Abstract
Objective
To assess trends in hemoglobin recovery among HIV-infected patients initiated on zidovudine-based combined antiretroviral therapy (cART) stratified by baseline hemoglobin level.
Methods
Hemoglobin data from non-pregnant adult patients initiating cART in rural north-central Nigeria between June 2009 and May 2011 was analyzed using a linear mixed effects model to assess the interaction between time, zidovudine-containing regimen, and baseline hemoglobin level on the outcome of subsequent hemoglobin level. Best fit curves were created for baseline hemoglobin in the 10th, 25th, 75th and 90th percentiles.
Results
We included 313 patients with 736 measures of hemoglobin in the analysis (239 on zidovudine and 74 on non-zidovudine-containing regimens). Median hemoglobin increased over time in both groups, with differences in hemoglobin response over time related to baseline hemoglobin levels and zidovudine use (p = 0.003). The groups of patients on zidovudine at the 10th and 90th percentiles had downward sloping curves while all other groups had upward trending hemoglobin levels.
Conclusion
Though hemoglobin levels increased overall for patients on zidovudine-containing regimens, for those in the 10th and 90th percentiles hemoglobin levels trended downward over time. These results have implications for decisions regarding when to initiate, switch from or avoid the use of zidovudine.
Keywords: zidovudine, AZT, anemia, hemoglobin, antiretroviral therapy
Background
Current World Health Organization (WHO) guidelines recommend zidovudine (ZDV) based antiretroviral therapy (ART) as an affordable first line treatment for HIV-infected patients in resource-limited settings, replacing stavudine-based regimens to reduce long-term toxicity.1 Zidovudine (abbreviated ZDV, also called AZT for its first name azidothymidine) is a known cause of hematologic toxicities, particularly anemia.2 Because of this, the WHO guidelines recommend not prescribing ZDV to patients having baseline hemoglobin levels less than 7g/dL.1 The impact of ZDV-associated anemia is variable in different settings. Whereas studies of HIV-infected patients in higher resourced settings have demonstrated recovery of hemoglobin levels after an initial decrease and overall improvement of baseline anemia in patients treated with ZDV-based regimens,3,4 data from resource-limited settings show mixed results related to ZDV risk. In a Ugandan cohort, starting a zidovudine-based regimen was not associated with increased risk of early severe anemia.5 However, a secondary analysis of a clinical trial and a multicenter cohort study including sites in Sub-Saharan Africa, Asia-Pacific and Latin America found associations between initial therapy containing ZDV and anemia.6,7 Variability in findings is expected given that the studies had different structures and outcomes, with some analyzing incident anemia in patients with normal hemoglobin and others looking at changes in hemoglobin levels among anemic patients initiating ZDV-based regimens.
For front-line treatment implementation, it would be useful to know expected hemoglobin responses to ZDV at varying baseline hemoglobin levels, especially in the context of settings with high baseline levels of anemia with multiple underlying causes (e.g., nutritional, AIDS-related, malaria and/or helminth associated).8–10 Nigeria is such a setting, with holoendemic malaria in much of the country, a significant burden of helminth infections, and nutritional deficiencies secondary to poverty. 11,12,13 Though ZDV is part of the recommended first-line regimen, Nigerian ART guidelines recommend avoiding the use of zidovudine or switching to another drug in persons having baseline hemoglobin values of less than 8 g/dL.14 We conducted a retrospective cohort study of HIV-infected patients in rural north-central Nigeria to track changes in hemoglobin levels during the first year of therapy in patients initiating ZDV-based regimens, stratified by baseline hemoglobin level.
Methods
Since 2008, the Vanderbilt Institute for Global Health (VIGH) and its non-governmental Nigerian incorporated affiliate, Friends in Global Health, LLC (FGH), have been implementing comprehensive HIV/AIDS services in rural Kwara and Niger states of Nigeria, with funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC). At the time of this study FGH was supporting HIV treatment services in five clinics: Sobi Specialist Hospital (Ilorin) and Lafiagi General Hospital in Kwara state; and Gawu Babangida Rural Hospital, Kuta Rural Hospital, and Umaru Yar Adua Hospital Sabon Wuse in Niger State. Prior to June 2010, patients eligible to initiate combined antiretroviral therapy (cART) included: those with WHO stage I or II disease with <200 CD4+ cells/μL, WHO stage III disease with <350 CD4+ cells/μL, or WHO stage IV regardless of CD4+ cell count. From June 2010 onward, patients having CD4+ counts <350 or WHO stage III or IV regardless of CD4+ cell count were eligible to initiate cART. Zidovudine-based regimens are used most commonly at the sites; alternate first line regimens use tenofovir, abacavir or stavudine as the primary nucleoside analog.
We used routinely collected PEPFAR program data for this analysis. After each clinic day, FGH data clerks entered data from national patient management and monitoring (PMM) forms that had been completed by clinicians, nurses, laboratory, and pharmacy staff into CAREWare™ (jProg®, New Orleans, LA). Routine audits of medical records were performed to ensure that forms were completed accurately and laboratory data were entered correctly. Data queries were generated for out-of-range and missing data. Each site addressed its data queries; clean data were extracted for the final analyses. All patients 15 years of age and older initiating cART between June 9, 2009 and May 25, 2011 who had at least two documented hemoglobin levels were eligible for analysis. Pregnant patients were excluded. Our main outcome was trends in hemoglobin values in HIV-infected patients on ZDV-based regimens, stratified by baseline hemoglobin level.
A linear mixed effects model was used to assess the interaction between time, ZDV-containing regimen, and baseline hemoglobin level (within 90 days of cART initiation) on the outcome of subsequent hemoglobin level while adjusting for sex and cotrimoxazole use.15 To account for non-linearity, baseline hemoglobin and time were transformed using natural cubic splines with three knots.16 Best fit curves depicting trajectories of hemoglobin were created for four strata of baseline hemoglobin: the 10th, 25th, 75th and 90th percentiles. If patients were switched off of ZDV, hemoglobin data was censored after the switch. Patients who died or were lost to follow-up contributed time until their last clinic visit. Patient demographics and clinical characteristics were compared by initial regimen using Wilcoxon rank sum and chi-squared tests, as appropriate. We employed R software 2.15.1 (www.r-project.org) for all data analyses. Analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses.
Ethics approvals for this secondary data analysis were obtained from the Vanderbilt University Institutional Review Board (#120004) and the Nigeria Health Research and Ethics Committee (#01/01/2007-03/02/2012).
Results
We identified 313 patients with 736 measures of hemoglobin who met inclusion criteria with 239 on ZDV-based initial regimens and 74 on non-ZDV regimens. The median age of patients was 34 years and 67% were female. There were no statistically significant differences between the ZDV and non-ZDV groups in either demographic profiles or baseline CD4 count. (Table) Body mass index was very slightly higher in the ZDV group (20.5 vs 20.1; p = 0.03). Baseline hemoglobin levels were higher for patients initiated on ZDV-based cART (median 10.4 g/dL versus 9.9 g/dL, p <0.001). There were 55 patients who were lost to follow-up and 11 patients who died during the first year on treatment, with no significant differences between the groups.
Table 1.
Summary of Patient Clinical Characteristics by ZDV at initiation
| No ZDV (n=74) | ZDV (n=239) | Combined (n=313) | P-valueb | |
|---|---|---|---|---|
| Agea | 34 (27, 43) | 33 (28, 42) | 34 (28, 42) | 0.858 |
| Female | 49 (66%) | 162 (68%) | 211 (67%) | 0.913 |
| BMI (kg/m2)c | 20.1 (17.1, 22.2) | 20.5 (19, 22.8) | 20.4 (18.4, 22.5) | 0.031 |
| Missing BMI (kg/m2), n(%) | 9 (12%) | 42 (18%) | 51 (16%) | |
| Functional status, n(%) | 0.648 | |||
| Missing | 1 (1%) | 3 (1%) | 4 (1%) | |
| Working | 67 (92%) | 221 (94%) | 288 (93%) | |
| Ambulatory | 5 (7%) | 14 (6%) | 19 (6%) | |
| Bedridden | 1 (1%) | 1 (<1%) | 2 (1%) | |
| CD4 count | 176 (98, 266) | 200 (91, 293) | 193 (97, 284) | 0.540 |
| Missing CD4 count, n(%) | 3 (4%) | 14 (6%) | 17 (5%) | |
| Hemoglobin at baseline | 9.9 (7.6, 11.2) | 10.4 (9.5, 11.9) | 10.3 (9.2, 11.8) | <0.001 |
| Missing hemoglobin, n(%) | 1 (1%) | 9 (4%) | 10 (3%) | |
| Hemoglobin category (percentiles), n(%) | ||||
| <7.7 | 23 (32%) | 10 (4%) | 33 (11%) | |
| 7.7–9.1 | 13 (18%) | 33 (14%) | 46 (15%) | |
| 9.2–10.2 | 7 (10%) | 69 (30%) | 76 (25%) | |
| 10.3–11.7 | 17 (23%) | 60 (26%) | 77 (25%) | |
| 11.8–13.0 | 8 (11%) | 38 (17%) | 46 (15%) | |
| >13.0 | 5 (7%) | 20 (9%) | 25 (8%) | |
| WHO stage, n(%) | 0.083 | |||
| I | 14 (19%) | 57 (24%) | 71 (23%) | |
| II | 16 (22%) | 43 (18%) | 59 (19%) | |
| III | 37 (50%) | 132 (55%) | 169 (54%) | |
| IV | 7 (9%) | 7 (3%) | 14 (4%) | |
| Death in 12 months | 3 (4%) | 8 (3%) | 11 (4%) | 0.99 |
| Lost in 12 months | 18 (24%) | 37 (15%) | 55 (18%) | 0.12 |
Continuous variables are reported as medians (interquartile range).
To compare the distribution of study characteristics for participants by ZDV at initiation, we employ chi-square tests. Similarly, we use a Wilcoxon rank sum test for continuous variables by ZDV at initiation.
Weight, functional status, CD4, WHO stage are collected at treatment initiation. Treatment data is collected in a window of 90 days before and 30 days after date of treatment initiation. Hemoglobin is also collected at treatment initiation, but we allow for a window of ± 90 days from date of treatment initiation.
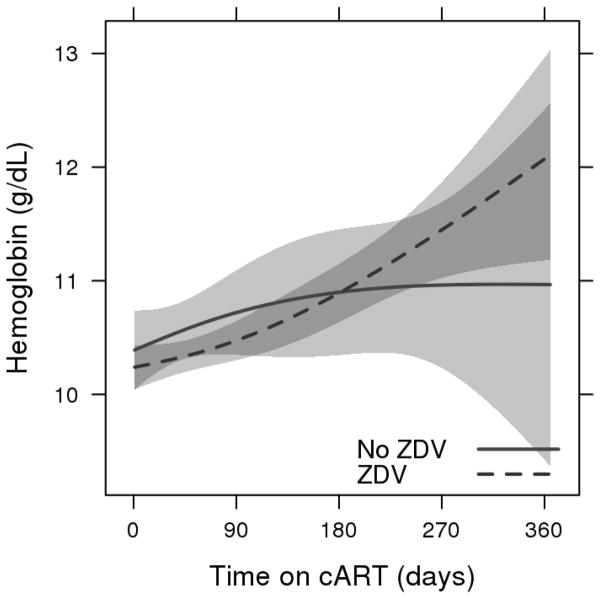
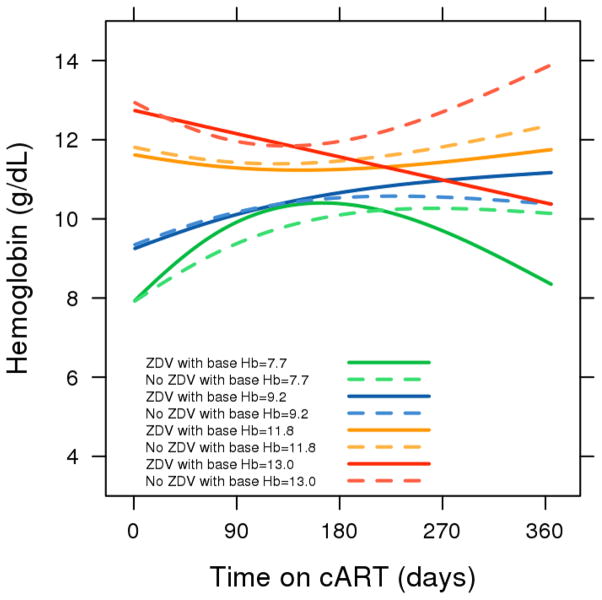
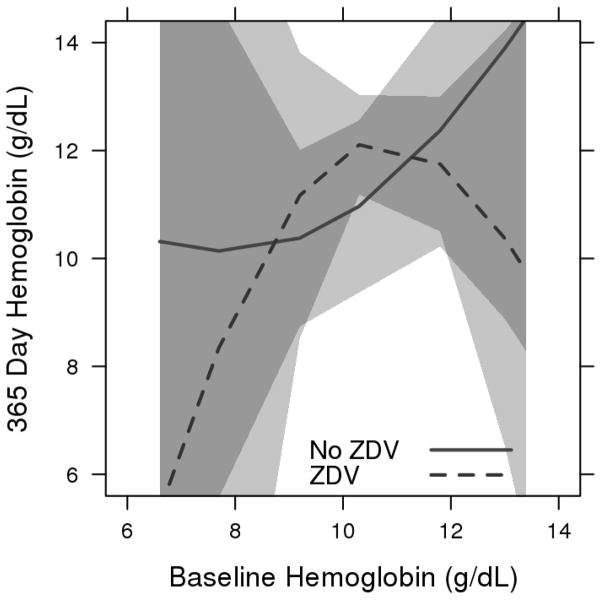
There was an increase in median hemoglobin response over one year both for patients on ZDV (+1.8 g/dL) and for patients not on ZDV (+0.7 g/dL), with overlapping confidence intervals. (Figure 1) After an initial increase, the group of patients on ZDV at the lowest baseline hemoglobin level (the 10th percentile at 7.7 g/dL) had a downward sloping curve starting at about 150 days on therapy. Patients on ZDV with initial hemoglobin levels at the 90th percentile (13.0 g/dL) also had a downward trajectory for hemoglobin, with estimated expected levels of 10.4 g/dL at 365 days. All other curves for both ZDV-based regimens and non-ZDV-based regimens had upward trending hemoglobin responses. (Figure 2) There was evidence of differences in hemoglobin response over time related to baseline hemoglobin levels and ZDV use (3-way interaction, p=0.003). Figure 3 depicts the relationship between baseline and 365-day hemoglobin, illustrating the lower year-end values at both ends of the baseline hemoglobin spectrum for patients receiving ZDV-based cART.
Figure 1.
Predicted mean hemoglobin response during the first year on zidovudine and non-zidovudine-based cART regimens
Estimated mean hemoglobin response with 95% confidence bands from a linear mixed model including sex, cotrimoxazole use, and a three-way interaction between time on cART, baseline hemoglobin, and cART regimen. Adjusted to median baseline hemoglobin=10.3 g/dL, female sex and cotrimoxazole use.
Figure 2.
Predicted mean hemoglobin response during the first year on zidovudine and non-zidovudine-based cART regimens stratified by baseline hemoglobin
Lines are drawn for zidovudine (solid) and non-zidovudine-based (dashed) regimens, and stratified by the 10th (7.7 g/dL, green), 25th (9.2 g/dL, blue), 75th (11.8 g/dL, yellow), and 90th (13.0 g/dL, red) percentiles of baseline hemoglobin. Adjusted to female sex and cotrimoxazole use.
Figure 3.
Predicted 365 day hemoglobin by baseline hemoglobin for zidovudine and non-zidovudine-based cART regimens
Estimated mean hemoglobin response at 365 days with 95% confidence bands. Adjusted to female sex and cotrimoxazole use.
Discussion
In the context of all that is known about the effect of ZDV on hemoglobin, this study sheds light on the relationship between the hemoglobin level at cART initiation and the changes in hemoglobin over time in real-world rural settings. For patients initiated on ZDV-based regimens, we found that though hemoglobin levels increased overall, for those in the 10th and 90th percentiles, hemoglobin levels trended downward over time. Patients starting ART at the lower end of hemoglobin values may well be sicker than other patients such that co-infections or nutritional disadvantages are extant. Our findings are consistent with a prior study in West Africa that found associations between the risk of grade 3 or 4 anemia with low baseline hemoglobin levels.17 For the patients starting ART at the highest levels of hemoglobin, a regression to the mean phenomenon is likely. In addition clinicians may not be as concerned about ZDV-related decreases in hemoglobin, feeling that these patients have a larger buffer zone before a switch off of ZDV may be indicated.
A strength of our study is its likely overall representativeness to many other African PEPFAR-supported initiatives, as it reflects real-world challenges in rural settings. Limitations of our study included the lack of data in our database on the use of blood transfusions for patients with severe anemia. Use of such a procedure would move findings in the lowest hemoglobin percentile group toward the null; our data show declining hemoglobin trends in this group nonetheless. The modest sample size and predominantly female study population could limit generalizability of findings. Similar studies are warranted in other patient populations. In addition, we examined only two hemoglobin data points in some patients, which may not be sufficient to reliably ascertain trends. We did not assess medication intake using validated measures of adherence, such as pill count or MIMS bottles. Nor did we have data on specific factors or comorbidities that may contribute to anemia. However, we think it likely that differences in adherence and the distribution of anemia related factors between ZDV and non-ZDV groups will be non-differential.
In conclusion, when ZDV-based regimens are used in settings where anemia is prevalent, one can have an expectation of improvement in hemoglobin levels over time, with the exception of those initiating cART in the lowest hemoglobin percentile (≤7.7 g/dL in our study), and less critically, in persons with the highest hemoglobin levels. Our findings can influence clinical decisions regarding at which levels of hemoglobin to start ZDV-based regimens, when to switch from ZDV and when to avoid ZDV altogether.
Acknowledgments
Funding acknowledgement
This publication was made possible by support from the President’s Emergency Plan for AIDS Relief (PEPFAR) through cooperative agreement No. 5U2GPS001063 from the HHS/Centers for Disease Control and Prevention (CDC), Division of Global HIV/AIDS. MA is supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health, award number R01HD075075. The study sponsors had no role in in the study design, in the collection, analysis or interpretation of data, in the writing of the manuscript or in the decision to submit the manuscript for publication. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention or the U.S. National Institutes of Health.
Footnotes
Declaration of Conflict of Interest
The authors declare there is no conflict of interest.
References
- 1.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 2.Richman D, Fischl M, Grieco M, Gottlieb M, Volberding P, Laskin O, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita S, Yoshimura K, Kimura T, Kamihira A, Takano M, Eto K, et al. Spontaneous recovery of hemoglobin and neutrophil levels in Japanese patients on long-term Combivir containing regimen. J Clin Virol. 2005;33:188–193. doi: 10.1016/j.jcv.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan P, Hanson D, Brooks J. Impact on hemoglobin of starting combination antiretroviral therapy with or without zidovudine in anemic HIV-infected patients. J Acquir Immune Defic Syndr. 2008;48:163–168. doi: 10.1097/QAI.0b013e3181685714. [DOI] [PubMed] [Google Scholar]
- 5.Kiragga A, Castelnuovo B, Nakanjako D, Manabe Y. Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. J Int AIDS Soc. 2010:13. doi: 10.1186/1758-2652-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ssali F, Stohr W, Munderi P, Reid A, Walker AS, Gibb DM, et al. Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antivir Ther. 2006;11:741–749. doi: 10.1177/135965350601100612. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Jaquet A, Bissagnene E, Musick B, Wools-Kaloustian K, Maxwell N, et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific and central and South America. J Int AIDS Soc. 2012:15. doi: 10.1186/1758-2652-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saracino A, Nacarapa EA, da Costa Massinga EA, Martinelli D, Scacchetti M, de Oliveira C, et al. Prevalence and clinical features of HIV and malaria co-infection in hospitalized adults in Beira, Mozambique. Malar J. 2012;11:241. doi: 10.1186/1475-2875-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modjarrad K, Zulu I, Redden DT, Njobvu L, Freedman DO, Vermund SH. Prevalence and predictors of intestinal helminth infections among human immunodeficiency virus type 1-infected adults in an urban African setting. Am J Trop Med Hyg. 2005;73:777–782. [PMC free article] [PubMed] [Google Scholar]
- 10.Walson JL, Stewart BT, Sangare L, Mbogo LW, Otieno PA, Piper BK, et al. Prevalence and correlates of helminth co-infection in Kenyan HIV-1 infected adults. PLoS Negl Trop Dis. 2010;4:e644. doi: 10.1371/journal.pntd.0000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onyenekwe C, Ele P, Ukibe N, Ezeani M, Ezechukwu C, Amilo G, et al. Assessment of White Blood Cell Count, Packed Cell Volume, Phagocytic Functions, Serum Albumin and Plasma Iron in HIV Infected Patients. Clinical Immunology. 2010;135:S110–S110. [Google Scholar]
- 12.Akinbo FO, Okaka CE, Omoregie R. Plasmodium falciparum and intestinal parasitic co-infections in HIV-infected patients in Benin City, Edo State, Nigeria. J Infect Dev Ctries. 2012;6:430–435. doi: 10.3855/jidc.1889. [DOI] [PubMed] [Google Scholar]
- 13.Igbedioh SO. Undernutrition in Nigeria: dimension, causes and remedies for alleviation in a changing socio-economic environment. Nutr Health. 1993;9:1–14. doi: 10.1177/026010609300900101. [DOI] [PubMed] [Google Scholar]
- 14.Federal Ministry of Health. National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. Abuja, Nigeria: Ministry of Health - Nigeria; 2010. [Google Scholar]
- 15.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 16.Hastie TJ. Generalized additive models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Danvers, MA: Chapman and Hall/CRC; 1992. [Google Scholar]
- 17.Moh R, Danel C, Sorho S, Sauvageot D, Anzian A, Minga A, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d’Ivoire. Antivir Ther. 2005;10:615–624. doi: 10.1177/135965350501000510. [DOI] [PubMed] [Google Scholar]