Abstract
The cyclooxygenase-2 (COX-2) enzyme and major lipid product, prostaglandin E2 (PGE2) are elevated in many solid tumors including those of the breast and are associated with a poor prognosis. Targeting this enzyme is somewhat effective in preventing tumor progression, but is associated with cardiotoxic secondary effects when used chronically. PGE2 functions by signaling through four EP receptors (EP1-4), resulting in several different cellular responses, many of which are pro-tumorigenic, and there is growing interest in the therapeutic potential of targeting EP4 and EP2. Other members in this signaling pathway are gaining more attention. PGE2 is transported out of and into cells by two unique transport proteins. Multiple Drug Resistance-Associated Protein 4 (MRP4) and Prostaglandin Transporter (PGT) modulate PGE2 signaling by increasing or decreasing the levels of PGE2 available to cells. 15-hydroxyprostaglandin dehydrogenase (15-PGDH) metabolizes PGE2 and silences the pathway in this manner (Figure 1). The purpose of this review is to summarize the extensive data supporting the importance of the COX-2 pathway in tumor biology with a focus on more recently described pathway members and their role in modulating PGE2 signaling. This review describes evidence supporting roles for MRP4, PGT and 15-PGDH in several tumor types with an emphasis on the roles of these proteins in breast cancer. Defining the importance of these latter pathway members will be key to developing new therapeutic approaches that exploit the tumor-promoting COX-2 pathway.
Introduction
Currently, cancer is the cause of 1 in 4 deaths in the United States (1). Breast cancer is the most frequently diagnosed cancer among women accounting for 23% of total cancer diagnoses and 14% of cancer-related deaths (1). Metastatic disease is the primary cause of cancer-related death, and therefore, components of the metastatic process are attractive therapeutic targets (2). Elevated expression of both the cyclooxygenase-2 (COX-2) enzyme and its major lipid product, prostaglandin E2 (PGE2) is detected in many solid tumors, including breast cancer (3–6). Elevated expression of these two molecules is associated with a poor prognosis. PGE2 initiates multiple signaling pathways upon binding to a family of four G-protein-coupled receptors (EP1-EP4). EP4 and/or EP2-signaling promotes several of the steps in the metastatic process (3, 4, 6, 7). Additionally, PGE2 signaling is one pathway that has been implicated in the formation and maintenance of cells responsible for repopulating a tumor after surgery and/or chemotherapy eradicates the majority of tumor cells; i.e tumor initiating or cancer stem-like cells (8–12). Much of the effort to exploit the COX-2 pathway therapeutically has focused on direct inhibition of the COX-2 enzyme or, more recently, on inhibition of EP4 signaling. Although non-steroidal anti-inflammatory drugs (NSAIDs) that inhibit COX enzymes show tumor preventative effects, long-term use of these therapies has been shown to increase the risk for severe cardiotoxic side effects. Tumors also adapt to these therapies by activating compensatory mechanisms such as modulating expression of pathway member proteins or diverting the COX-2 substrate, arachidonic acid, to lipoxygenases, another inflammatory pathway not targeted by COX inhibitors (13). Therefore, new therapeutic targets in this oncogenic pathway need to be identified.
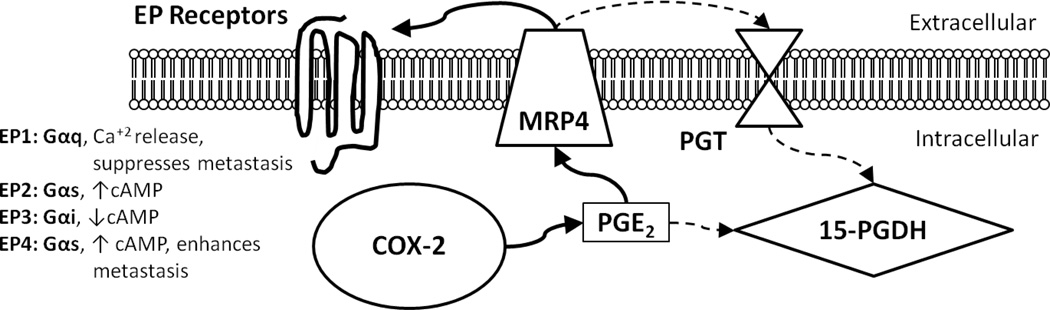
Other components of the COX-2 pathway regulate the intracellular and extracellular levels of PGE2 available to mediate cell signaling. Multiple Drug Resistance-Associated Protein 4 (MRP4) and Prostaglandin Transporter (PGT) modulate PGE2 signaling by respectively increasing or decreasing the levels of extracellular PGE2 available to cells. 15-hydroxyprostaglandin dehydrogenase (15-PGDH) metabolizes PGE2 intracellularly into an inactive form and silences the signaling pathway in this manner (Figure 1). The purpose of this review is to briefly summarize the extensive literature supporting the importance of the COX-2 pathway to tumor biology with a focus on describing our more limited understanding of the role that MRP4, PGT and 15-PGDH may play in tumor progression.
Figure 1. Schematic of PGE2 synthesis, transport, signaling, and metabolism.
The cyclooxygenase-2 (COX-2) enzyme is the rate-limiting step in the production of prostaglandin E2 (PGE2) from arachidonic acid. PGE2 is exported from the cell via multiple drug resistance-associated protein 4 (MRP4). In an autocrine or paracrine manner, extracellular PGE2 binds any of its four cognate EP receptors, EP1-4, and initiates diverse signaling pathways via activation of G-proteins. Alternatively, PGE2 is imported via the prostaglandin transporter (PGT) and inactivated by 15-hydroxyprostaglandin dehydrogenase (15-PGDH). The bold arrows indicate members of this pathway up-regulated in cancer while the dotted arrows indicate members of this pathway down-regulated in cancer.
Cyclooxygenase-2 and Prostaglandin E2
The cyclooxygenase enzyme is expressed in two forms, cyclooxygenase (COX)-1 and COX-2 (3–5). While COX-1 is constitutively expressed in most tissues, COX-2 is induced by inflammatory stimuli; moreover, aberrant expression of COX-2 is commonly found in solid tumors (3, 4, 14). COX-2 is responsible for the rate-limiting step in the conversion of arachidonic acid to prostaglandins. Both COX-1 and COX-2 enzymes are targets for NSAIDs (e.g., aspirin, ibuprofen, and indomethacin) while COX-2 is uniquely inhibited by drugs in the coxib family (e.g., celecoxib, rofecoxib, valdecoxib). Elevated COX-2 expression is found in nearly half of all breast cancer samples and is associated with a worse prognosis (15, 16). A more recent analysis confirmed that COX-2 is often elevated, but did not detect significant differences in COX-2 expression among different molecular subtypes of breast cancer, suggesting that COX-2 targeting may be broadly applicable in diverse breast cancers (5, 17).
Prostaglandin E2 EP Receptor Signaling
PGE2 initiates diverse signaling pathways in cells upon binding to four G-protein coupled receptors, termed EP1 through EP4 (18). Gαq is activated upon PGE2 binding to the EP1 receptor while EP2 and EP4 receptors activate Gαs, and EP3 is linked to Gαi. EP activation is linked to multiple effector pathways including phosphoinositide-3-kinase (PI3K), protein kinase B (AKT), protein kinase A (PKA), epidermal growth factor receptor (EGFR), Src kinase and Ras/MEK/ERK (18). EP4 and EP2 are strongly implicated in multiple processes that contribute to cancer progression (18). Downstream cellular responses induced by PGE2 signaling include angiogenesis, anti-apoptosis, proliferation, migration, invasion, immune evasion, epithelial-mesenchymal transition (EMT), and support of a cancer stem-like cell phenotype, all of which are associated with the established or emerging hallmarks of cancer (3, 12, 18–21). Kundu et al. reported increased levels of COX-2, PGE2 and EP4 in malignant and metastatic breast cells with stem-like properties compared to the bulk population (12). The ability of tumor cells to form mammospheres (an indication of breast cancer stem cell phenotype) was disrupted by several EP4 antagonists corresponding to a loss of phenotypic markers of stem-like behavior (12). The tumor-initiating capacity of putative breast cancer stem cells was markedly reduced in mice treated with EP4 antagonists (12). The vast majority of signaling through EP receptors has been described in plasma membrane bound receptors; however, there is growing evidence of functional nuclear or perinuclear localized EP receptors (18, 22). Nuclear localization of EP receptors has been detected in both normal and malignant tissues and tissue context may play an important role in determining the function of these receptors. When detected by immunohistochemistry in malignant breast cancer, nuclear EP1 correlated with a better prognosis than malignancies with no nuclear staining (23). Conversely, in cholangiocarcinoma nuclear EP1 was shown to enhance the activation of signal transducer and activator of transcription-3 (STAT3) and induce tumor cell growth (24).
MRP4
Multiple Drug Resistance-associated Protein 4 (MRP4/ABCC4) is the fourth member of the C subfamily of ATP-binding cassette (ABC) proteins (25, 26). MRP4 was discovered for its role in mediating drug resistance in several tumor types. MRP4 exports a wide range of exogenous compounds including anti-retroviral compounds, anti-HIV compounds, camptothecins, methotrexate, and ceftins (27, 28). Endogenous substrates for MRP4 include prostaglandins, leukotrienes, cyclic nucleotides (cAMP and cGMP) and glutathione-conjugated molecules such as bile acids and steroid hormones (27–29). Elevated expression of MRP4 has also been detected in drug-naïve tumors including neuroblastoma, prostate cancer, pancreatic cancer, and acute myeloid leukemia, all in which MRP4 expression is associated with a worse prognosis (28–32). While some of these tumor types utilize MRP4 in defined mechanisms such as exporting identified substrates, the role of MRP4 in other malignancies remains elusive (29, 32). MRP4 has been identified as the main efflux transporter of PGE2, but whether this function is important to malignant behavior is not known (4, 27, 29, 33). Autocrine signaling by PGE2 promotes cell survival and proliferation in neuroblastoma, one of the tumor types strongly associated with elevated MRP4 expression (28, 30, 34). We have detected a gradient of MRP4 expression in breast cancer cell lines that parallels metastatic potential, suggesting that MRP4 contributes to malignant behavior in breast cancer (unpublished observations). MRP4 is able to transport other prostanoids, but since PGE2 is the main product of COX-2 in breast cancer, we and others have hypothesized that altering the expression or activity of MRP4 in breast cancer should modulate the impact of PGE2 in this disease (27, 29, 35, 36).
MRP4 Regulation
ABCC4 is the gene that encodes MRP4 (27, 29, 37). Transcription of ABCC4 mRNA is regulated by several signaling pathways including the aryl hydrocarbon receptor (AhR), glucocorticoid receptor (GR), nuclear factor (erythroid-derived 2)-like 2 (Nrf2), peroxisome proliferator-activated receptor alpha (PPARα), and the N-myc oncogene (34, 38, 39). Interestingly, the anti-inflamatory lipid lipoxin A4 (LXA4) inhibits MRP4 mRNA and protein expression through estrogen receptor alpha (40). Micro-RNAs (miRNAs) miR-124a and miR-506 suppress MRP4 protein translation in a tissue-specific manner (41). MRP4 expression is also regulated by alternative splicing and nonsense mediated decay of a truncated mRNA transcript (42). Once translated, MRP4 is localized to the apical membrane of polarized cells, following interaction with Na+/H+ Exchanger Regulatory Factor 1 (NHERF1) (43). In the absence of NHERF1 expression, MRP4 is found at the basolateral membrane of polarized cells. It should be noted that loss of epithelial cell polarity is one of the key characteristics of tumor progression (44). Interestingly, in non-polarized cells, NHERF1 protein controls MRP4 internalization from the membrane through interaction with the C-terminal PDZ domain on MRP4 (45). Sorting nexin 27 (SNX27) is another protein shown to interact with the PDZ domain on MRP4 in a similar mechanism (46). This internalization mechanism attenuates the activity of MRP4 by removing MRP4 from the cell membrane and trafficking it to the late endosome (45).
MRP4 Inhibition
Numerous pharmacologic inhibitors have been identified for MRP4, but like other multidrug resistance (MDR) inhibitors, none have shown clear clinical benefit in reversing drug resistance (29). Inhibitors of MRP4 include NSAIDs such as indomethacin and sulindac, cyclic nucleotide analogs such as thiopurines, and phosphodiesterase inhibitors including sildenafil and dipyridamole (27, 28). These inhibitory molecules are assumed to function by competing with the substrate-binding site of MRP4 to prevent the transport of the intended substrate. The leukotriene D4 antagonist MK571 inhibits the activity of MRP4 possibly by stimulating internalization (27, 28, 45, 47). Recently, two additional MRP4 inhibitors have been identified from a compound library and are under investigation for specificity and off-target effects (48).
PGT: PGE2 Import
While MRP4 exports PGE2 to the extracellular space, internalization and inactivation of PGE2 is performed by two distinct proteins. PGE2 is imported into cells via the prostaglandin transporter (PGT) and subsequently oxidized to 15-keto-PGE2 by the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH, Fig. 1). Both of these steps are required for efficient inactivation of PGE2 and suppression of signaling (49). PGT functions as an antiporter by exchanging a lactate anion for PGE2, which is negatively charged at physiological pH (50). Since most tumor cells have high intracellular lactate as a byproduct of glycolysis, the efflux of lactate facilitates the influx of PGE2 from the pericellular space (3, 33). Importing PGE2 from the pericellular space attenuates signaling by preventing PGE2 from binding to and activating EP receptors on the cell surface (33, 51). PGT could function in a tumor cell autonomous manner; however, transport function in other cells in the tumor microenvironment may also be important in controlling the extracellular levels of PGE2. In fact, PGT expressed on adipocytes decreased the levels of extracellular PGE2 and subsequent PGE2-induced gene transcription in vitro (51). Since adipocytes comprise a large proportion of the non-tumor cells in the breast cancer microenvironment, PGT expression could be tumor-preventative by suppressing PGE2 signaling (52).
PGT Regulation
Since the role of PGT is to assist in the resolution of PGE2 signaling, it is often expressed during resolution of an inflammatory response. However, in the environment of chronic inflammation, PGT is expressed at lower levels than in normal tissue (53). It is unclear whether the reduction of PGT expression causes chronic inflammation by decreasing PGE2 clearance, or is a result of the sustained pro-inflammatory environment. Cancer is often referred to as “a wound that never heals” since many wound healing pathways are consistently activated in a tumor. PGT expression is increased in diabetic skin and contributes to slower healing in this tissue (53). Hyperglycemia induced PGT expression in human dermal microvascular endothelial cells, and increased clearance of PGE2 from a wound site suppressed the rate of healing (53). Inhibition of PGT with T26A, a recently developed novel inhibitor of PGT, increased wound healing in a dose-dependent manner (53, 54). β-catenin has also been associated with decreased PGT expression in intestinal epithelium and colorectal tumor cells (55). Aberrant β-catenin signaling is found in many solid tumors and contributes to proliferative and migratory phenotypes found in aggressive cancer. In colorectal cancer cell lines in which PGT expression was lower than in normal cells, PGT expression was induced by treatment with the DNA demethylating agent 5-aza-2’-deoxycytidine and the histone deacetylase inhibitor trichostatin A (33). This suggests that PGT expression is suppressed in colorectal tumors by an epigenetic mechanism in a direct or indirect manner. Since PGT is a mediator of intracellular lactate, it would be reasonable to expect PGT to be highly expressed in cells with overly active glycolysis; however, other lactate transporter proteins such as monocarboxylate transporter 1 (MCT1) that cotransport lactate molecules along with H+ ions are highly expressed in cancer thereby relieving lactate stress in the tumor cell and avoiding the clearance of PGE2 from the microenvironment (56).
MRP4 and PGT: PGE2 Export and Import
Holla, et al. recognized the inverse relationship between PGT and MRP4 in the regulation of PGE2 transporters in colorectal cancer (33). They examined colorectal tumor samples and found that MRP4 is increased and PGT is decreased, when compared to adjacent normal tissue. Eruslanov et al. show that tumor-derived factors in conditioned media not only suppressed the expression of PGT and 15-PGDH, but also enhanced the expression of MRP4 in myeloid cells (57). The identity of the molecule(s) in the conditioned media responsible for these effects has not been elucidated. These changes in PGE2 transporter expression led to increased levels of PGE2 in the microenvironment and enhanced PGE2 signaling and consequently led to the conversion of myeloid cells into M2-polarized macrophages or myeloid-derived suppressor cells (MDSCs), both of which support an immune-tolerant tumor microenvironment.
15-PGDH: PGE2 Inactivation
15-hydroxyprostaglandin dehydrogenase (15-PGDH) is considered to be a tumor-suppressor since decreased expression of this enzyme is associated with increased tumorigenesis in breast, colon, lung, and pancreas (58, 59). In breast cancer, 15-PGDH expression is correlated with estrogen receptor (ER) expression, and loss of 15-PGDH in tumors may be due to 15-PGDH promoter hypermethylation (58). Loss of 15-PGDH expression in the colon attenuates the effect of celecoxib, a specific COX-2 inhibitor, on PGE2 concentration (60). This combinatory effect is likely due to celecoxib not binding to the COX-1 enzyme which would still be able to produce low levels of PGE2 in the colon. This pharmacogenetic interaction is important to consider when pharmacologically targeting PGE2 levels. 15-PGDH and COX-2 have been shown to be reciprocally regulated, likely at the post-transcriptional level (59). Recently, miR-21 has emerged as a PGE2-induced micro-RNA negative regulator of 15-PGDH expression in cholangiocarcinoma (61). 15-PGDH expression is enhanced by increasing transcription and mRNA stability as well as decreasing protein turnover. The diverse range of inducers of 15-PGDH expression includes growth factors (TGF-β), interleukin-4 (IL-4), steroids, NSAIDs, mitogen activated protein kinase kinase (MEK) inhibitors, histone deactylase (HDAC) inhibitors, and DNA demethylating agents (62–64).
Conclusion
There is abundant evidence to support the targeting of COX-2 and PGE2 signaling in cancer therapy; however, prolonged inhibition of the COX-2 enzyme itself increases the risk for cardiotoxic secondary effects (3, 4, 65). This brings to the forefront the necessity to discover new, more specific targets in this oncogenic pathway.
Elevated PGE2 in aggressive tumors could arise from an imbalance in COX-2 and 15-PGDH expression as well as MRP4 and PGT expression in multiple cell types in the tumor microenvironment. Preventing activation of the EP4 receptor by PGE2 decreases the metastatic potential of breast cancer cells and reduces tumor-initiating potential of breast cancer stem cells in a preclinical model (7, 12). Since EP4 activation occurs when extracellular PGE2 binds the EP4 receptor, decreasing the level of PGE2 in the extracellular space should attenuate signaling through this receptor as well as metastatic activity of the tumor cell. Inhibiting MRP4 expression and activity should decrease PGE2 export from cells reducing the pro-tumor effects of PGE2 in the tumor microenvironment. As an alternative approach, Subbaramaiah et al. have shown that decreasing extracellular PGE2 by overexpressing the prostaglandin transporter (PGT) in adipocytes suppresses gene transcripts downstream of EP receptor activation (44).
Elevated COX-2 is detected in approximately 50% of primary breast tumors, but in spite of this widely reported finding, PGE2 can be detected in the majority of malignant breast tissues suggesting that additional factors other than COX-2 levels determine tissue PGE2 concentrations (3, 5, 6). Elevated MRP4 expression, or reduced PGT or 15-PGDH activity could provide a mechanism for sustained PGE2 signaling in the tumor microenvironment even in the context of low COX-2 expression. The recent literature supports the further investigation of MRP4, as well as PGT and 15-PGDH as potential therapeutic targets in breast and other malignancies.
Acknowledgements
Work in the authors’ laboratory was supported by the U.S. Veterans Administration.
Contributor Information
Tyler J. Kochel, Email: tkoch001@umaryland.edu, University of Maryland, Baltimore. Baltimore, MD, USA..
Amy M. Fulton, Email: afulton@umaryland.edu, Department of Pathology, University of Maryland, Baltimore. Baltimore, MD, USA, Baltimore Veterans Affairs Medical Center. Baltimore, MD, USA..
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S. [Accessed June 30, 2014];SEER Cancer Statistics Review, 1975–2011. http://seer.cancer.gov/csr/1975_2011.
- 3.Greenhough A, et al. The COX-2/PGE pathway: key roles in the hallmarks of cancer and adaptation to the tumor microenvironment. Carcinogenesis. 2009;30(3):377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 4.Menter DG, Dubois RN. Prostaglandins in cancer cell adhesion, migration, and invasion. Int J Cell Biol. 2012;2012:723419. doi: 10.1155/2012/723419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glover JA, Hughes CM, Cantwell MM, Murray LJ. A systematic review to establish the frequency of cyclooxygenase-2 expression in normal breast epithelium, ductal carcinoma in situ, microinvasive carcinoma of the breast and invasive breast cancer. Br. J Cancer. 2011;105(1):13–17. doi: 10.1038/bjc.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kundu N, et al. Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Res. Treat. 2009;117(2):235–242. doi: 10.1007/s10549-008-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E Receptor EP4 Antagonism Inhibits Breast Cancer Metastasis. Cancer Research. 2006;66(6):2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 8.Dohadwala M, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277(52):50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71(2):614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H-J, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012;2(9):840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kundu N, et al. Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Res. Treat. 2014;143(1):19–31. doi: 10.1007/s10549-013-2779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh R, Marks DJB, Sales K, Winslet MC, Seifalian AM. Cyclooxygenase/lipoxygenase shunting lowers the anti-cancer effect of cyclooxygenase-2 inhibition in colorectal cancer cells. World J Surg Oncol. 2012;10:200. doi: 10.1186/1477-7819-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Half E, et al. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62(6):1676–1681. [PubMed] [Google Scholar]
- 15.Ristimäki A, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62(3):632–635. [PubMed] [Google Scholar]
- 16.Ristimäki A. Cyclooxygenase 2: from inflammation to carcinogenesis. Novartis Found. Symp. 2004;256:215–221. discussion 221-226, 259-269. [PubMed] [Google Scholar]
- 17.Zhao X, et al. Cyclooxygenase-2 expression during immortalization and breast cancer progression. Cancer Res. 2008;68(2):467–475. doi: 10.1158/0008-5472.CAN-07-0782. [DOI] [PubMed] [Google Scholar]
- 18.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011;30(3–4):449–463. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C-W, et al. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72(5):1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya M, et al. Nuclear localization of prostaglandin E2 receptors. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15792–15797. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, et al. Prostaglandin E Receptor EP1 Suppresses Breast Cancer Metastasis and Is Linked to Survival Differences and Cancer Disparities. Molecular Cancer Research. 2010;8(10):1310–1318. doi: 10.1158/1541-7786.MCR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han C, et al. Modulation of Stat3 activation by the cytosolic phospholipase A2alpha and cyclooxygenase-2-controlled prostaglandin E2 signaling pathway. J. Biol. Chem. 2006;281(34):24831–24846. doi: 10.1074/jbc.M602201200. [DOI] [PubMed] [Google Scholar]
- 25.Dean M, Rzhetsky A, Allikmets R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Research. 2001;11(7):1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 26.Masereeuw R, Russel FGM. Regulatory pathways for ATP-binding cassette transport proteins in kidney proximal tubules. AAPS J. 2012;14(4):883–894. doi: 10.1208/s12248-012-9404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russel FGM, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends in Pharmacological Sciences. 2008;29(4):200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Huynh T, Norris MD, Haber M, Henderson MJ. ABCC4/MRP4: a MYCN-regulated transporter and potential therapeutic target in neuroblastoma. Front Oncol. 2012;2:178. doi: 10.3389/fonc.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10(2):147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 30.Rasmuson A, et al. Autocrine Prostaglandin E2 Signaling Promotes Tumor Cell Survival and Proliferation in Childhood Neuroblastoma. PLoS ONE. 2012;7(1):e29331. doi: 10.1371/journal.pone.0029331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montani M, et al. Multidrug resistance protein 4 (MRP4) expression in prostate cancer is associated with androgen signaling and decreases with tumor progression. Virchows Arch. 2013;462(4):437–443. doi: 10.1007/s00428-013-1390-8. [DOI] [PubMed] [Google Scholar]
- 32.Copsel S, et al. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J. Biol. Chem. 2011;286(9):6979–6988. doi: 10.1074/jbc.M110.166868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holla VR, Backlund MG, Yang P, Newman RA, DuBois RN. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prev Res. 2008;1(2):93–99. doi: 10.1158/1940-6207.CAPR-07-0009. [DOI] [PubMed] [Google Scholar]
- 34.Henderson MJ, et al. ABCC multidrug transporters in childhood neuroblastoma: clinical and biological effects independent of cytotoxic drug efflux. J. Natl. Cancer Inst. 2011;103(16):1236–1251. doi: 10.1093/jnci/djr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid G, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furugen A, et al. Contribution of multidrug resistance-associated proteins (MRPs) to the release of prostanoids from A549 cells. Prostaglandins Other Lipid Mediat. 2013;106:37–44. doi: 10.1016/j.prostaglandins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Abla N, et al. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. Journal of Pharmacology and Experimental Therapeutics. 2008;325(3):859–868. doi: 10.1124/jpet.108.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S, Weerachayaphorn J, Cai S-Y, Soroka CJ, Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(1):G126–G135. doi: 10.1152/ajpgi.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aleksunes LM, Yeager RL, Klaassen CD. Application of multivariate statistical procedures to identify transcription factors that correlate with MRP2, 3, and 4 mRNA in adult human livers. Xenobiotica. 2009;39(7):514–522. doi: 10.1080/00498250902952514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gori I, et al. Augmented epithelial multidrug resistance-associated protein 4 expression in peritoneal endometriosis: regulation by lipoxin A(4) Fertil. Steril. 2013;99(7):1965–1973. doi: 10.1016/j.fertnstert.2013.01.146. e2. [DOI] [PubMed] [Google Scholar]
- 41.Markova SM, Kroetz DL. ABCC4 is Regulated by microRNA-124a and microRNA-506. Biochem. Pharmacol. doi: 10.1016/j.bcp.2013.10.017. [published online ahead of print: October 30, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamba JK, et al. Nonsense mediated decay downregulates conserved alternatively spliced ABCC4 transcripts bearing nonsense codons. Hum. Mol. Genet. 2003;12(2):99–109. doi: 10.1093/hmg/ddg011. [DOI] [PubMed] [Google Scholar]
- 43.Hoque MT, Conseil G, Cole SPC. Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem. Biophys. Res. Commun. 2009;379(1):60–64. doi: 10.1016/j.bbrc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Lee M, Vasioukhin V. Cell polarity and cancer – cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121(8):1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 45.Hoque MT, Cole SPC. Down-regulation of Na+/H+ exchanger regulatory factor 1 increases expression and function of multidrug resistance protein 4. Cancer Res. 2008;68(12):4802–4809. doi: 10.1158/0008-5472.CAN-07-6778. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi H, et al. Sorting nexin 27 interacts with multidrug resistance-associated protein 4 (MRP4) and mediates internalization of MRP4. J. Biol. Chem. 2012;287(18):15054–15065. doi: 10.1074/jbc.M111.337931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochemical And Biophysical Research Communications. 1995;208(1):345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- 48.Cheung L, Flemming CL, Watt F, Masada N, Yu DMT, Huynh T, et al. High-throughput screening identifies Ceefourin 1 and Ceefourin 2 as highly selective inhibitors of multidrug resistance protein 4 (MRP4) Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.05.023. In Press. [DOI] [PubMed] [Google Scholar]
- 49.Nomura T, Lu R, Pucci ML, Schuster VL. The Two-Step Model of Prostaglandin Signal Termination: In Vitro Reconstitution with the Prostaglandin Transporter and Prostaglandin 15 Dehydrogenase. Molecular Pharmacology. 2004;65(4):973–978. doi: 10.1124/mol.65.4.973. [DOI] [PubMed] [Google Scholar]
- 50.Chan BS, Endo S, Kanai N, Schuster VL. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am J Physiol Renal Physiol. 2002;282(6):F1097–F1102. doi: 10.1152/ajprenal.00151.2001. [DOI] [PubMed] [Google Scholar]
- 51.Subbaramaiah K, Hudis CA, Dannenberg AJ. The prostaglandin transporter regulates adipogenesis and aromatase transcription. Cancer Prev Res (Phila) 2011;4(2):194–206. doi: 10.1158/1940-6207.CAPR-10-0367. [DOI] [PubMed] [Google Scholar]
- 52.Subbaramaiah K, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2(4):356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Syeda MM, et al. Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis. The American Journal of Pathology. 2012;181(1):334–346. doi: 10.1016/j.ajpath.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Schuster VL, Chi Y, Chang Y-T, Min J. Prostaglandin transporter inhibitors. 2012 [Google Scholar]
- 55.Smartt HJM, et al. β-catenin negatively regulates expression of the prostaglandin transporter PGT in the normal intestinal epithelium and colorectal tumour cells: a role in the chemopreventive efficacy of aspirin? Br. J. Cancer. 2012;107(9):1514–1517. doi: 10.1038/bjc.2012.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinheiro C, et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56(7):860–867. doi: 10.1111/j.1365-2559.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 57.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE2 catabolism in myeloid cells. J. Leukoc. Biol. 2010;88(5):839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf I, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Research. 2006;66(15):7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 59.Tong M, Ding Y, Tai H-H. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis. 2006;27:2170–2179. doi: 10.1093/carcin/bgl053. [DOI] [PubMed] [Google Scholar]
- 60.Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proceedings of the National Academy of Sciences. 2009;106(23):9409. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu L, Byrnes K, Han C, Wang Y, Wu T. miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth. Mol Cancer Res. 2014 doi: 10.1158/1541-7786.MCR-13-0419. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tai H-H. Prostaglandin catabolic enzymes as tumor suppressors. Cancer Metastasis Rev. 2011;30(3–4):409–417. doi: 10.1007/s10555-011-9314-z. [DOI] [PubMed] [Google Scholar]
- 63.Chi X, Freeman BM, Tong M, Zhao Y, Tai H-H. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is up-regulated by flurbiprofen and other non-steroidal anti-inflammatory drugs in human colon cancer HT29 cells. Arch. Biochem. Biophys. 2009;487(2):139–145. doi: 10.1016/j.abb.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 64.Tai H-H, Chi X, Tong M. Regulation of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by non-steroidal anti-inflammatory drugs (NSAIDs) Prostaglandins Other Lipid Mediat. 2011;96(1–4):37–40. doi: 10.1016/j.prostaglandins.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clinical Cancer Research. 2010;16(5):1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]