Abstract
Background
Tendon-bone healing after rotator cuff repair occurs by fibrovascular scar tissue formation, which is weaker than a normal tendon-bone insertion site. Growth factors play a role in tissue formation and have the potential to augment soft tissue healing in the perioperative period.
Questions/purposes
Our study aim was to determine if rhPDGF-BB delivery on a collagen scaffold can improve tendon-to-bone healing after supraspinatus tendon repair compared with no growth factor in rats as measured by (1) gross observations; (2) histologic analysis; and (3) biomechanical testing.
Methods
Ninety-five male Sprague-Dawley rats underwent acute repair of the supraspinatus tendon. Rats were randomized into one of five groups: control (ie, repair only), scaffold only, and three different platelet-derived growth factor (PDGF) doses on the collagen scaffold. Animals were euthanized 5 days after surgery to assess cellular proliferation and angiogenesis. The remaining animals were analyzed at 4 weeks to assess repair site integrity by gross visualization, fibrocartilage formation with safranin-O staining, and collagen fiber organization with picrosirius red staining, and to determine the biomechanical properties (ie, load-to-failure testing) of the supraspinatus tendon-bone construct.
Results
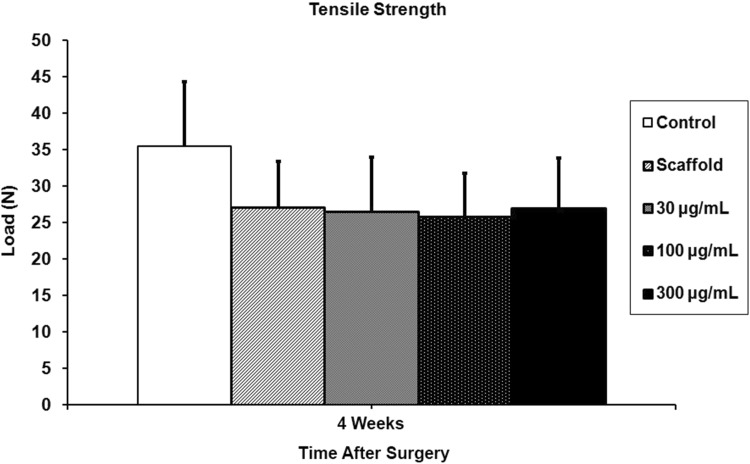
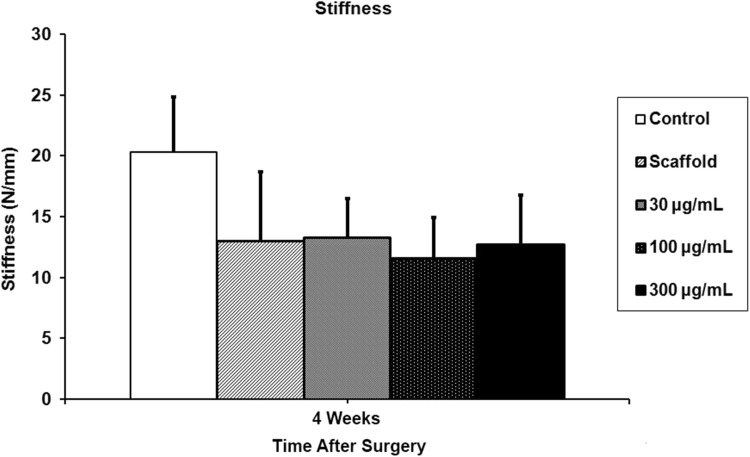
The repaired supraspinatus tendon was in continuity with the bone in all animals. At 5 days, rhPDGF-BB delivery on a scaffold demonstrated a dose-dependent response in cellular proliferation and angiogenesis compared with the control and scaffold groups. At 28 days, with the numbers available, rhPDGF-BB had no effect on increasing fibrocartilage formation or improving collagen fiber maturity at the tendon-bone insertion site compared with controls. The control group had higher tensile loads to failure and stiffness (35.5 ± 8.8 N and 20.3 ± 4.5 N/mm) than all the groups receiving the scaffold, including the PDGF groups (scaffold: 27 ± 6.4 N, p = 0.021 and 13 ± 5.7 N/mm, p = 0.01; 30 µg/mL PDGF: 26.5 ± 7.5 N, p = 0.014 and 13.3 ± 3.2 N/mm, p = 0.01; 100 µg/mL PDGF: 25.7 ± 6.1 N, p = 0.005 and 11.6 ± 3.3 N/mm, p = 0.01; 300 µg/mL PDGF: 27 ± 6.9 N, p = 0.014 and 12.7 ± 4.1 N/mm, p = 0.01).
Conclusions
rhPDGF-BB delivery on a collagen scaffold enhanced cellular proliferation and angiogenesis during the early phase of healing, but this did not result in either a more structurally organized or stronger attachment site at later stages of healing. The collagen scaffold had a detrimental effect on healing strength at 28 days, and its relatively larger size compared with the rat tendon may have caused mechanical impingement and extrinsic compression of the healing tendon. Future studies should be performed in larger animal models where healing occurs more slowly.
Clinical Relevance
Augmenting the healing environment to improve the structural integrity and to reduce the retear rate after rotator cuff repair may be realized with continued understanding and optimization of growth factor delivery systems.
Introduction
Rotator cuff repair surgery is one of the most common surgical procedures performed by orthopaedic surgeons, accounting for nearly 250,000 cases yearly in the United States alone. Although the surgery is performed routinely, prior studies have reported a relatively high rate of failure of tendon healing after repair [3, 11, 12, 14]. Previous basic science work has shown that the native supraspinatus tendon-bone insertion site is not recreated after a rotator cuff repair [4, 13, 24]. Instead, the tendon heals to the bone with fibrovascular interface scar tissue, which has inferior biomechanical properties compared with native tissue. The observation may explain the high percentage of patients who experience recurrent rotator cuff tears after repair [10]. Given the poor healing response, attention has turned to methods to augment the biologic response after rotator cuff repair to decrease the retear rate after surgery and improve patient satisfaction and clinical outcomes.
Because cytokines play an important role in cell proliferation, differentiation, chemotaxis, and matrix synthesis through autocrine or paracrine signaling, cytokines have the potential to improve rotator cuff tendon-bone healing. A recent study reported expression of transforming growth factor-β, insulin-like growth factor-1, basic fibroblast growth factor, and platelet-derived growth factor (PDGF) in a rabbit supraspinatus tendon full-thickness defect model [18]. There was sequential expression of the factors in fibroblasts, blood cells, and vascular endothelial cells, and it was found that PDGF was mildly expressed between Days 7 and 14 postsurgery. The data demonstrated that cytokines have a role in tissue formation and support the potential for growth factors to improve tendon-to-bone healing.
PDGF-BB is of particular interest because it is the one isoform that is a universal ligand for the PDGF-tyrosine kinase receptors [27]. PDGF-BB has been found to act as a mitogen and chemotactic cytokine that can potentially enhance ligament and tendon healing in small animal models [5, 16, 22]. In a rat model of rotator cuff repair, delivery of cells expressing PDGF-BB with a polyglycolic acid scaffold showed restoration of normal crimp patterning and collagen bundle alignment compared with suture repair only [28]. The studies demonstrate that improved healing with PDGF depends on dosage, timing, and delivery vehicle used.
We sought to determine whether rhPDGF-BB delivery on a collagen scaffold could improve tendon-to-bone healing after supraspinatus tendon repair in rats compared with no growth factor as measured by (1) gross observations; (2) histological analysis; and (3) biomechanical testing.
Materials and Methods
Study Design
We chose the rat rotator cuff to study tendon-to-bone healing based on previous work demonstrating anatomic similarities with the human shoulder [25]. The Institutional Animal Care and Use Committee approved the protocols for animal experimentation described in our study. Ninety-five mature, male Sprague-Dawley rats underwent unilateral detachment followed by immediate transosseous repair of the right supraspinatus tendon. In the preoperative holding area, the animal technicians randomly assigned the rats into one of five groups (n = 19 per group): control (transosseous repair), scaffold only (Type I collagen scaffold placed at the repair site), and three PDGF doses (30 µg/mol/L, 100 µg/mol/L, 300 µg/mol/L) on the collagen scaffold. The rats were euthanized at 5 days (n = 3 per group; 15 total rats) and 28 days (n = 16 per group; 80 total rats) after surgery, and tissue was allocated for either histologic analysis or biomechanical testing. The project manager (DK) randomly assigned the animals to either histological analysis or biomechanical testing at the conclusion of every operative day.
Surgical Technique
All surgical procedures were performed using a previously described protocol [8]. Briefly, a longitudinal incision was made on the anterolateral aspect of the shoulder and the deltoid was split. The supraspinatus tendon was sharply transected from its insertion site at the greater tuberosity and a Mason-Allen stitch was placed through the cut end of the tendon. The tuberosity was decorticated until bleeding was noted, and the insertion site was débrided of all soft tissue and fibrocartilage. Two bone tunnels were created at the anterior and posterior portions of the insertion site in a crossed fashion using a 22-gauge needle. The suture ends from the tendon were passed through the bone tunnels and tied over the humeral cortex to ensure reapproximation of the repaired tendon to the bone. The repair site was irrigated with saline before application of the Type I collagen scaffold with or without rhPDGF-BB solution at varying doses.
Under sterile conditions, the rhPDGF-BB was prepared on the day of surgery with 20 mmol/L sodium acetate buffer and rhPDGF-BB stock concentration of 300 µg/mol/L. For the high-dose PDGF group (300 µg/mol/mL or 6 µg PDGF), 20 µL of rhPDGF-BB stock solution was adsorbed onto the scaffold before placement and fixation onto the repair site. For the medium-dose PDGF group (100 µg/mol/mL or 2 µg PDGF), 66 µL of 20 mmol/L sodium acetate and 34 µL of 300 µg/mol/L rhPDGF-BB were mixed in a sterile Eppendorf tube and a 20-µL aliquot of the solution was prepared for adsorption onto the scaffold. For the low-dose PDGF group (30 µg/mol/mL or 0.6 µg PDGF), 90 µL of buffer was mixed with 10 µL of 300 µg/mol/L rhPDGF-BB, and then a 20-µL aliquot of the solution was adsorbed onto the collagen sponge. For the scaffold group (0 µg PDGF), 20 µL of the buffer was adsorbed onto the sponge before placement and fixation onto the repair site.
The rhPDGF-BB delivered with a Type I collagen scaffold (BioBlanket Surgical Mesh; Kensey Nash, Exton, PA, USA) was placed and fixed on the bursal surface of the repaired tendon-bone insertion site with two simple, interrupted stitches using 4-0 Ethibond suture (Ethicon, Somerville, NJ, USA) (Fig. 1). This collagen scaffold is γ-irradiated, noncrosslinked bovine collagen sponge that is FDA-approved for the reinforcement and repair of soft tissue [9]. The sponge was sized to a circular patch (5-mm diameter, 1.8-mm thickness) with a punch biopsy to cover the repaired enthesis and greater tuberosity repair site. As described, three doses of rhPDGF-BB were administered locally through the collagen scaffold (30 µg/mL, 100 µg/mL, and 300 µg/mL), representing three experimental groups. The fourth experimental group received only the Type I collagen scaffold carrier without rhPDGF-BB, whereas the control group only underwent tendon repair. Postoperatively, the rats were allowed ad libitum cage activity after surgery.
Fig. 1A–B.
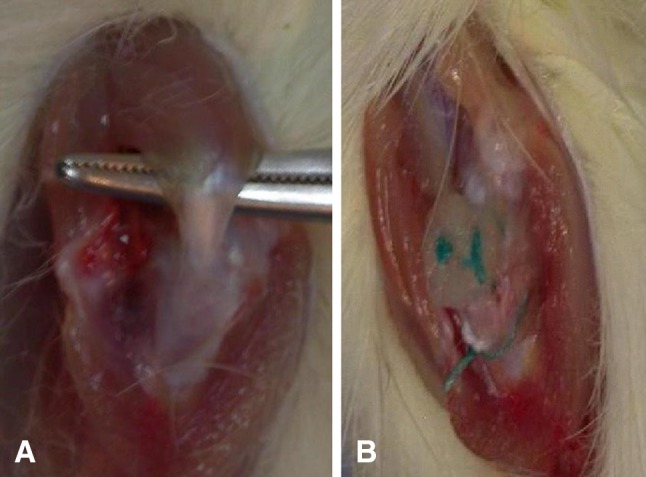
(A) Identification of the supraspinatus tendon and (B) fixation of a collagen patch on the bursal surface of the tendon-bone insertion site is shown.
Histologic Analysis
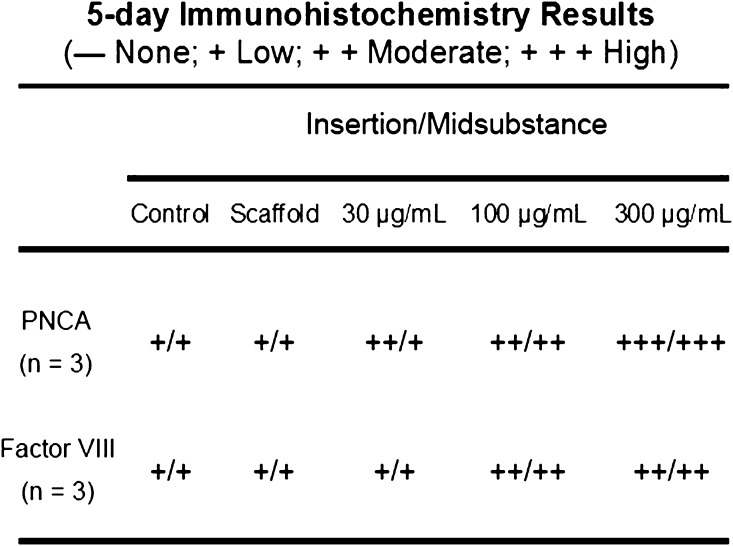
A total of 35 animals (n = 7 per group) were allocated for histologic analysis with 15 rats (n = 3 per group) euthanized at 5 days postsurgery and 20 rats (n = 4 per group) euthanized at 28 days postsurgery. Tissue specimens were fixed in 10% neutral-buffered formalin for 48 hours. After fixation, tissues were decalcified in Immunocal® (Decal, Tallman, NY, USA) for 24 hours and washed in phosphate-buffered saline solution. The tissues were subsequently dehydrated and embedded in paraffin. Five-micrometer-thick sections of the repaired supraspinatus tendon-greater tuberosity construct were cut in the coronal plane. The 5-day tissue sections, from both the tendon-bone insertion site and the tendon midsubstance, were stained with hematoxylin and eosin as well as immunohistochemical localization for proliferating cell nuclear antigen (PCNA) and Factor VIII to qualitatively assess cellular proliferation and angiogenesis, respectively. Qualitative assessments were determined by assigning a histologic grade corresponding to the number of cells per high-power field immunolocalized to the tendon-bone insertion site and tendon midsubstance as 0 cells (none), one to three cells (low), four to six cells (moderate), or more than seven cells (high). Three observers (DK, LVG, SAR) performed the immunohistochemical assessments together as a group and arrived at a consensus. The 28-day tissue sections were stained with hematoxylin and eosin, safranin-O, and picrosirius red.
Using light microscopy (Eclipse E800; Nikon, Melville, NY, USA), 28-day tissue sections stained with safranin-O were analyzed to determine the total area of new fibrocartilage formation at the supraspinatus tendon-bone interface. Digital images of the stained tissue sections were taken using a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI, USA). Image J (National Institutes of Health, Bethesda, MD, USA) software was used to determine the area of new fibrocartilage formation by manually outlining the metachromasia at the tendon-bone repair site on the safranin-O-stained slides at a total magnification of × 40 on three sequential coronal sections. Total area of metachromasia was recorded for each specimen as squared micrometers and the mean ± SD for each group was determined [19].
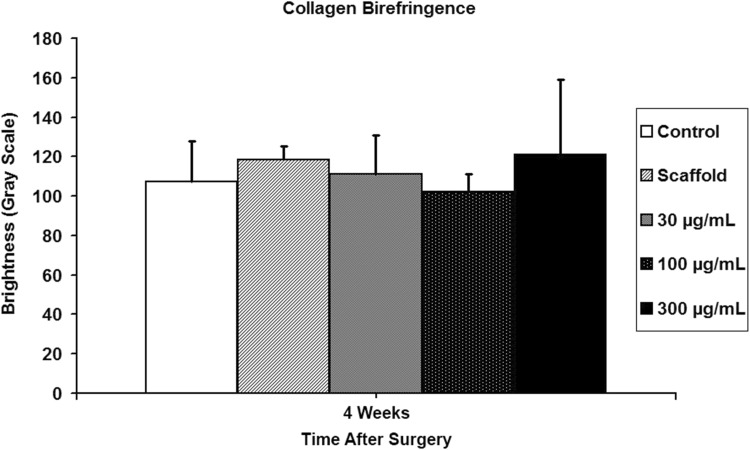
Using polarized light microscopy, 28-day tissue sections were stained with picrosirius red for semiquantitative analysis of collagen fiber continuity and organization at the tendon end adjacent to the repaired supraspinatus tendon-bone insertion site. By quantifying the collagen birefringence under polarized light, differences in collagen deposition and maturation in the healing tendon could be detected. Measurements were taken as described in work previously done in our laboratory [8].
Biomechanical Testing
Sixty animals were euthanized 28 days postsurgery and allocated for biomechanical testing (n = 12 per group). Of the 60 specimens, 58 were tested to characterize the structural properties of the supraspinatus tendon-bone constructs. Two of the specimens were unavailable for tensile testing, one because of a proximal humerus fracture noted before the 28-day observation period and another as a result of tissue damage during dissection. Each shoulder was removed from the −80º C freezer and thawed at room temperature on the day of biomechanical testing. The supraspinatus tendon attached to the humerus was carefully dissected from surrounding tissues, and the supraspinatus muscle belly was removed from the tendon. The specimen was placed in a custom-designed uniaxial testing system. The tendon was secured in a screw clamp using sandpaper and cyanoacrylate, whereas the humerus was secured in a vise grip to prevent fracture through the physis. The specimen was preloaded to 0.1 N and then loaded to failure at a rate of 14 µm/sec corresponding to approximately 0.4% strain per second. The ultimate load at failure and the failure site were recorded. Displacement was measured using a 1-µm resolution micrometer system attached to the linear stage. The linear region of the load-displacement curve was used to calculate stiffness for each specimen.
Data Analysis
The primary outcome for our investigation was biomechanical testing of tendon attachment strength with secondary outcomes of histomorphometric analysis. A power study was performed before conducting the investigation, which was based on prior work evaluating rotator cuff tendon healing in rats [8]. Cohen et al. [8] reported an average stiffness of 14.9 N/mm at 28 days after repair with a SD of 5.0 N/mm for rats undergoing transosseous repair of the supraspinatus tendon. In our study, a 40% increase in stiffness would be considered clinically important, and so we set this as the difference we wished to be able to detect. Using these estimations, a power of 0.80 is achieved using 12 specimens per group with alpha set at 0.05 for biomechanical testing. The power calculation was performed using SigmaStat® software (Systat Software, San Jose, CA, USA), and the numbers were consistent with other studies using biomechanical testing of rat soft tissues.
Statistical analysis of total area of metachromasia, collagen birefringence, and structural properties was performed using two-way analysis of variance with appropriate post hoc testing with the Holm-Sidak method. Significance was set at p < 0.05.
Results
Gross Observations
At 5 days, there was poorly organized tissue between the tendon-bone insertion site in all groups with the scaffold groups, including the PDGF-treated groups, exhibiting white, fibrous tissue at the repair site compared with the control group. At 28 days, the tendon-bone interface tissue in the scaffold group and three PDGF groups exhibited more fibrous tissue compared with the control group, making it difficult to dissect out the supraspinatus tendon and the enthesis from the biceps tendon and infraspinatus tendon without the use of a dissecting microscope. The scaffold was not visible and appeared well incorporated with the native tissue. No tendons in any group had failed to heal because the repaired tendon was in continuity with the bone in all animals.
Histologic Analysis
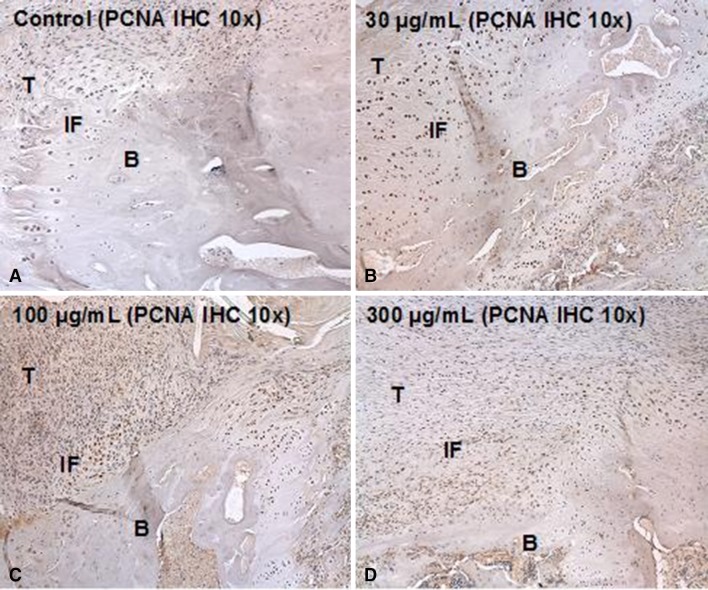
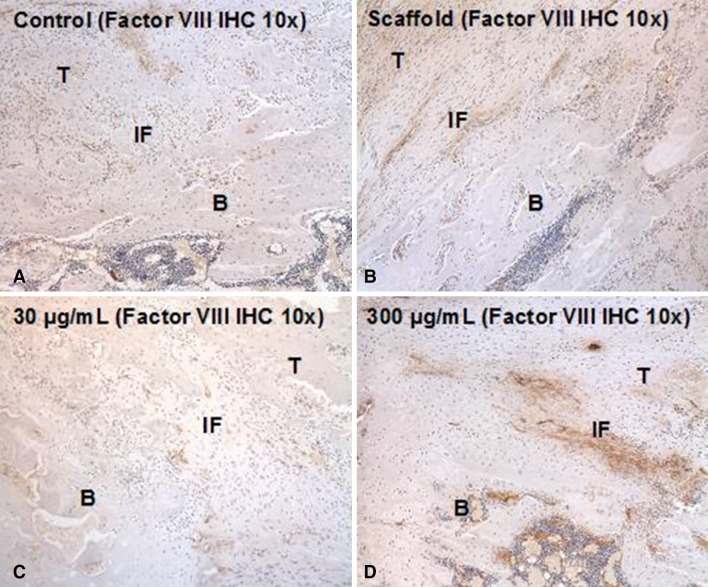
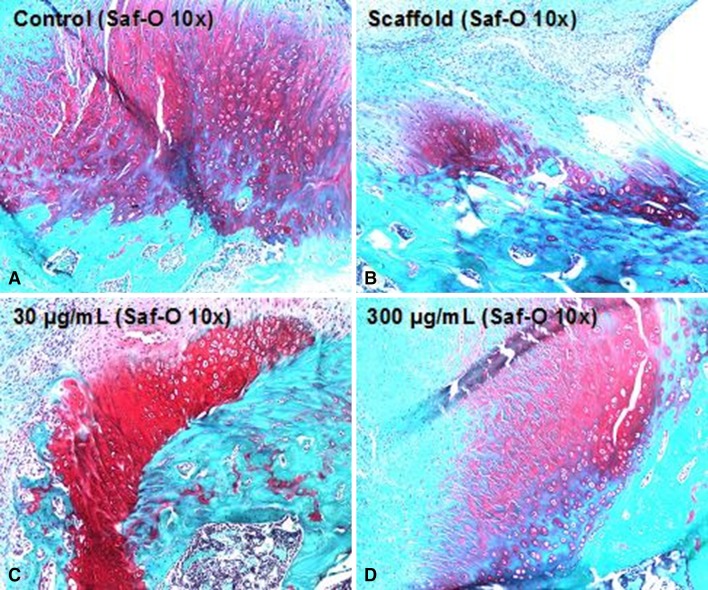
At 5 days postsurgery there was a dose-dependent response in both cellular proliferation and angiogenesis at both the tendon-bone insertion site and tendon midsubstance in groups receiving rhPDGF-BB on a Type I collagen scaffold (Fig. 2). The 300-µg/mol/L PDGF group had both the greatest number of actively dividing cells localized with PCNA immunohistochemistry and more endothelial cells localized with Factor VIII immunohistochemistry compared with all other experimental groups (Figs. 2, 3, 4). At 28 days postsurgery, rhPDGF-BB delivery on a collagen scaffold did not increase the area of glycosaminoglycan staining at the tendon-bone interface. Although the 300-µg/mL PDGF group had a mean total area of metachromasia of 147,786 ± 138,704 µm2 compared with 84,649 ± 76,500 µm2 for the scaffold group (p = 0.58), the large variability among specimens within each group resulted in no differences with the numbers available (Fig. 5). Some of the specimens in the high-dose PDGF group (ie, 300 µg/mL) contained large areas of newly formed fibrocartilage at the healing attachment site (Fig. 6). At 28 days postsurgery, there was no difference in collagen birefringence between the control group (107 ± 21 gray-scale units [GSU]) and all groups receiving the scaffold, including the PDGF groups (scaffold: 118 ± 7 GSU, p = 0.77; 30 µg/mL PDGF: 111 ± 19 GSU, p = 0.58; 100 µg/mL PDGF: 104 ± 9 GSU, p = 0.67; 300 µg/mL PDGF: 121 ± 36 GSU, p = 0.86) (Fig. 7).
Fig. 2.
At early time points, a dose-dependent increase in both cellular proliferation and angiogenesis at the enthesis and tendon midsubstance occurred with the delivery of rhPDGF-BB on a Type I collagen scaffold.
Fig. 3A–D.
Actively dividing cells were localized with PCNA immunohistochemistry (IHC) at both the tendon-bone insertion site and tendon midsubstance to assess cellular proliferation 5 days after surgery. Representative histologic sections (total magnification, × 100) of the (A) repair, (B) low-dose PDGF, (C) medium-dose PDGF, and (D) high-dose PDGF groups are provided. T = tendon; B = bone; IF = interface.
Fig. 4A–D.
Endothelial cells localized with Factor VIII immunohistochemistry (IHC) at both the tendon-bone insertion site and tendon midsubstance were used to assess angiogenesis 5 days postsurgery. Representative histologic sections (total magnification, × 100) of the (A) repair, (B) scaffold, (C) low-dose PDGF, and (D) high-dose PDGF groups. T = tendon; B = bone; IF = interface.
Fig. 5.
At 28 days postsurgery, rhPDGF-BB delivery on a collagen scaffold did not improve the area of new fibrocartilage formation at the enthesis compared with the repair group.
Fig. 6A–D.
Representative images (total magnification, × 100) of the (A) repair, (B) scaffold, (C) low-dose PDGF, and (D) high-dose PDGF groups stained with safranin-O (Saf-O) to assess fibrocartilage formation at the enthesis.
Fig. 7.
At 28 days postsurgery, rhPDGF-BB delivery on a collagen scaffold did not improve collagen fiber continuity and orientation at the enthesis compared with the repair and scaffold-only groups.
Biomechanical Testing
The control group, which underwent detachment and transosseous repair, had higher load-to-failure and stiffness (35.5 ± 8.8 N and 20.3 ± 4.5 N/mm) than all groups receiving the scaffold, including those treated with rhPDGF-BB (scaffold: 27 ± 6.4 N, p = 0.021 and 13 ± 5.7 N/mm, p = 0.01; 30 µg/mL PDGF: 26.5 ± 7.5 N, p = 0.014 and 13.3 ± 3.2 N/mm, p = 0.01; 100 µg/mL PDGF: 25.7 ± 6.1 N, p = 0.005 and 11.6 ± 3.3 N/mm, p = 0.01; 300 µg/mL PDGF: 27 ± 6.9 N, p = 0.014 and 12.7 ± 4.1 N/mm, p = 0.01) (Figs. 8, 9). The ultimate load-to-failure for all groups receiving the scaffold was 24% lower compared with the control group, and stiffness was 34% lower compared with the control group. Fifty of the 58 specimens failed at the tendon-bone insertion site during biomechanical testing, and the remaining eight specimens failed at the supraspinatus tendon midsubstance. Only the specimens that failed at the enthesis are reported.
Fig. 8.
The control group had a significantly higher ultimate load-to-failure than all groups receiving the scaffold, including those treated with rhPDGF-BB.
Fig. 9.
Stiffness of the supraspinatus tendon-bone constructs was significantly reduced in all groups receiving the scaffold compared with controls.
Discussion
Biologic augmentation of the healing tendon-bone insertion site using growth factors holds promise for improving the structural integrity and reducing the retear rate after rotator cuff repair. In our rat rotator cuff repair model, we found that rhPDGF-BB delivery on a Type I collagen scaffold demonstrated a dose-dependent response in cellular proliferation and angiogenesis compared with control (ie, repair only) and scaffold (ie, Type I collagen sponge only) groups by 5 days postsurgery. However, by 28 days postsurgery, rhPDGF-BB had no effect on the area of fibrocartilage, collagen fiber maturity and orientation at the tendon-bone interface, or biomechanical properties compared with the control group (ie, repair only). In fact, the scaffold may have had a detrimental effect on healing strength at 28 days. One plausible explanation for the superior attachment strength in the control group is that the size of the scaffold (5-mm diameter, 1.8-mm thickness) may have been too large relative to the size of the rat supraspinatus tendon (3.06 ± 0.6 mm) and thus acted as a barrier to tendon-bone healing. However, using a smaller patch was not surgically feasible, and thus the relatively large scaffold may have caused mechanical impingement and extrinsic compression of the healing tendon [26].
There are several limitations to this study. First, we used an acute injury and repair model to study the healing of rotator cuff tendons rather than in a chronic, degenerative state, which is typically seen in the human condition. The acute, traumatic environment represents an idealized scenario for determining whether biologic augmentation of a rotator cuff repair is possible, and this was our rationale for delivering PDGF in this setting. The growth factor may not augment healing in an acute repair model as a result of the favorable healing potential in this setting; rather, PDGF may have a more positive effect in an environment where the healing response is impaired (ie, chronic condition). Second, application of the scaffold as an onlay graft (ie, scaffold over repair site) rather than as an interpositional graft (ie, scaffold between tendon and bone at repair site) may not have allowed for optimal growth factor delivery. The scaffold was used as an onlay graft because this is how the scaffold technology has been designed, approved by the FDA, and used in clinical situations. Third, the immunohistological assays used at early time points to assess cell proliferation and angiogenesis were qualitative in nature. This may have led to possible assessor bias because we did not perform any intra- or interobserver reliability tests of these measurements. A fourth limitation is that the histomorphometric assays at later time points (ie, fibrocartilage formation and collagen organization) were likely to be underpowered to detect meaningful differences with such a small sample size (n = 4 per group) and the wide variability between specimens. One way to address this issue is to allocate additional animals for histomorphometric analysis. Another limitation of our study is the unknown release kinetics of rhPDGF-BB from the scaffold. Elution studies were not performed to demonstrate how long the growth factor would be present during the healing process. However, in vitro studies on the effect of PDGF on repair of meniscal defects have demonstrated that diffusion of PDGF from an absorbable collagen sponge was rapid with approximately 50% being released from the collagen sponge within the first hour [2]. After 5 days of incubation, 8% of the PDGF was present in the meniscus, 11% in the collagen sponge, and 62% in the culture medium. In light of these data, it is possible that there was an inadequate concentration of rhPDGF-BB to augment healing in our model.
In vivo effects of PDGF-BB on tendon and ligament healing have been mixed, most likely because various doses of PDGF have been administered with different delivery vehicles to augment healing in either intraarticular or extraarticular environments. After medial collateral ligament rupture in rabbits, 20 µg of PDGF-BB with fibrin sealant delivered on the ligamentous surface demonstrated greater cellularity and vascularity, increased cross-sectional area, and greater ultimate load and energy absorbed to failure at 6 weeks postsurgery compared with the fibrin sealant group [16]. In a rat patellar tendon-defect model, a single bolus injection of 1 µg PDGF into the wound site led to greater cellular proliferation at 2 weeks for early supplementation and increased peak load, cross-sectional area, and pyridinoline content for later supplementation [5]. In contrast, in a rabbit model of partial anterior cruciate ligament (ACL) laceration treated with 20 µg of PDGF in fibrin sealant applied to the injured ligament at the time of surgery, there was significantly decreased maximum load and stiffness of the femur-ACL-tibia complex, by 56% and 41%, respectively, compared with no treatment to the injured ACL [20]. After ACL reconstruction with flexor tendon autograft in sheep, 60 µg of PDGF mixed with poly(D, L-lactide), coated on sutures at the time of surgery, increased failure load at early time points compared with the control group [31]. At 12 and 24 weeks postsurgery, no difference in failure load was seen between groups. The absence of an effect of PDGF on healing in these intraarticular models may be attributable, at least in part, to the presence of synovial fluid and inadequate vascularity.
The location of the patch may impede healing from the bursal side by preventing infiltration of fibroblasts and small vessels from the subacromial bursa to the repair site [29]. Recent work in a large animal model (sheep) of rotator cuff repair demonstrated improved healing based on histology and ultimate load to failure when the scaffold device was used as an interpositional graft rather than as an overlay graft to deliver PDGF [15]. The finding supports our claim that bursal patch placement may have been detrimental to healing in the rat shoulder, and a larger animal model may be preferable where the healing environment is slower, longer, and similar to the human condition.
The scaffold device in our study was observed to incorporate favorably with the host tissue by 4 weeks. However, the resulting host repair tissue deposition and remodeling did not lead to strengthened repair with or without rhPDGF-BB delivery. Only two prospective, randomized controlled studies have been performed using any of the FDA-approved scaffold devices with variable healing rates being observed depending on the scaffold material. The first clinical trial investigating repair of large and massive chronic rotator cuff tears with porcine small intestinal submucosa (Restore Orthobiologic Implant; DePuy, Warsaw, IN, USA) augmentation did not improve the rate of tendon-bone healing compared with the nonaugmented repair group (27% [four of 15] versus 60% [nine of 15]) [17]. Rather, 20% (three of 15) of the patients in the scaffold augmentation group had a severe, sterile, postoperative inflammatory reaction. A more recent clinical trial that investigated the use of human dermis (GraftJacket; Wright Medical, Arlington, TX, USA) for augmentation of chronic two-tendon tears found that patients who received an augmented repair had better healing rates at 2-year followup than those who underwent repair only (85% [17 of 20] versus 40% [six of 15]) [1]. No adverse events related to the human dermis scaffold were observed. Despite favorable graft-host tissue incorporation, scaffold technology has not been optimized to consistently improve healing and function after rotator cuff repair, and additional basic science and clinical trials are needed to better understand the safety, mechanism of action, efficacy, and surgical indications for the numerous commercial scaffold devices currently FDA-approved for rotator cuff repair.
Another possible reason for lack of improvement in structural properties may be insufficient doses of rhPDGF-BB and the inappropriate temporal availability of this growth factor. The highest dose in our study was three times less than the dose previously reported in studies investigating the effects of PDGF-BB on soft tissue healing [16, 20]. A recent study investigating growth factor expression profile after rotator cuff surgery in a rabbit model noted that PDGF-BB expression was most prominent during Days 7 through 14 postsurgery [18]. If an insufficient dose of rhPDGF-BB was administered too early in the healing process, then the growth factor may have been unavailable at later time points to adequately promote matrix synthesis to augment the repair.
Our study demonstrates the two major technical challenges of growth factor delivery in in vivo models: (1) how to temporally and spatially apply different growth factors for best effect; and (2) how to deliver therapeutic molecules to target cells in a specific and sustained manner. Two strategies that hold promise for improving tendon-bone healing include gene transfer of PDGF-BB into tenocytes that can then be delivered to the repair site [23, 30] and the design of new biodegradable material carriers for local and sustained delivery of cytokines at the healing enthesis [6, 7, 21]. Further studies, in larger animal models, where the healing environment is slower, are needed to optimize the delivery of this potentially useful growth factor to possibly augment structural healing and reduce the retear rate after rotator cuff repair.
Acknowledgments
We acknowledge Stephen Goldman PhD, for providing the collagen sponge material (ie, Type I collagen scaffold) from Kensey Nash, Inc (Exton, PA, USA). We also thank Ran Stark MD, for his assistance with the animal surgeries.
Footnotes
The institution of one of the authors (SAR) has received less than USD 10,000, in any one year, funding from BioMimetic Therapeutics, Inc (Franklin, TN, USA). One or more of the authors is a paid consultant for Biomet (Warsaw, IN, USA) (LVG) and Smith & Nephew (Memphis, TN, USA) (SAR), has stock or stock options in Cayenne Medical (Scottsdale, AZ, USA) (SAR), and is on the medical/orthopaedic publications editorial/governing board of the HSS Journal (LVG).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF, Laboratory for Soft Tissue Research Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of mensical defects in vitro. In Vitro Cell Dev Biol Anim. 2005;41:305–310. doi: 10.1290/0503018.1. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/S1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 5.Chan BP, Fu SC, Qin L, Rolf C, Chan KM. Supplementation-time dependence of growth factors in promoting tendon healing. Clin Orthop Relat Res. 2006;448:240–247. doi: 10.1097/01.blo.0000205875.97468.e4. [DOI] [PubMed] [Google Scholar]
- 6.Chen RR, Silva EA, Yuen WW, Brock AA, Fischback C, Lin AS, Guldberg RE, Mooney DJ. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21:3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 7.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 8.Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362–369. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- 9.Coons DA, Barber AF. Tendon graft substitutes—rotator cuff patches. Sports Med Arthrosc. 2006;14:185–190. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;304:43–53. [PubMed] [Google Scholar]
- 12.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair: a preliminary study. J Bone Joint Surg Am. 1999;81:1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Harryman DT, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA. Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed] [Google Scholar]
- 15.Hee CK, Dines JS, Dines DM, Roden CM, Wisner-Lynch LA, Turner AS, McGilvray KC, Lyons AS, Puttlitz CM, Santoni BG. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med. 2011;39:1630–1639. doi: 10.1177/0363546511404942. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, Schmidt CC. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. Am J Sports Med. 1998;26:549–554. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- 17.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears: a randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Koike Y, Trudel G, Curran D, Uhthoff HK. Delay of supraspinatus repair by up to 12 weeks does not impair enthesis formation: a quantitative histologic study in rabbits. J Orthop Res. 2006;24:202–210. doi: 10.1002/jor.20031. [DOI] [PubMed] [Google Scholar]
- 20.Kong HJ, Kim ES, Huang YC, Mooney DJ. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res. 2008;25:1230–1238. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 21.Kondo E, Yasuda K, Yamanaka M, Minami A, Tohyama H. Effects of administration of exogenous growth factors on biomechanical properties of the elongation-type anterior cruciate ligament injury with partial laceration. Am J Sports Med. 2005;33:188–196. doi: 10.1177/0363546504266979. [DOI] [PubMed] [Google Scholar]
- 22.Letson AK, Dahners LE. The effect of combinations of growth factors on ligament healing. Clin Orthop Relat Res. 1994;308:207–212. [PubMed] [Google Scholar]
- 23.Nakamura N, Shino K, Natsuume T, Horibe S, Matsumoto N, Kaneda Y, Ochi T. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998;5:1165–1170. doi: 10.1038/sj.gt.3300712. [DOI] [PubMed] [Google Scholar]
- 24.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon healing using a mixture of osteoinductive growth factors: an experimental study in sheep. J Bone Joint Surg Am. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 25.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/S1058-2746(96)80070-X. [DOI] [PubMed] [Google Scholar]
- 26.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, 3rd, Carpenter JE. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 27.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Uggen JC, Dines J, Uggen CW, Mason JS, Razzano P, Dines D, Grande DA. Tendon gene therapy modulates the local repair environment in the shoulder. J Am Osteopath Assoc. 2005;105:20–21. [PubMed] [Google Scholar]
- 29.Uhthoff HK, Sano H, Trudel G, Ishii H. Early reactions after reimplantation of the tendon of supraspinatus into bone: a study in rabbits. J Bone Joint Surg Br. 2000;82:1072–1076. doi: 10.1302/0301-620X.82B7.9986. [DOI] [PubMed] [Google Scholar]
- 30.Wang XT, Liu PY, Tang JB. Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. J Hand Surg. 2004;29:884–890. doi: 10.1016/j.jhsa.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Weiler A, Förster C, Hunt P, Falk R, Jung T, Unterhauser FN, Bergmann V, Schmidmaier G, Haas NP. The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:881–891. doi: 10.1177/0363546503261711. [DOI] [PubMed] [Google Scholar]