. The timing of microsurgical reconstruction in lower extremity trauma. Microsurgery. 2008;28:632–634. doi: 10.1002/micr.20551. [DOI] [PubMed] [Google Scholar]
- 31.Langfitt M, Webb LX, Onwuchuruba C, Callahan M, Smith TL. Microvascular effects of subatmospheric pressure in striated muscle. J Reconstr Microsurg. 2013;29:117–123. doi: 10.1055/s-0032-1329924. [DOI] [PubMed] [Google Scholar]
- 32.Leininger BE, Rasmussen TE, Smith DL, Jenkins DH, Coppola C. Experience with wound VAC and delayed primary closure of contaminated soft tissue injuries in Iraq. J Trauma. 2006;61:1207–1211. doi: 10.1097/01.ta.0000241150.15342.da. [DOI] [PubMed] [Google Scholar]
- 33.Li RG, Ren GH, Tan XJ, Yu B, Hu JJ. Free flap transplantation combined with skin grafting and vacuum sealing drainage for repair of circumferential or sub-circumferential soft-tissue wounds of the lower leg. Med Sci Monit. 2013;19:510–517. doi: 10.12659/MSM.883963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindstedt S, Malmsjo M, Ingemansson R. Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thorac Surg. 2007;84:568–573. doi: 10.1016/j.athoracsur.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 35.Liu DS, Sofiadellis F, Ashton M, MacGill K, Webb A. Early soft tissue coverage and negative pressure wound therapy optimises patient outcomes in lower limb trauma. Injury. 2012;43:772–778. doi: 10.1016/j.injury.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Tan G, Luan F, Tang X, Kang P, Tu C, Pei F. The use of external fixation combined with vacuum sealing drainage to treat open comminuted fractures of tibia in the Wenchuan earthquake. Int Orthop. 2012;36:1441–1447. doi: 10.1007/s00264-011-1404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Jadad AR, Tugwell P. Assessing the quality of randomized controlled trials: current issues and future directions. Int J Technol Assess Health Care. 1996;12:195–208. doi: 10.1017/S0266462300009570. [DOI] [PubMed] [Google Scholar]
- 38.Morykwas MJ, Argenta LC. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc. 1997;6:279–288. [PubMed] [Google Scholar]
- 39.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Morykwas MJ, David LR, Schneider AM, Whang C, Jennings DA, Canty C, Parker D, White WL, Argenta LC. Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil. 1999;20:15–21. doi: 10.1097/00004630-199901001-00003. [DOI] [PubMed] [Google Scholar]
- 41.Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47:547–551. doi: 10.1097/00000637-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Moues CM, van den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg. 2007;60:672–681. doi: 10.1016/j.bjps.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 43.Moues CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 44.Mullner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg. 1997;50:194–199. doi: 10.1016/S0007-1226(97)91369-2. [DOI] [PubMed] [Google Scholar]
- 45.Parrett BM, Matros E, Pribaz JJ, Orgill DP. Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures. Plast Reconstr Surg. 2006;117:1315–1322. doi: 10.1097/01.prs.0000204959.18136.36. [DOI] [PubMed] [Google Scholar]
- 46.Patzakis MJ, Bains RS, Lee J, Shepherd L, Singer G, Ressler R, Harvey F, Holtom P. Prospective, randomized, double-blind study comparing single-agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. J Orthop Trauma. 2000;14:529–533. doi: 10.1097/00005131-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Rinker B, Amspacher JC, Wilson PC, Vasconez HC. Subatmospheric pressure dressing as a bridge to free tissue transfer in the treatment of open tibia fractures. Plast Reconstr Surg. 2008;121:1664–1673. doi: 10.1097/PRS.0b013e31816a8d9d. [DOI] [PubMed] [Google Scholar]
- 48.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 49.Stannard JP, Volgas DA, Stewart R, McGwin G, Jr, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23:552–557. doi: 10.1097/BOT.0b013e3181a2e2b6. [DOI] [PubMed] [Google Scholar]
- 50.Steiert AE, Gohritz A, Schreiber TC, Krettek C, Vogt PM. Delayed flap coverage of open extremity fractures after previous vacuum-assisted closure (VAC) therapy: worse or worth? J Plast Reconstr Aesthet Surg. 2009;62:675–683. doi: 10.1016/j.bjps.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 51.Tejwani NC, Webb LX, Harvey EJ, Wolinsky PR. Soft-tissue management after trauma: initial management and wound coverage. Instr Course Lect. 2011;60:15–25. [PubMed] [Google Scholar]
- 52.Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum-assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg. 2003;37:28–33. doi: 10.1080/713796078. [DOI] [PubMed] [Google Scholar]
- 53.Webb LX, Dedmond B, Schlatterer D, Laverty D. The contaminated high-energy open fracture: a protocol to prevent and treat inflammatory mediator storm-induced soft-tissue compartment syndrome (IMSICS) J Am Acad Orthop Surg. 2006;14:S82–S86. doi: 10.5435/00124635-200600001-00019. [DOI] [PubMed] [Google Scholar]
- 54.Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg. 2004;52:276–279. doi: 10.1097/01.sap.0000111861.75927.4d. [DOI] [PubMed] [Google Scholar]
- 55.Weitz-Marshall AD, Bosse MJ. Timing of closure of open fractures. J Am Acad Orthop Surg. 2002;10:379–384. doi: 10.5435/00124635-200211000-00001. [DOI] [PubMed] [Google Scholar]


