Abstract
Objectives
Weight loss is common in advanced dementia, but regulators and clinicians are uncertain how often it is treatable. Study objectives were to describe: 1) quality of care for feeding problems in advanced dementia, and 2) probability and predictors of weight loss and mortality.
Design
Prospective cohort
Setting
24 nursing homes
Participants
256 residents with advanced dementia and feeding problems, and family surrogates
Measurements
Family reported on quality of feeding care at enrollment and 3 months. Chart reviews at enrollment, 3, 6 and 9 months provided data on feeding problems, treatments, weight loss of >5% in 30 days or >10% in 6 months, and mortality. Organizational variables were obtained from administrator surveys and publically reported data.
Results
Residents with advanced dementia and feeding problems had an average age of 85; 80% had chewing and swallowing problems, 11% weight loss and 48% poor intake. Family reported feeding assistance of moderate quality; 23% felt the resident received less assistance than needed. Mortality risk was significant; 8% died within 3 months, 17% within 6 months and 27% within 9 months. Residents with advanced dementia and stable weight had a 5.4% rate of significant weight loss and a 2.1% risk of death over 3 months. Residents with advanced dementia and weight loss had a 38.9% chance of stabilizing weight over the next 3 months, but also had a 19.2% chance of dying. Weight loss was the only independent predictor of death.
Conclusion
Weight loss is a predictor of death in advanced dementia. Treatments can often stabilize weight, but weight loss should be used to trigger discussion of goals of care and treatment options.
Keywords: dementia, nutrition, nursing home
Background
Dementia is a syndrome caused by multiple diseases, resulting in deficits in memory and other cognitive domains. Dementia has profound effects on health and quality of life for over 5 million Americans and 36 million people worldwide, and creates substantial burdens for their caregivers.1 This incurable condition has a variable life expectancy, progressing to death over 3–12 years.2,3,4 In its later stages dementia is characterized by complex problems with gait, continence, feeding and self-care, as well as loss of communication skills and inability to recognize friends and family.5 The 6-month mortality risk in advanced dementia is 28%; superimposed illnesses, including infection and nutritional decline, are proximate causes of death.6,7,8
In early dementia taste and smell dysfunction, medications or major depression may trigger anorexia and weight loss, but prolonged, irreversible feeding problems are uncommon.9 Advanced dementia results in sparse speech, incontinence, and dependency for all activities of daily living, including need for eating assistance.10,11 Feeding problems are nearly universal in advanced dementia –CASCADE, a prospective study of nursing home residents with advanced dementia reported 86% of residents had problems with food intake or swallowing.12 Feeding problems in advanced dementia have varied mechanisms – apraxia or visuospatial dysfunction affect mechanics of eating, distracting behaviors interfere with intake, swallowing is prolonged and poorly coordinated, and dysphagia causes choking, or results in food avoidance. Volicer et al studied feeding problems in 71 patients with advanced Alzheimer’s disease and reported that 25% required set up or cueing, 18% required physical assistance, 25% refused or spit out food, and 32% had dysphagia.13
Nursing home care includes supportive feeding, and nutritional decline in this population is generally assumed to be treatable. When feeding problems persist the resident can develop weight loss, which in turn may trigger family distress, consideration of tube feeding, and external review of quality of care. The rate of weight loss is tracked as a CMS nursing home quality measure, promoting efforts to stabilize or reverse this problem.14 However, feeding problems and weight loss are also common during the terminal phase of many incurable diseases, including advanced dementia. In a study of 792 persons who died in long-term care facilities, 72% of residents had reduced intake of food and water in their final month of life.15
Clinicians and regulators lack data on the natural history of feeding problems in advanced dementia, and therefore remain uncertain whether weight loss should be regarded as reversible, or as a predictor of terminal decline. Using prospective data for a cohort of nursing home residents with advanced dementia and feeding problems, we designed a study with the following aims: 1) to describe the quality of care for feeding problems in advanced dementia from the perspective of family caregivers, 2) to describe the probability and predictors of persistent weight loss or mortality.
Methods
The study enrolled a prospective cohort of nursing home residents with advanced dementia and feeding problems. Subjects were enrolled as part of a cluster randomized trial of a decision aid intervention on feeding options in dementia care; detailed methods and results of the parent study have been published.16,17 Surrogates in the intervention sites received and reviewed the decision aid, compared to usual information and decision-making in the control sites. All outcomes were collected and analyzed at the level of the individual, and data collection was identical for intervention and control participants.
Setting and Participants
The study was conducted in 24 North Carolina nursing homes. Nursing home residents with advanced dementia and feeding problems were enrolled with their surrogate decision-makers from August 2007 through July 2009. To be eligible, residents had to be aged ≥65 and have advanced dementia. A diagnosis of dementia was confirmed by chart review, and dementia severity staged 6–7 on the Global Deterioration Scale (GDS) by the nurse who most often provided care.5,18 This stage of dementia is characterized by dependency for most activities of daily living, limited language function, and loss of recognition of familiar persons. Eligibility also required chart evidence of at least one feeding problem, defined as poor intake, dysphagia or weight loss. Poor intake meant taking < 75% of meals over the past 14 days. Dysphagia was defined as chart evidence for difficulty swallowing, choking on food or liquid, dehydration, dysphagia, or aspiration. Weight loss was defined as >5% of body weight in 30 days or >10% in 6 months preceding the chart abstraction, a definition derived from variables of the Minimum Data Set (MDS). Residents were ineligible if they had a feeding tube, a “Do Not Tube Feed” order, were enrolled in hospice, or had weight loss associated with diuresis.
Eligible surrogates were identified as the resident’s guardian, Health Care Power of Attorney, or the primary family contact and most likely to be involved in clinical decision-making. Surrogates who responded to an informational letter gave informed consent for themselves and the resident.
Data Collection
Trained research assistants completed in-person interviews with surrogates at enrollment, and telephone interviews at 3 months. Using a structured chart abstraction tool, they reviewed nursing home records at enrollment, 3, 6 and 9 months. When a resident died, the next chart review was completed on schedule but the next surrogate interview used modified wording and permitted more flexible scheduling to acknowledge death and bereavement.
Measures
Data to meet the first aim came from surrogate interviews supplemented by information on actual feeding approaches from chart abstraction. During baseline interviews, surrogates provided data on their own demographics and their relationship to the resident. At baseline and 3-month interviews they answered a series of structured questions about their perceptions of the quality of care for feeding problems. Using Likert-scaled responses from 1=always to 4=never, surrogates responded to 5 items which rated the quality of feeding assistance provided by staff. They reported whether or not a variety of feeding treatments were used, and whether family, staff or both provided assistance with eating for the resident, and whether the amount of assistance was “the right amount, more assistance than needed, or less assistance than needed.” Descriptive data on use of feeding treatments came from review of medical orders and nursing, physician and dietary notes for information on hospice, new feeding tubes, new orders not to use feeding tubes, and new oral feeding treatments including diet modification, dysphagia diets, high calorie supplements, specialized staff assistance, specialized utensils, and positioning.
To meet the second aim, research assistants collected data on the outcomes of weight loss and mortality from nursing home charts at 3, 6 and 9 months. Weight loss was defined as >5% of body weight in 30 days or >10% in 6 months. Evidence for weight loss was collected from the corresponding section of the Minimum Data Set (MDS), or from weight records recorded by nursing home staff for the interval of interest. To define the timing of weight loss during follow-up we used the date of MDS completion. If the MDS date was missing but the weight loss outcome was recorded, we replaced the MDS date with the final date of the follow-up interval. In 3 cases the chart review interval was later than the most recent MDS, and therefore the final date of the follow-up interval was used rather than the MDS date. If the resident had died the date of death was also recorded. This method resulted in complete data on vital status for all residents.
Organizational level and individual level variables were then considered as potential predictors of the outcomes of death or persistent weight loss. Organizational level variables were obtained at baseline on a written administrator survey, and from the nursing home’s quality data on the Medicare Nursing Home Compare website.19 Organizational variables included nursing home staffing with a nurse practitioner or physician assistant (full-time, part-time, or none), business model (for-profit, not-for-profit), ownership (corporate, hospital, independent), number of beds, number of Medicare-certified beds, presence or absence of special services (dementia unit, feeding aides, or assisted dining program), percent of residents who were African American, rates of tube feeding, weight loss and Do-Not-Resuscitate (DNR) orders, number of licensed staff hours per resident, certified nursing assistant (CNA) staff hours per resident, deficiencies, and overall quality ratings. Individual-level variables on residents came from nursing home chart review at enrollment. These variables included resident age, gender and race, functional status using the MDS Activities of Daily Living scale (range 0–28, higher scores indicate greater dependence), and Mitchell’s MDS prognostic risk score for advanced dementia (range 0–19, higher scores indicate increased 6-month mortality risk).20 Additional individual-level variables included presence or absence of swallowing problems, weight loss, or poor intake at time of enrollment, and documentation of aspiration, pneumonia or dehydration events in the 3 months preceding enrollment.
Statistical Analysis
Simple frequency distributions were used to describe the characteristics of residents with advanced dementia, and their surrogates. Surrogates’ responses to interview questions were summarized with categorical response options. Responses to Likert-scaled questions were reported as the average of all responses; missing values were imputed to the mean response.
Cumulative frequency and percentage were used to summarize the likelihood of persistent weight loss and death at 3, 6 and 9 months. Exact time of patient’s survival and transition between stable weight and weight loss were modeled by Markov renewal process, which uses Cox proportional hazards regression for a disease progression with multiple states.21 To define other possible predictors of death and of future weight loss, nursing home organizational level variables and resident individual level variables were examined in bivariate comparisons for association with each of these up to 9 months. Variables associated with each outcome at the p<0.10 level in bivariate comparisons were then included in multivariate models to identify independent predictors of each outcome. This analysis was further adjusted for the presence or absence of weight loss at the time of study enrollment, since pre-existing weight loss is a strong predictor of future weight loss and of death.
Human Subjects
All research procedures were approved by the Institutional Review Board (IRB) of the University of North Carolina School of Medicine, and for individual research sites by the IRBs of Alamance Regional Hospital and the East Carolina University Brody School of Medicine. A Data Safety Monitoring Committee reviewed preliminary data, adverse events and mortality every 6 months throughout enrollment and data collection.
Results
During the study period, research assistants screened 972 residents with dementia and identified 425 who were eligible. The most common reasons for ineligibility included: not having advanced dementia (n=241), death (n=78) or moving (n=63) prior to mailing of the invitation letter, or no qualifying surrogate (n=45).17 Of 425 eligible resident-surrogate dyads, 256 were enrolled (60%). Surrogates who did not consent to participate were similar to those who enrolled. Baseline data was complete for all enrolled residents and surrogates, and for 99% of residents and surrogates at 3 months and 86% at 9 months.
Nursing home residents with advanced dementia and feeding problems had an average age of 85, 77% were women, 70% were white and 29% African American. (Table 1) Eighty percent had problems with swallowing at baseline, 11% had weight loss and 48% had chart documentation of poor intake. Swallowing problems were more common for residents with dementia severity GDS 7 vs. GDS 6 (88% vs 77%, p=0.03), but rate of weight loss and poor intake were similar. Using baseline MDS data, residents’ average prognostic score of 4.29 predicted a 23% mortality risk within 6 months.20 Most surrogates were daughters (45%) or sons (24%) of the resident.
Table 1.
Characteristics of Residents with Advanced Dementia and Their Surrogates
| Characteristic | Cohort (n=256) |
|---|---|
| Age | 85.3 |
| Gender | |
| Female | 77% |
| Marital Status | |
| Never Married | 6% |
| Married | 18% |
| Widowed | 67% |
| Separated / Divorced | 9% |
| Race | |
| White | 70% |
| African American | 29% |
| American Indian | 1% |
| Hispanic / Latino | 1% |
| Feeding problems | |
| Difficulty with chewing/swallowing | 80% |
| Weight loss | 11% |
| Poor intake | 48% |
| MDS Prognostic score* (0–19) | 4.29 |
| Surrogate’s Relationship to Resident | |
| Spouse | 8% |
| Daughter | 45% |
| Son | 24% |
| Grandchildren | 4% |
| Nephews & Nieces | 8% |
| Siblings | 5% |
| Other Family | 3% |
| Non-Family | 2% |
Minimum Dataset Prognostic score ranges from 0–19, with high scores indicating increased 12-month mortality risk
Surrogates’ Perceptions of the Quality of Care for Feeding Problems
Family surrogates reported their observations of the quality of care for feeding problems during baseline interviews, and again at 3 months. Baseline responses did not differ significantly from 3 month responses, so only 3 month data are reported. (Table 2) Surrogates believed that 73% of residents with advanced dementia and feeding problems had difficulty taking adequate food and water. Most surrogates felt very or somewhat involved in decisions about the resident’s nutrition – only 20% reported no involvement. Personal feeding assistance, high calorie drinks and modified diets were the most common interventions, reported for over 70% of residents; only one resident had a feeding tube placed in 3 months’ follow-up. Using a 1–4 scale for which lower ratings indicate better quality, family rated the quality of feeding assistance as moderate; they gave the best rating for food consistency (1.44) and the worst rating for the choice of foods the resident could enjoy (2.17). According to surrogates, direct assistance came primarily from nursing home staff for 59% of residents, primarily from family and friends for 8%, and from both sources for 12%; however, 21% of residents had no direct feeding assistance. Asked to rate the amount of assistance provided, 23% of family caregivers felt the resident with dementia was getting less feeding assistance than needed.
Table 2.
Quality of Care for Feeding Problems at 3 Months
| Cohort (n=256) | |
|---|---|
| Resident has difficulty taking enough food or water | 73% |
| Cumulative number of new feeding tubes 3 months | 1 |
| Cumulative number of new feeding tubes 6 months | 2 |
| Cumulative number of new feeding tubes 9 months | 4 |
| Hospice enrollment at 3 months | 4% |
| Treatments used at 3 months | |
| Assistance with eating by mouth | 77% |
| High calorie drinks | 75% |
| Special diet | 71% |
| Special positioning | 42% |
| Utensils that are easier to hold | 11% |
| Who assists with eating/drinking at 3 months | |
| Mostly NH staff | 59% |
| Mostly family or friend | 8% |
| Equally NH staff and family/friend | 12% |
| No one | 21% |
| Surrogate involvement in feeding treatment decisions at 3 months | |
| Not at all | 20% |
| Somewhat involved | 40% |
| Very involved | 39% |
| Don’t know or refused | 1% |
| Quality of assisted feeding at 3 months (rated 1=always to 4=never) | |
| Staff responds in quick manner | 1.79 |
| Staff responds in a caring manner | 1.71 |
| Staff responds in an effective manner | 1.81 |
| Foods they enjoy | 2.17 |
| Foods easy to chew/swallow | 1.44 |
| Amount of help eating/drinking at 3 months (%) | |
| Right amount | 76% |
| More assistance than needed | 2% |
| Less assistance than needed | 23% |
Probability and Predictors of Weight Loss for Residents with Advanced Dementia and Feeding Problems
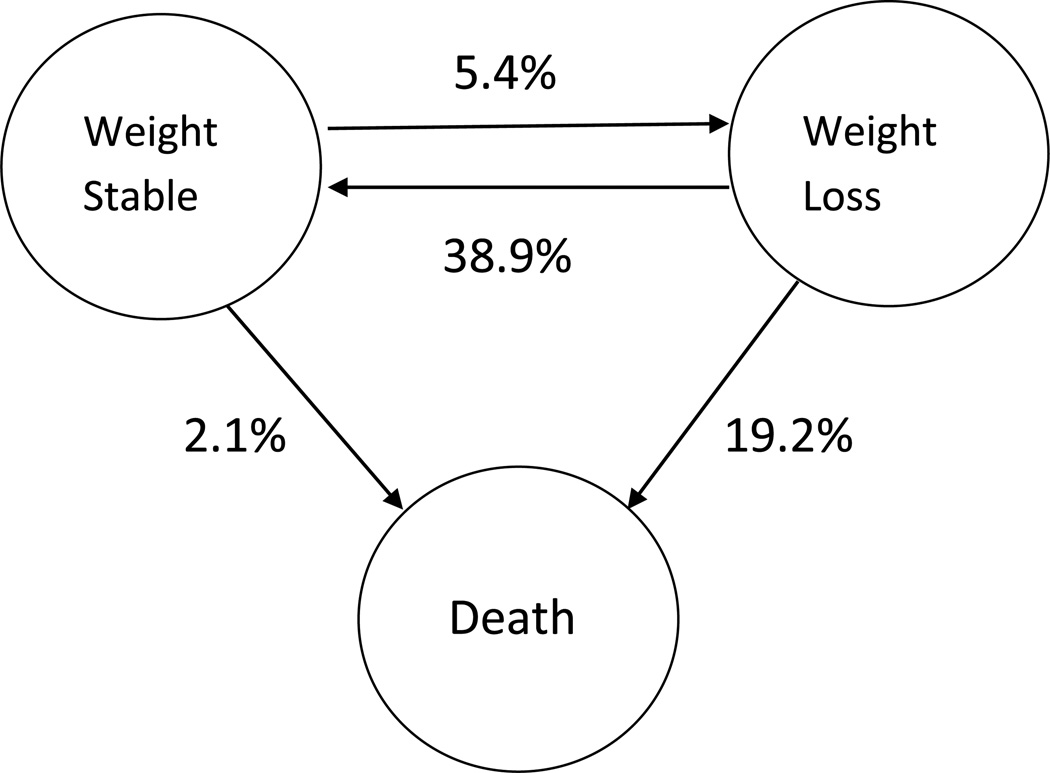
In this cohort of nursing home residents with advanced dementia and feeding problems, 9–11% had significant weight loss during each 3-month interval of follow-up. In initial bivariate analyses, no organizational variables were associated with residents’ risk of weight loss. Among individual resident characteristics, only African-American race and presence of a swallowing problem were predictive of weight loss in multivariate analysis. (Table 3) In the dynamic analyses, a resident with advanced dementia whose weight was stable had a 5.4% chance of developing significant weight loss over the next 3 months. (Figure 1) If a resident with advanced dementia had already experienced weight loss, they had a 38.9% chance of stabilizing weight over the next 3 months, but also had a 19.2% risk of death.
Table 3.
Hazard Ratios for Weight Loss and Mortality Outcomes
| Weight loss Hazard ratio (95% CI)+ |
Mortality Hazard ratio (95% CI)+ |
|
|---|---|---|
| African-American race | 2.7 (1.1–6.5) | 0.6 (0.3–1.1) |
| Swallowing problem | 3.6 (2.1–6.4) | 0.9 (0.5–1.5) |
Analyses adjusted for resident age, gender, race, swallowing problem, and prior observed aspiration; analyses based on stable weight at enrollment
Figure 1.
Three Month Outcome Probabilities for Advanced Dementia with Feeding Problems
A resident with advanced dementia and stable weight has a 5.4% chance of developing weight loss over the next 3 months, and a 2.1% chance of dying during that time interval. A resident with advanced dementia and weight loss has a 38.9% chance of stabilizing weight over the next 3 months, and a 19.2% chance of dying during that same time interval.
Probability and Predictors of Death for Residents with Advanced Dementia and Feeding Problems
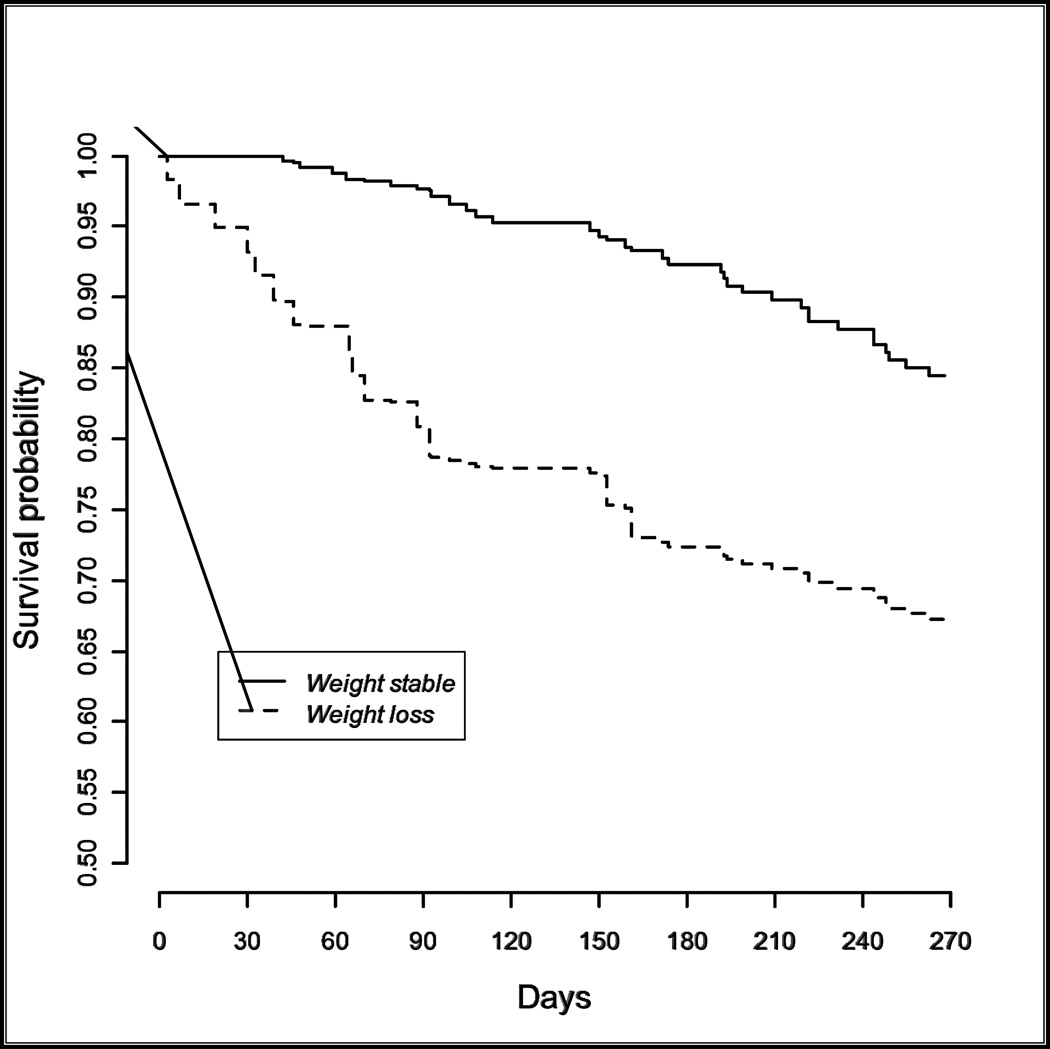
Mortality was significant for this cohort of residents with advanced dementia and feeding problems; 8% died within 3 months, 17% within 6 months and 27% within 9 months follow-up. A resident with advanced dementia whose weight was stable had a 2.1% chance of death over the next 3 months, while a resident with advanced dementia and weight loss had an 19.2% risk of death during the next 3 months. (Figure 1) In multivariate analysis weight loss at enrollment was the only independent predictor of mortality. (Figure 2)
Figure 2.
Survival Probability for Advanced Dementia with or without Weight Loss
P<0.001 for comparison of survival probabilities for weight stable vs. weight loss
Discussion
Feeding problems are nearly universal in advanced dementia; this prospective study helps clinicians and family caregivers know what to expect during the months after feeding problems develop. Each quarter, approximately 10% of nursing home residents with advanced dementia and feeding problems had weight loss. Residents received assisted feeding and supplements – tube feeding was rare in this cohort. During the next 3 months nearly 40% of residents with weight loss stabilized, while 20% died. Thus, in advanced dementia weight loss may be a useful trigger to intensify assistance with eating by mouth, and to prompt a compassionate discussion of goals of care and end-of-life treatment wishes.
Which residents with advanced dementia are likely to develop weight loss? In this study African Americans and persons with a documented swallowing problem were more likely to lose weight. A causal linkage between swallowing problems and weight loss is clearly plausible; the association with African American race – ethnicity may be related to differential care or other unmeasured factors associated with racial clustering within sites, although we were unable to discern an independent effect of facility characteristics in our analyses. Also, it is important to note that in this analysis African American race – ethnicity was not associated with increased mortality.
Study limitations should be considered when reviewed our results. This analysis is secondary to the original purpose of data collection for a randomized controlled trial of a decision aid on feeding options, and some data such as detailed weight timelines were not collected. The study sites – 24 nursing homes – were purposefully diverse to be representative of the organization, size, and racial composition of nursing homes. However, these sites were willing and capable of engaging in a major research study and therefore are likely to be somewhat more administratively stable and may provide higher quality care than the average nursing home organization. Enrolled residents all had advanced dementia, but more precise data on dementia subtypes was not available. Data on the primary outcomes of weight loss and mortality were uniformly available in health records; however, data on the quality of assisted feeding was dependent on surrogate reports rather than direct observation, and validity may vary by frequency of visits. Nursing home records typically use weight and nursing assistants’ records of the percentage of meals eaten by residents to track nutritional status. Future studies of nutritional decline in advanced dementia could be improved by direct measurement of early markers of intake and nutrition such as calorie counts, serum proteins and biometrics.
This study has implications for clinicians, family caregivers and advocates or regulators who are concerned with quality of dementia care. Family caregivers in this study express awareness of the use of assisted oral feeding, modified diets, and high calorie supplements. One in four family caregivers believe assistance with feeding was less than needed, and an important subset report helping during meals. Since tube feeding was very rare family caregivers and clinicians may understand these outcomes to be representative of assisted feeding outcomes for persons with advanced dementia. For clinicians advising family caregivers, if a nursing home resident with advanced dementia has problems with intake, but no weight loss, risk of weight loss or death during the next 3 months is very low. However, if a nursing home resident with advanced dementia develops significant weight loss, they have a 40% probability of stabilizing weight but also a 20% risk of death in the next 3 months. Concurrent with enhanced assisted feeding interventions, weight loss in this population may be useful to trigger a family meeting to discuss goals of care and end-of-life treatment preferences. Finally, advocates and regulators may benefit from understanding that weight loss in advanced dementia can sometimes be stabilized through nutritional support, yet it also is a strong predictor of impending mortality. High quality care for this population includes provision of nutrition, and compassionate preparation for the final phase of life.
ACKNOWLEDGMENTS
| Elements of Financial/Personal Conflicts |
LH, ME, FCL | TSC | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Employment or Affiliation | x | x | ||
| Grants/Funds | x | x | ||
| Honoraria | x | x | ||
| Speaker Forum | x | x | ||
| Consultant | x | x | ||
| Stocks | x | x | ||
| Royalties | x | x | ||
| Expert Testimony | x | x | ||
| Board Member | x | x | ||
| Patents | x | x | ||
| Personal Relationship | x | x | ||
For “yes”, provide a brief explanation: Dr. Carey has provided expert testimony in legal cases involving feeding tubes.
Funding source: NIH-National Institute for Nursing Research R01 NR009826
Sponsor’s Role: The sponsor had no direct role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Footnotes
ClinicalTrials.gov registration: University of North Carolina-Chapel Hill, NCT01113749. http://clinicaltrials.gov/ct2/show/NCT01113749
Author Contributions: Study concept and design (LCH, ME, FCL, TSC), acquisition of subjects and/or data (LCH, ME, FCL, TSC), analysis and interpretation of data (LCH, ME, FCL, TSC), and preparation of manuscript (LCH, ME, FCL, TSC).
References
- 1.Alzheimer’s Facts and Figures on the Alzheimer’s Association website. http://www.alz.org/alzheimers_disease_facts_and_figures.asp; viewed 9/11/2012.
- 2.Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013 doi: 10.1002/gps.3946. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Beydoun MA, Beydoun HA, Kaufman JS, An Y, Resnick SN, O’Brien R, Ferrucci L, Zonderman AB. Apolipoprotein E ε4 allele interacts with sex and cognitive status to influence all-cause and cause-specific mortality in U.S. older adults. J Am Geriatr Soc. 2013;61:525–534. doi: 10.1111/jgs.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie J, Brayne C, Matthews FE Medical Research Council Cognitive Function and Ageing Research collaborators. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336:258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisberg B, Ferris SH, De Leon MJ, et al. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 6.Morrison RS, Siu AL. Survival in end-stage dementia following acute illness. JAMA. 2000;284:47–52. doi: 10.1001/jama.284.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc. 1997;45:1054–1059. doi: 10.1111/j.1532-5415.1997.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 8.Brandt HE, Dliens L, Ooms ME, et al. Symptoms, signs, problems and diseases of terminally ill nursing home patients. Arch Intern Med. 2005;165:314–320. doi: 10.1001/archinte.165.3.314. [DOI] [PubMed] [Google Scholar]
- 9.Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer’s diseases. Am J Psych. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- 10.Reisberg B, Ferris SH, De Leon MJ, et al. The global deterioration scale for assessment of primary degenerative dementia. Am J Psych. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 11.Reisberg B. Functional Assessment Staging (FAST) Psychopharm Bull. 1988;24:653–659. [PubMed] [Google Scholar]
- 12.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volicer L, Seltzer B, Rheaume Y, et al. Eating difficulties in patients with probable dementia of the Alzheimer type. J Geriatr Psych and Neurol. 1989;2:188–195. doi: 10.1177/089198878900200404. [DOI] [PubMed] [Google Scholar]
- 14.Teno JM, Mor V, Kabumoto GT, et al. Every numerator needs a denominator: measuring weight loss as a quality indicator. J Am Geriatr Soc. 2002;50:S11. [Google Scholar]
- 15.Hanson LC, Eckert JK, Dobbs D, et al. Symptom experience of dying long term care residents. J Am Geriatr Soc. 2008;56:91–98. doi: 10.1111/j.1532-5415.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanson LC, Gilliam R, Lee TJ. Successful clinical trials research in nursing homes: The Improving Decision Making Study. Clinical Trials. 2010;7:735–743. doi: 10.1177/1740774510380241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson LC, Carey TS, Caprio AJ, et al. Improving decision making for feeding options in dementia care: a randomized trial. J Am Geriatr Soc. 2011;59:2009–2016. doi: 10.1111/j.1532-5415.2011.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 19.Nursing Home Compare website ratings. http://www.medicare.gov/NursingHomeCompare/search.aspx?bhcp=1 last viewed 1 February 2013.
- 20.Mitchell SL, Kiely DK, Hamel MB, Park PS, Morris JN, Fries BE. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291:2734–2740. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 21.Dabrowska DM, Sun G, Horowitz MM. Cox regression in a Markov renewal model: an application to the analysis of bone marrow transplant data. J Amer Statist Assoc. 1994;89:867–877. [Google Scholar]