Abstract
Objectives
Succinic semialdehyde dehydrogenase (SSADH) deficiency is a gamma-aminobutyric acid (GABA) degradative defect. Epilepsy affects half of patients. The murine model is associated with a transition from absence to convulsive seizures in the third week, with fatal status epilepticus.
Methods
The clinical phenotype is reported from a patient database. Flumazenil-Positron Emission Topography (FMZ-PET) and Transcranial Magnetic Stimulation (TMS) were used to study GABA neurotransmission. Electrocorticography, single cell electrophysiology, and radioligand binding studies are reported from animal studies.
Results
Generalized seizures predominate, including tonic-clonic, atypical absence, and myoclonic. EEG discharges are typically generalized spike-wave. MRI shows a dentatopallidoluysian pattern. Sudden Unexpected Death in Epilepsy Patients (SUDEP) has occurred and the associated neuropathology reveals chronic excitotoxic injury in gloubus pallidus. Investigations using FMZ-PET and TMS support downregulation of GABAA and GABAB activity, respectively, in patients. Gamma-hydroxybutyrate (GHB) induces spike-wave discharges in homozygous null mice via GHB and GABAB-mediated mechanisms. These resemble absence seizures and are abolished by a GABAB receptor antagonist. Decreased binding of GABAA and GABAB receptor antagonists has been demonstrated in P19 and P14 null mice, respectively. Downregulation of GABAA and GABAB receptor subunits is observed by P14. GABAA and GABAB mediated potentials are reduced from P8–P14.
Conclusion
Generalized epilepsy and epileptiform discharges are characteristic of SSADH deficiency. Spontaneous absence seizures appear in null mice by the third week, which may be induced by GHB or GABAB activity. Subsequent overuse dependent downregulation of GABAA and GABAB receptor activity may be associated with hyperexcitability concomitant with the transition to generalized seizures.
Keywords: SSADH deficiency, epilepsy, absence seizures, generalized convulsive seizures, GABA, GHB
Introduction
SSADH deficiency is an autosomal recessively inherited disorder in GABA degradation that has been reported in approximately 450 people. The enzyme SSADH is involved in the breakdown of GABA to succinic acid via the intermediate succinic semialdehyde, and in its absence, GABA is alternatively converted to GHB (Figure 1). There is a resultant build up of both GABA and GHB in physiological fluids and brain parenchyma. SSADH deficiency is considered likely underdiagnosed due to the heterogeneity in clinical manifestations of the disease, and the high level of clinical suspicion required to test for the disease using specific-ion monitoring for GHB aciduria [1]. Clinical manifestations of the disease include intellectual deficiency, hypotonia, behavioral problems, seizures and ataxia. Most patients present with developmental delay with a mean age of diagnosis of two years, although patients have been diagnosed as late as the third decade. Epilepsy is present in approximately half of affected individuals. Neuroimaging demonstrates increased T2-weighted MRI signal in the globus pallidus (Figure 2), cerebellar dentate nucleus and subthalamic nucleus, along with cerebral and cerebellar atrophy (Figure 3).
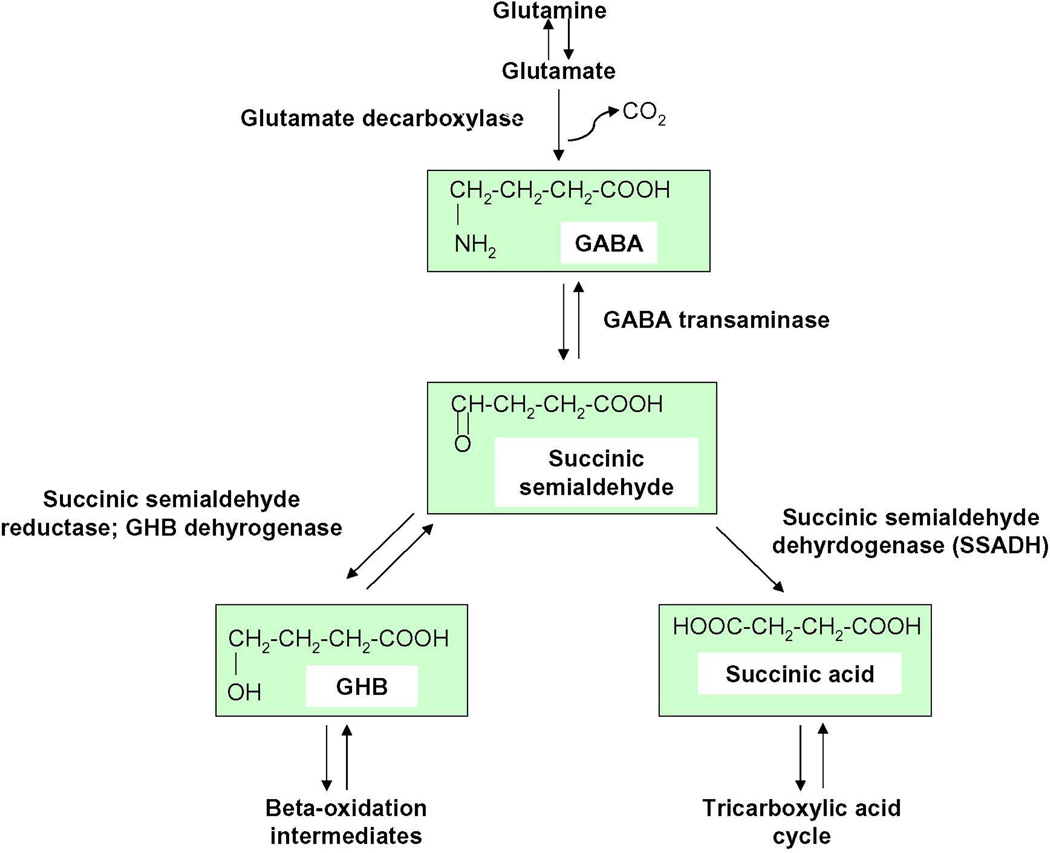
Figure 1. GABA degradation pathway.
GABA is normally converted via GABA-transaminase to succinate semialdhyde, which is then broken down to succinic acid by succinate semialdehyde dehydrogenase (SSADH). In the absence of SSADH, succinate semialdehyde is converted to GHB rather than succinic acid, and this leads to a build up of both GHB and GABA in the brain.
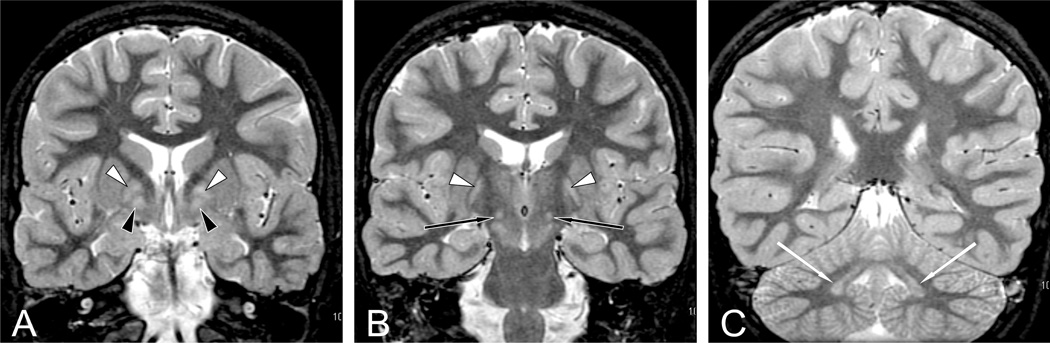
Figure 2. Dentatopallidoluysian Pattern in SSADH Deficiency.
Coronal short tau inversion recovery sections from MRI in patient with SSADH deficiency showing bilateral symmetric homogenous signal abnormalities in each globus pallidus pars lateralis (white arrow, A and B), pars medialis (black arrow, A), subthalamic nucleus (black arrows, B), and dentate nucleus (white arrows, C). (Reprinted with permission from Pearl et al: Neurology 2009;73:423–9.)
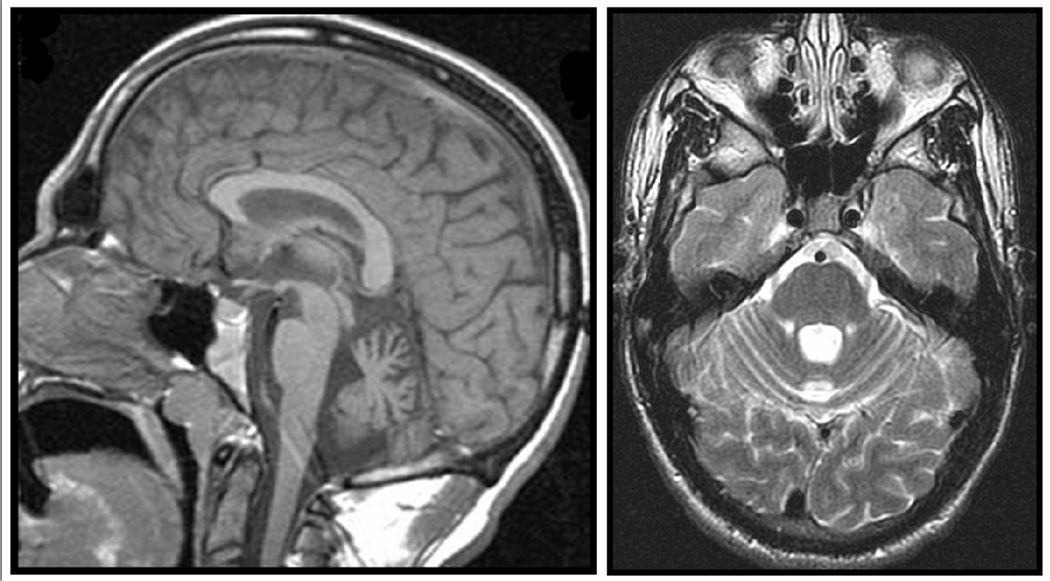
Figure 3. MRI of patient with SSADH deficiency- midsagittal and axial.
Midsaggittal (left) and axial (right) 1.5 T MRI demonstrating cerebellar atrophy predominantly affecting the midline vermis. (Reproduced with permission from Acosta et al. J Child Neurol 2010 May 5. [Epub ahead of print].)
The enzymatic deficiency and affected gene locus (Aldh5A1) have been previously identified, and the development of a transgenic mouse model has allowed further study of the physiologic changes produced by SSADH deficiency. The homozygous null mutant animal model expresses a characteristic phenotype with failure-to-thrive, ataxia, and a progressive epileptic disorder characterized by a transition from absence to generalized convulsive seizures and ultimately fatal status epilepticus by the third week of life [2]. The transition from absence to convulsive activity could be analogized to the human epilepsy syndromes of generalized childhood absence or juvenile absence epilepsy as well as juvenile myoclonic epilepsy. The prevalence of seizures in this hyper-GABAergic condition may be viewed as paradoxical. This paper addresses the seizure and EEG data associated with human SSADH deficiency and ongoing research in the human condition and murine model aimed at elucidating the mechanisms underlying epilepsy in this metabolic disorder.
Human Phenotype
Patient Vignette
A 5-year-old boy presented with severe hyperactivity and a new onset generalized convulsion. Neurological examination revealed a minimally verbal, nondysmorphic boy with mild hypotonia, hyporeflexia, and gait ataxia. Psychoeducational testing showed mild intellectual disability (Wechsler Intelligence Scales for Children: verbal 55, performance 65) and deficient adaptive behaviors on the Vineland Adaptive Behavioral Scales. EEG revealed mild background slowing during wakefulness with intermittent generalized spike-wave discharges that were activated during sleep. Valproate was begun but was associated with lethargy. MRI was obtained, demonstrating increased T2-weighted signal in the globus pallidus. Urine organic acids revealed 4-hydroxybutyric aciduria. This led to enzymatic quantification of SSADH enzymatic activity in leucocytes, which confirmed the diagnosis of SSADH deficiency. The patient was treated with lamotrigine with fair seizure control, and has had persistent problems with expressive aphasia, obsessive-compulsive disorder, and anxiety. Now age 25, the patient lives in a group home with other young adults, most of whom have been diagnosed with cerebral palsy, intellectual disabilities, or autism.
Human SSADH deficiency is usually characterized by a relatively nonprogressive encephalopathy manifest in the first two years of life by hypotonia and developmental delay associated with mild ataxia and hyporeflexia. Virtually constant characteristics are intellectual disability and profound expressive language deficits. Psychiatric symptoms tend to be the most disabling and are characterized by inattention and hyperactivity, and sometimes aggression in early childhood, and anxiety and obsessive-compulsive behaviors in adolescence and adulthood [3, 4]. Intermittent decompensation as seen in other metabolic disorders is not the usual presentation, although this is a subgroup (approximately 10% of reported patients) with a more severe phenotype including a degenerative course characterized by regression and prominent extrapyramidal manifestations [5].
A clinical database using systematic questionnaires of 68 patients indicates that developmental delay is a universal presentation. Common clinical features include intellectual disability, behavior problems, and motor dysfunction (Tables 1 and 2). In order to address the long-term outlook, we reported on 33 patients (52% males) over ten years of age [3]. The mean age of this patient cohort is 17.1 years +/− 6.4 years (range 10.1–39.6 years). The mean age when symptoms first appeared was 11 months (range 0–44 months) and the mean age at diagnosis was 6.6 years, although some individuals were not diagnosed until age 25 years.
Table 1.
Clinical features of SSADH deficiency (n=68)
| Number | Percentage | |
|---|---|---|
| Developmental delay | 67 | 98.5% |
| Mental retardation | 65 | 95.6% |
| Hypotonia | 57 | 83.8% |
| Behavior problems | 47 | 69.1% |
| Seizures | 34 | 50.0% |
| Ataxia | 50 | 73.5% |
Table 2.
Neuropsychiatric disturbances in patients with SSADH deficiency (n=43)
| Number | Percentage | |
|---|---|---|
| Aggression | 9 | 20.9% |
| Anxiety | 17 | 39.5% |
| Hallucinations | 5 | 11.6% |
| Hyperactivity | 24 | 55.8% |
| Inattention | 31 | 72.1% |
| Obsessive compulsive disorder | 16 | 37.2% |
| Sleep disturbances | 28 | 65.1% |
| Pervasive developmental disorders/autism | 5 | 11.6% |
Nearly half of patients develop epilepsy, usually with generalized tonic-clonic and atypical absence seizures (Table 3) [6]. The epilepsy may be difficult to control, with some patients having sporadic but recurrent episodes of generalized convulsive status epilepticus. We are aware of one patient who died with a diagnosis of SUDEP, and the neuropathology findings are discussed below. Electroencephalographic findings include background slowing, epileptiform abnormalities (usually generalized and sometimes multifocal), and occasionally photosensitivity and electrographic status epilepticus of sleep (Table 4; Figure 4). Heterozygosity for SSADH deficiency has been reported in one family with a parent and sibling having generalized spike-wave discharges, photosensitivity, and absence and myoclonic seizures [7].
Table 3.
Seizure activity in patients with SSADH deficiency (n=34)
| Number | Percentage | |
|---|---|---|
| Generalized tonic-clonic | 18 | 52.9% |
| Absence | 17 | 50.0% |
| Myoclonic | 8 | 23.5% |
| Unspecified | 3 | 8.8% |
| Other (Anterior temporal lobe epilepsy, atonic partial, febrile) | 16 | 47.1% |
Table 4.
EEG findings in patients with SSADH deficiency (n=40)
| Number | Percentage | |
|---|---|---|
| Normal EEG | 17 | 42.5% |
| Abnormal EEG | 23 | 57.5% |
| Background abnormal/slowing | 12 | 30.0% |
| Spike discharges | 11 | 27.5% |
| Electrographic status epilepticus during slow wave sleep | 1 | 2.5% |
| Photosensitivity | 3 | 7.5% |
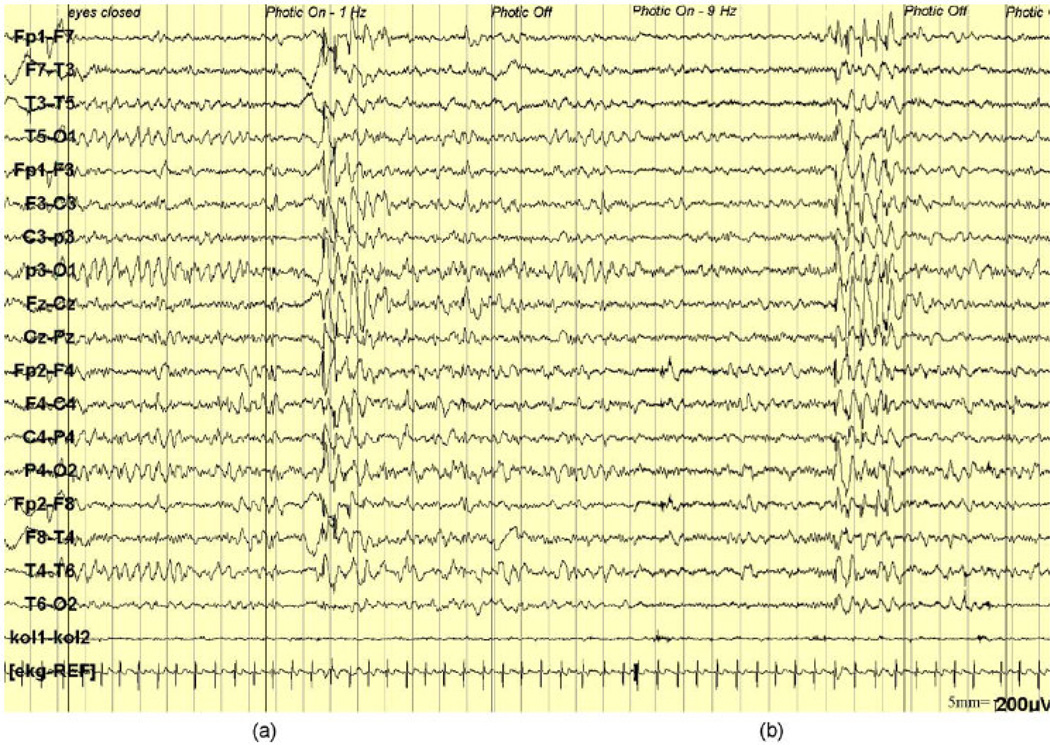
Figure 4. EEG abnormalities in SSADH deficiency.
(a) Intermittent photic stimulation (IPS) at 1 Hz causes bilateral 2.5 Hz spike-waves with frontocentral predominance. (b) 9 Hz IPS causes bilateral, diffuse spike-and-wave discharges (Reproduced with permission from: Dervent et al: Clin Neurophysiol 2004;115:1417–22.)
Sleep disorders are common and manifest by excessive daytime somnolence or disorders of initiating and maintaining sleep [6]. We reported sleep study data in ten patients with overnight polysomnograms and diurnal multiple sleep latency studies with prolonged latency to stage REM latency (mean 272 ± 89 min), and reduced stage REM percentage (mean 8.9%,range 0.3% – 13.8%) [8]. Half of patients showed a decrease in daytime mean sleep latency indicating excessive daytime somnolence. Thus, there appears to be a reduction in REM sleep in the disorder. Similarly, animal models have demonstrated that hyperGABAergic states, e.g. via inhibition of GABA transaminase using L-cycloserine, are associated with reduction of REM sleep and prolongation of the transition phase between sleep stages NREM and REM [9].
Radiographic Findings
We have identified a pallidodentatoluysian pattern of increased T2-weighted signal intensity involving the globus pallidi, cerebellar dentate nuclei, and subthalamic nuclei bilaterally and symmetrically. Other findings include cerebral atrophy, cerebellar atrophy, delayed myelination, and T2-hyperintensities in subcortical white matter, thalamus, and brainstem [10, 11]. While the pallidal hyperintensity is usually homogeneous and equally affects the internal and external portions, we have had occasional patients with heterogeneous and even asymmetric involvement. Magnetic resonance spectroscopy will show spectra for routine single and multi-voxel studies of N-acetyl aspirate, choline, and lactate, but specialized protocols that allow editing for small molecules has shown elevated levels of GABA and related compounds (including GHB and homocarnosine) in patients but not obligate heterozygotes [12, 13]. Fluorodeoxyglucose PET studies have shown decreased cerebellar glucose metabolism in patients with cerebellar atrophy demonstrated on structural MRI [1, 14].
Neuropathology
Recently the first post-mortem examination of SSADH deficiency became available in the patient diagnosed with SUDEP as noted above. The underlying diagnosis of SSADH deficiency had been made retrospectively in a 19-year old deceased female following the confirmed diagnosis in her living sister. Her living sister had been followed with a history of developmental delay and borderline cognition (IQ = 70), until presenting with seizures and a subsequent diagnosis of SSADH deficiency. The decedent presented with developmental delay, seizure onset at age thirteen years, and death at age nineteen after having experienced escalating convulsive seizure activity. A pathogenic homozygous mutation of Aldh5A1 (p.Gly409Asp) was detected in the living sister, and subsequently confirmed in the decedent. Both parents were confirmed as heterozygote carriers. The major neuropathological finding was striking discoloration of the globus pallidi, which is remarkably consistent with imaging findings in the condition. On microscopic examination, there was hyperemia and granular perivascular calcification of the globus pallidus and superior colliculus, consistent with chronic excitotoxic injury, without significant neuronal loss or gliosis of CA1 of the hippocampus, the area which would have been considered most vulnerable to epileptic or hypoxic injury [15].
Laboratory and Biochemical Findings
Laboratory screening is done with urine organic acids, which consistently show elevated GHB although specific ion monitoring is sometimes necessary to detect an otherwise subtle peak that is close on the spectrograph to the larger and normal urea peak [1]. Specimens also frequently show dicarboxylic aciduria and elevated 3,4-dihydroxyhexanoic acid. Routine laboratory tests as well as plasma amino acids and studies of bioenergetics and mitochondrial screening are noninformative. All physiologic fluids will be elevated in GHB, and in the absence of exogenous administration, confirmation of the enzymatic deficiency should be obtained through either enzymatic quantification, available in leucocytes, or mutational analysis via sequencing of the gene Aldh5A1.
The disorder is associated with characteristic alterations in neurotransmitter concentrations, although CSF neurotransmitter analysis is not required to make the diagnosis. Cerebrospinal fluid has been studied for GABA concentrations and related metabolites [16]. There is a two- to four-fold increase in GABA and 30-fold increase of GHB in CSF [17]. There are also elevated levels of homocarnosine and a trend toward low glutamine levels, implicating dysfunction of the neuronal-glial shuttle wherein glutamine is synthesized only in astroglia with subsequent glutamate and GABA formation in neurons.
Pathophysiology of Epilepsy in SSADH Deficiency
The pathophysiology of absence seizures raises the importance of altered GABAA and GABAB mechanisms, which represent fundamental defects in SSADH deficiency. Typical absence seizures usually present as staring episodes associated with three-per-second, generalized spike-wave discharges on EEG. Atypical absence seizures have relatively similar semiology but are associated with a slower spike-wave frequency (1.5–2.5 Hz) and tend to occur in the setting of a symptomatic neurodevelopmental disorder. Absence seizures are caused by alteration of the thalamocortical circuitry involving thalamic relay neurons, thalamic reticular neurons, and cortical pyramidal neurons. Thalamic relay neurons activate cortical pyramidal neurons in either a tonic mode causing wakefulness or REM sleep, or a burst mode that uses T-type calcium channels to produce non-REM sleep. The thalamic reticular neurons hyperpolarize the thalamic relay neurons through GABAB receptors and cause burst firing during wakefulness, which may lead to absence seizures. The thalamic reticular neurons are in turn inhibited by neighboring reticular neurons through activation of GABAB receptors. Therefore, both GABAA and GABAB receptors are involved in absence epilepsy, with the former mediating the inhibitory post-synaptic potentials regulating thalamocortical behavior and the latter synchronizing thalamocortical circuitry [18].
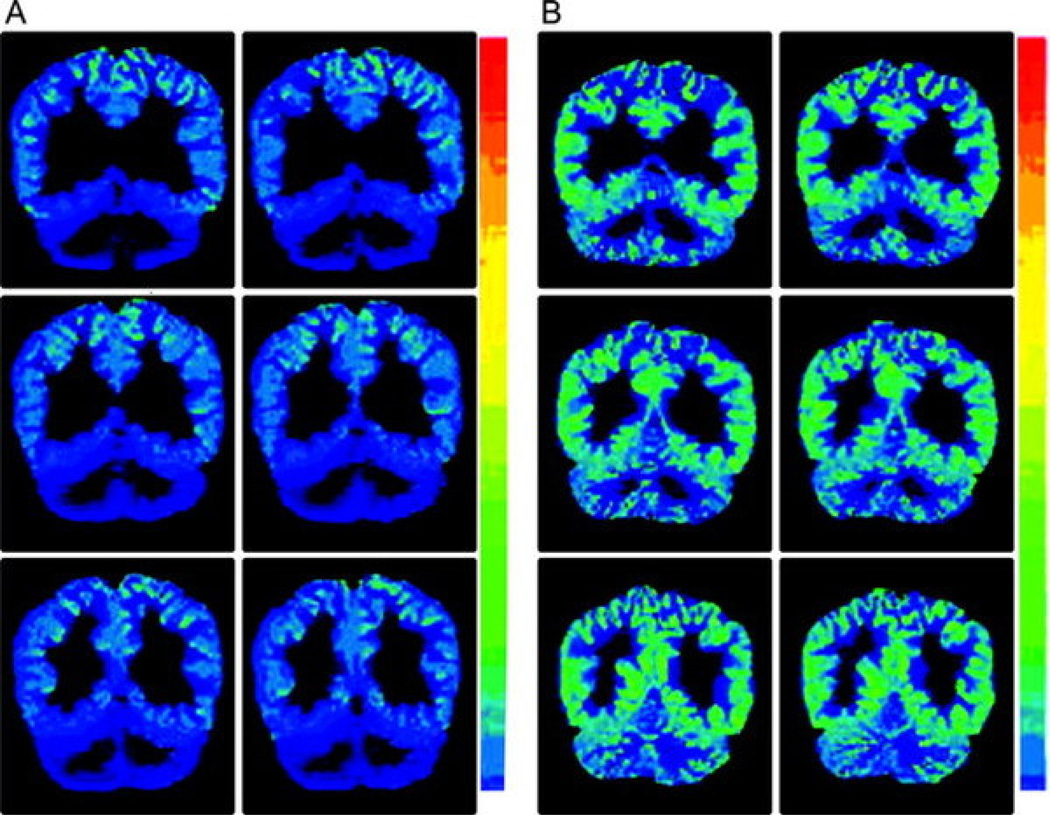
GABAB mediated activity is responsible for physiologic de-inactivation of T-type calcium channels that lead to the spike discharge in absence seizures. Hence, excessive GABAB activity may be predicted to exacerbate absence seizures, as seen with administration of vigabatrin, raising overall GABA levels, or more specifically baclofen, a GABAB receptor agonist. Further, decreased GABAergic activity could potentially be associated with a transition from absence to generalized convulsive seizures later in life. PET has been performed using the benzodiazepine receptor antagonist FMZ in SSADH deficient patients, unaffected parent heterozygotes and healthy adult controls [19]. Results showed a significant reduction in FMZ binding in the SSADH deficient individuals in comparison to the controls in all regions of interest: basal ganglia, amygdala, hippocampus, cerebellar vermis, and frontal, parietal and occipital cortices (Figure 5). There was no noted difference in binding between the controls and the heterozygotes. This demonstrates a decrease in the number of GABAA-benzodiazepine binding sites in the central nervous system of patients with SSADH deficiency, and suggests overuse-dependent down-regulation of GABAAR activity.
Figure 5. Decreased GABAA binding on FMZ-PET in SSADH deficiency.
FMZ-PET shows marked reduction of cortical binding potential of [11C]-flumazenil in patient with SSADH deficiency (A) versus heterozygote control (B). (Reproduced with permission from Pearl et al. Neurology 2009;73:423–9.)
Additionally TMS with single and paired-pulse stimuli was performed on eight sets of SSADH deficient individuals and their heterozygous parents [20]. Loss of long interval intracortical inhibition and a reduced cortical silent period were noted in affected individuals when compared to heterozygotes and healthy controls, consistent with diminished GABABR cortical activity. Hence, there is evidence of desensitization of both GABAA and GABAB receptor activity in vivo, suggesting less GABA mediated inhibition and potentially subsequent predisposition to convulsive seizures.
Murine Model
Absence seizures may be evoked in mice with direct application of GHB [21]. In SSADH deficiency, there is accumulation of both GABA and GHB, as well as dysregulation of multiple amino acids (glutamate, glutamine, alanine, aspartate, serine, taurine, cystathionine, methionine, homocarnosine, arginine) [22]. Of all interventions, NCS-382, a GHB receptor antagonist, has had the highest survival rate in the mutant animal model [1]. Absence seizures may also be artificially induced in the SSADH (−/−) mouse model using baclofen, a GABABR agonist, or GHB, which acts via both GHB and GABAB receptor mediated mechanisms to produce 7Hz spike-wave discharges in thalamocortical circuitry [2]. Absence seizures are abolished in the mouse model with the use of the GABABR antagonist CGP35348 [2] or the antiepileptic ethosuximide, which causes voltage dependant blockade of T-type calcium currents. It has also been demonstrated that although the increase in whole-brain GABA contents in SSADH(−/−) mice does not change spontaneous inhibitory postsynaptic currents, a measure of synaptic GABAergic connections, it does increase tonic inhibition in the neocortex through presumed extrasynaptic GABAA receptors [23]. This implies that the increased extracellular GABA levels may act through extrasynaptic GABAA receptors to cause excessive cortical GABAergic neurotransmission, thus aiding in absence seizure production in SSADH deficiency.
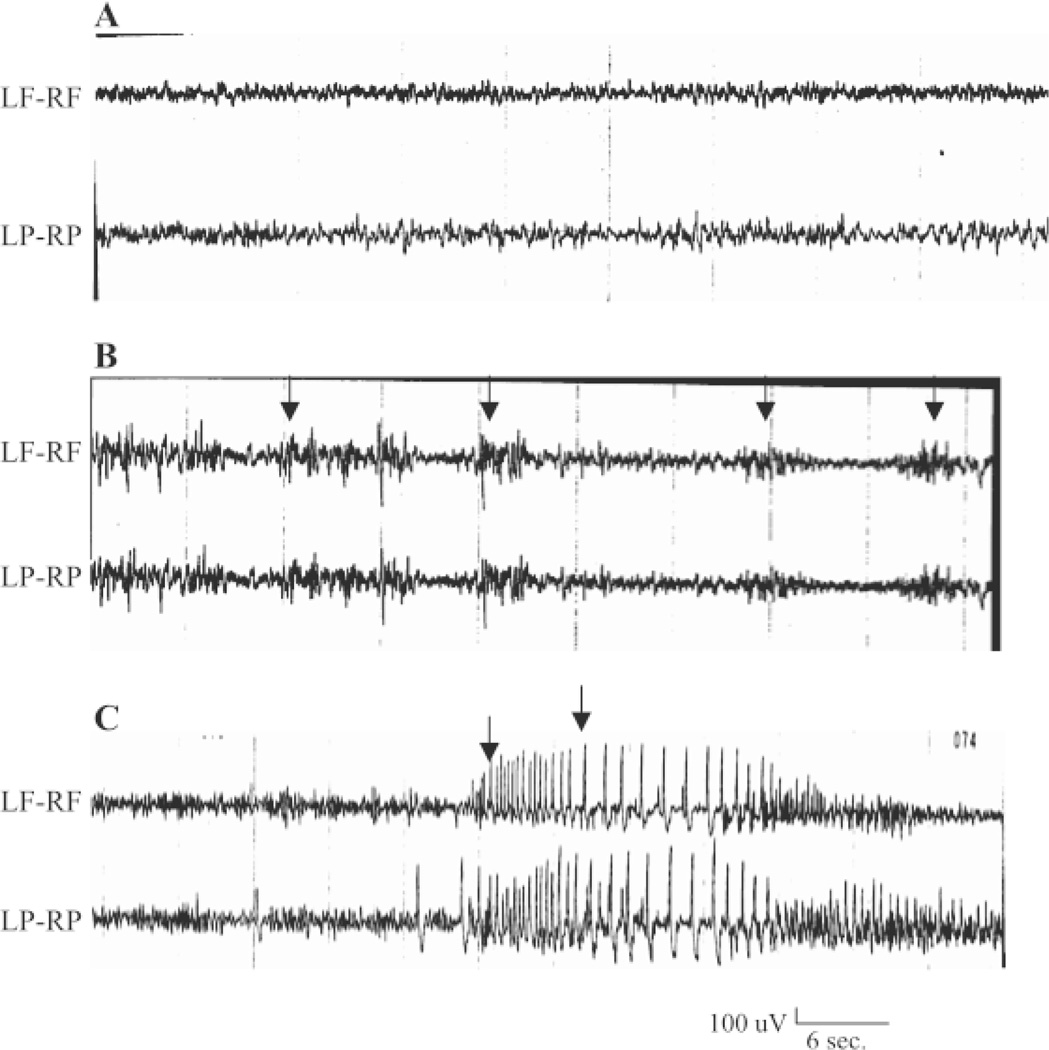
The transition from absence to generalized convulsive seizures has been demonstrated in the SSADH(−/−) mouse using electrocorticogram (ECoG) recordings in P16 and P20 mice. Compared to an uneventful baseline ECoG of P16 wild type (+/+) mice, P16 SSADH (−/−) mice displayed 250–300µV, 5–7Hz spike-and-wave discharges (SWD) lasting 3–6s in duration (Figure 6. This activity was accompanied by a frozen stare and vibrissal twitching, and was consistent with absence epilepsy. P20 SSADH null mice were also tested using ECoG, and rather than the SWD seen in absence seizures, a generalized 600µV discharge at 5Hz followed by 1.5–2Hz SWD were observed, which was indicative of tonic-clonic seizures. Such generalized tonic-clonic seizures were considered to be the cause of mortality in these SSADH null mice due to the later development of convulsive status epilepticus [2].
Figure 6. Transition from absence to tonic-clonic seizures in P20 SSADH(−/−) mice.
(A) Baseline electrocorticogram (ECoG) recordings in P16 wild-type (+/+) mice show uneventful 35–50 uV and 5–7 Hz oscillations. (B) ECoG of P16 SSADH (−/−) mice reveals 250–300 uV, 5–7 Hz spike-and-wave discharges (SWD) lasting 3–6s duration. This was associated with frozen stare and vibrissal twitching, suggesting absence seizures. (C) ECoG recording in P20 SSADH(−/−) mice show a transition from absence to a generalized 600 uV at 5 Hz followed by 1.5 to 2 Hz SWD associated with tonic-clonic seizures (arrow). LF= left frontal, RF= right frontal, LP= left parietal, RP= right parietal. (Reproduced with permission from: Cortez et al. Pharmacol Biochem Behav 2004;79:547–53.)
The mechanism for induction of absence seizures by GHB in the mouse model does not explain the development of convulsive seizures. A postulate is that absence seizures may evolve into generalized convulsive seizures through overuse-dependent down-regulation of both GABAA and GABAB receptors in the presence of high levels of GABA and GHB. This could result in a breakdown of GABA-mediated inhibition, consequently causing cortical hyperexcitability and rendering an epileptogenic state.
Decreased binding of GABAAR antagonist [35S]
Tert-butylbicyclophosphorothionate has been demonstrated in the cortex, hippocampus and thalamus of P19 SSADH(−/−) mice compared to SSADH(+/+) mice before the onset of tonic-clonic seizures [24]. There is specific downregulation of the GABAAR subunit β2 demonstrated through immunohistochemical staining of the SSADH(−/−) mouse brain. Spontaneous rhythmic activity was recorded in 67% of SSADH(−/−) mice, but no spontaneous discharges were seen in the wild type model. Extracellular field recordings in hippocampal CA1 pyramidal layers of P14 SSADH null mice show larger amplitudes of evoked postsynaptic field potentials and an increased number of population spikes. Additionally, a significant reduction in input resistance and resting membrane potential of hippocampal neurons is seen in P14 but not P9 SSADH(−/−) mice. These findings suggest a hyperexcitable state in the pyramidal cells of SSADH deficient mice after the second week of life. GABAAR-mediated IPSPs are also noted to be reduced in hippocampal neurons in both P8 and P14 SSADH null mice. It is hypothesized that this demonstrated decrease in surface GABAAR activity in SSADH deficient mice may be due to increased receptor endocytosis in the presence of increased GABA levels and prolonged occupancy of GABAA receptors [24].
There is parallel evidence for GABAB downregulation in the null mouse [9]. Decreased binding of the GABABR antagonist CGP54626A is seen throughout the brain, and particularly in the hippocampus of both P7 and P14 SSADH(−/−) mice when compared to wild type. Moreover, there is a significant decrease in GABAB mediated synaptic potentials in both P7 and P14 SSADH(−/−) mice compared to wild type mice. Prolonged agonist treatment of GABAB receptors appears to result in increased endocytosis of GABABR with resultant decrease in receptor function, again supporting use-dependent GABAB downregulation, which may mediate the development of generalized convulsive seizures.
Treatment Strategies and Clinical Trials
Treatment for SSADH deficiency remains problematic and no consistently successful therapy has emerged [25]. Treatment is symptomatic and targeted to clinical deficits. Options include anxiolytic agents or selective serotonin reuptake inhibitors (SSRIs) and related medications for obsessive-compulsive disorder. Appropriate antiepileptics are chosen for generalized epilepsy other than avoidance of valproate due to its ability to inhibit any residual SSADH enzymatic activity [26]. Hence, worsening of the patient in the vignette provided (vide supra) was attributed in retrospect to the metabolic diagnosis. Antiepileptics used successfully for maintenance therapy have been lamotrigine, levetiracetam, and topiramate.
Vigabatrin, an irreversible inhibitor of GABA-transaminase, is a logical choice because it will inhibit the conversion of GABA to GHB, a putative pathogen in this condition. A video-manuscript report of two adolescent brothers demonstrates exercise-induced paroxysmal dyskinesias, manifest by a prominently lurching gait, which showed some improvement following vigabatrin therapy [27]. Vigabatrin, however, has not been a consistently helpful therapeutic for these patients, and there have been many reports of lack of effect or, worse, worsening of symptoms ranging from seizure control to alertness. Further, safety concerns persist regarding retinal toxicity of vigabatrin, especially in this patient population that is unable to report visual field defects. In clinical trials, 30% of patients treated with vigabatrin for epilepsy report visual field defects following one year of treatment [28, 29, 30] MRI signal changes caused by vigabatrin, particularly prominent in the GABA-rich thalamus and basal ganglia, pose additional concerns regarding vigabatrin use in SSADH deficiency [8, 9]. While vigabatrin will lead to at least transient decreases in CSF GHB levels [31], there may be a deleterious effect related to attendant increases in CSF (and brain) GABA levels [13].
Clinical trials have been proposed and initiated with several agents. Taurine, an amino acid with several possible neurotropic roles, lengthened survival in the SSADH mutant mouse using either intraperitoneal or oral application [32]. Taurine is associated with an observed safe level in humans at 3 grams/day, and higher dosages have been tested without significant adverse effects. In a single case report available as an abstract, taurine was reported to improve gait, coordination and energy of a 2 ½-year-old boy with SSADH deficiency [33]. The patient was given 4 grams/day (~ 200 mg/kg) over one year. Higher doses were associated with insomnia. At 9 months teachers reported improved behavior, peer interactions, increased level of activity and coordination. At 12 months, the boy’s MRI was interpreted as improved. No correlation was found between improved behavior and urine GHB levels. The case was neither controlled or blinded, but a clinical trial is in progress at our center.
Animal work in the SSADH mutant model has suggested benefit from treatment with SGS 742, a GABAB receptor antagonist. Earlier studies had shown promise with a similar compound, CGP-35348 [32, 34]. SGS 742 improves electrocorticographic epileptiform activity in the mutant mouse model. Phase II human studies in adults with mild cognitive impairment have shown significantly improved attention, reaction time, visual information processing, and working memory as well as a good safety profile [35]. Planned clinical trials of this agent in SSADH deficiency are dependent upon access to the compound.
Conclusions
SSADH deficiency is a rare neurometabolic disorder characterized by developmental delay, hypotonia, cerebellar ataxia, and hyporeflexia in early childhood. Epilepsy affects approximately half of patients, and generalized epileptiform abnormalities are common on EEG. The severity of the epilepsy is variable, although some patients have experienced repeated status epilepticus and SUDEP has been reported. ESES has been documented, and a less common but more severe phenotype includes active myoclonic seizures. In the murine model, early absence seizures transition into generalized convulsive seizures, and ultimately lethal status epilepticus in the third week of life. Correlation of murine and human data suggest that absence seizures are related to excessive GHB and GABAB mediated activity, and generalized convulsive seizures may be an effect of overuse dependent down-regulation of GABAA and GABAB receptor activity. The latter would result in a hyperexcitable state and thus epileptogenesis in this hyper-GABAergic disorder.
Acknowledgements
This work is supported by National Institutes of Health NS 40270/HD58553 (KMG, PLP), Pediatric Neurotransmitter Disease Association (KMG, PLP), Delman Fund for Pediatric Neurology Research (PLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was presented in the Joint Meeting of Infantile Seizure Society (13th Annual Meeting) and Taiwan Child Neurology Society (14th Annual Meeting), International Symposium on Epilepsy in Neurometabolic Diseases, Taipei, Taiwan, March 26–28, 2010.
References
- 1.Pearl PL, Gibson KM, Acosta MT, Vezina LG, Theodore WH, Rogawski MA, et al. Clinical spectrum of succinic semialdehyde dehydrogenase deficiency. Neurology. 2003;60:1413–1417. doi: 10.1212/01.wnl.0000059549.70717.80. [DOI] [PubMed] [Google Scholar]
- 2.Cortez MA, Wu Y, Gibson KM, Snead OC., 3rd Absence seizures in succinic semialdehyde dehydrogenase deficient mice: a model of juvenile absence epilepsy. Pharmacol Biochem Behav. 2004;79:547–553. doi: 10.1016/j.pbb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Knerr I, Gibson KM, Jakobs C, Pearl PL. Neuropsychiatric morbidity in adolescent and adult succinic semialdehyde dehydrogenase deficiency patients. CNS Spectr. 2008;13:598–605. doi: 10.1017/s1092852900016874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearl PL, Gibson KM. Clinical aspects of the disorders of GABA metabolism in children. Curr Opin Neurol. 2004;17:107–113. doi: 10.1097/00019052-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Pearl PL, Capp PK, Novotny EJ, Gibson KM. Inherited disorders of neurotransmitters in children and adults. Clin Biochem. 2005;38:1051–1058. doi: 10.1016/j.clinbiochem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Pearl PL, Jakobs C, Gibson KM. Valle D, Beaudet A, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, editors. Disorders of beta- and gamma-amino acids in free and peptide-linked forms. Online molecular and metabolic bases of inherited disease. 2007 Online: www.ommbid.com. [Google Scholar]
- 7.Dervent A, Gibson KM, Pearl PL, Salomons GS, Jakobs C, Yalcinkaya C. Photosensitive absence epilepsy with myoclonia and heterozygosity for succinic semialdehyde dehydrogenase (SSADH) deficiency. Clin Neurophysiol. 2004;115:1417–1422. doi: 10.1016/j.clinph.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Pearl PL, Shamin S, Theodore WH, Gibson KM, Forester K, Combs SE, et al. Polysomnographic abnormalities in succinic semialdehyde dehydrogenase (SSADH) deficiency. Sleep. 2009;36:1645–1648. doi: 10.1093/sleep/32.12.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzzi A, Wu Y, Frantseva MV, Velazquez JLP, Cortez MA, Liu C, et al. Succinic semialdehyde dehydrogenase deficiency: GABAB receptor-mediated function. Brain Res. 2006;1090:15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 10.Yalçinkaya C, Gibson KM, Gündüz E, Koçer N, Fiçicioğlu C, Küçükercan I. MRI findings in succinic semialdehyde dehydrogenase deficiency. Neuropediatrics. 2000;31:45–46. doi: 10.1055/s-2000-15298. [DOI] [PubMed] [Google Scholar]
- 11.Ziyeh S, Berlis A, Korinthenberg R, Spreer J, Schumacher M. Selective involvement of the globus pallidus and dentate nucleus in succinic semialdehyde dehydrogenase deficiency. Pediatr Radiol. 2002;32:598–600. doi: 10.1007/s00247-002-0717-4. [DOI] [PubMed] [Google Scholar]
- 12.Escalera GI, Ferrer I, Marina LC, Sala PR, Salomons GS, Jakobs C, et al. Succinic semialdehyde dehydrogenase deficiency: decrease in 4-OH-butyric acid levels with low doses of vigabatrin. An Pediatr (Barc) 2010;72:128–132. doi: 10.1016/j.anpedi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Pearl PL, Gropman A. Monitoring gamma-hydroxybutyric acid levels in succinate-semialdehyde dehydrogenase deficiency. Ann Neurol. 2004;55:599. doi: 10.1002/ana.20084. [DOI] [PubMed] [Google Scholar]
- 14.Al-Essa MA, Bakheet SM, Patay ZJ, Powe JE, Ozand PT. Clinical, fluorine-18 labeled 2-fluoro-2-deoxyglucose positron emission tomography (FDG PET), MRI of the brain and biochemical observations in a patient with 4-hydroxybutyric aciduria; a progressive neurometabolic disease. Brain Dev. 2000;22:127–131. doi: 10.1016/s0387-7604(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 15.Knerr I, Gibson KM, Murdoch G, Salomons GS, Jakobs C, Combs S, et al. Neuropathology in succinic semialdehyde dehydrogenase deficiency. Pediatr Neurol. 2010;42:255–258. doi: 10.1016/j.pediatrneurol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson KM, Schor DS, Gupta M, Guerand WS, Senephansiri H, Burlingame TG, et al. Focal neurometabolic alterations in mice deficient for succinate semialdehyde dehydrogenase. J Neurochem. 2002;81:71–79. doi: 10.1046/j.1471-4159.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- 17.Pearl PL, Novotny EJ, Acosta MT, Jakobs C, Gibson KM. Succinic semialdehyde dehydrogenase deficiency in children and adults. Ann Neurol. 2003;54:S73–S80. doi: 10.1002/ana.10629. [DOI] [PubMed] [Google Scholar]
- 18.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 19.Pearl PL, Gibson KM, Quezado Z, Dustin I, Taylor J, Trzcinski S, et al. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009;73:423–429. doi: 10.1212/WNL.0b013e3181b163a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearl PL, Gibson KM, Cortez MA, Wu Y, Carter Snead O, 3rd, Knerr I, et al. Succinic semialdehyde dehydrogenase deficiency: Lessons from mice and men. J Inherit Metab Dis. 2009;32:343–352. doi: 10.1007/s10545-009-1034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snead OC., 3rd The gamma-hydroxybutyrate model of absence seizures: correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges. Neuropharmacology. 1991;30:161–167. doi: 10.1016/0028-3908(91)90199-l. [DOI] [PubMed] [Google Scholar]
- 22.Gupta M, Polinsky M, Senephansiri H, Snead OC, Jansen EE, Jakobs C, et al. Seizure evolution and amino acid imbalances in murine succinate semialdehyde dehydrogenase (SSADH) deficiency. Neurobiol Dis. 2004;16:556–562. doi: 10.1016/j.nbd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Drasbek KR, Vardya I, Delenclos M, Gibson KM, Jensen K. SSADH deficiency leads to elevated extracellular GABA levels and increased GABAergic neurotransmission in the mouse cerebral cortex. J Inherit Metab Dis. 2008;31:662–668. doi: 10.1007/s10545-008-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Buzzi A, Frantseva M, Velazquez JP, Cortez M, Liu C, et al. Status epilepticus in mice deficient for succinate semialdehyde dehydrogenase: GABAA receptor-mediated mechanisms. Ann Neurol. 2006;59:42–52. doi: 10.1002/ana.20686. [DOI] [PubMed] [Google Scholar]
- 25.Bishnoi M, Chopra K, Kulkarni SK. Progesterone attenuates neuroleptic-induced orofacial dyskinesia via the activity of its metabolite, allopregnanolone, a positive GABA(A) modulating neurosteroid. Prog Neuropsychopharmacol Biol Pychiatry. 2008;32:451–461. doi: 10.1016/j.pnpbp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Shinka T, Ohfu M, Hirose S, Kuhara T. Effect of valproic acid on the urinary metabolic profile of a patient with succinic semialdehyde dehydrogenase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;792:99–106. doi: 10.1016/s1570-0232(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 27.Leuzzi V, Di Sabato ML, Deodato F, Rizzo C, Boenzi S, Carducci C, et al. Vigabatrin improves paroxysmal dystonia in succinic semialdehyde dehydrogenase deficiency. Neurology. 2007;68:1320–1321. doi: 10.1212/01.wnl.0000259537.54082.6d. [DOI] [PubMed] [Google Scholar]
- 28.Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–618. doi: 10.1212/wnl.50.3.614. [DOI] [PubMed] [Google Scholar]
- 29.Spence SJ, Sankar R. Visual field defects and other ophthalmological disturbances associated with vigabatrin. Drug Saf. 2001;24:385–404. doi: 10.2165/00002018-200124050-00005. [DOI] [PubMed] [Google Scholar]
- 30.Vanhatalo S, Nousiainen I, Eriksson K, Rantala H, Vainionpaa L, Mustonen K, et al. Visual field constriction in 91 Finnish children treated with vigabatrin. Epilepsia. 2002;43:748–756. doi: 10.1046/j.1528-1157.2002.17801.x. [DOI] [PubMed] [Google Scholar]
- 31.Ergezinger K, Jeschke R, Frauendienst-Egger G, Korall H, Gibson KM, Schuster VH. Monitoring of 4-hydroxybutyric acid levels in body fluids during vigabatrin treatment in succinic semialdehyde dehydrogenase deficiency. Ann Neurol. 2003;54:686–689. doi: 10.1002/ana.10752. [DOI] [PubMed] [Google Scholar]
- 32.Gupta M, Greven R, Jansen EE, Jakobs C, Hogema BM, Froestl W, et al. Therapeutic intervention in mice deficient for succinate semialdehyde dehydrogenase (gamma-hydroxybutiric aciduria) J Pharmacol Exp Ther. 2002;302:180–187. doi: 10.1124/jpet.302.1.180. [DOI] [PubMed] [Google Scholar]
- 33.Saronwala A, Tournay A, Gargus JJ. Taurine treatment of succinate semialdehyde dehydrogenase (SSADH) deficiency reverses MRI-documented globus lesions and clinical syndrome. Am Coll Med Genet; 15th Ann Clinical Genet Meeting; March 12–16 2008; Phoenix AZ USA. p. 103. [Abstract]. [Google Scholar]
- 34.Hogema BM, Gupta M, Senephansiri H, Burlingame TG, Taylor M, Jakobs C, et al. Pharmacologic rescue of lethal seizures in mice deficient in succinate semialdehye dehydrogenase. Nat Genet. 2001;29:212–216. doi: 10.1038/ng727. [DOI] [PubMed] [Google Scholar]
- 35.Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, et al. SGS742: the first GABA(B) receptor antagonist in clinical trials. Biochem Pharmacol. 2004;68:1479–1487. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]