Abstract
Growth factors and their downstream receptor tyrosine kinases (RTKs) mediate a number of biological processes controlling cell function. Adaptor (docking) proteins, which consist exclusively of domains and motifs that mediate molecular interactions, link receptor activation to downstream effectors. Recent studies have revealed that Grb2-associated-binders (Gab) family members (including Gab1, Gab2, and Gab3), when phosphorylated on tyrosine residues, provide binding sites for multiple effector proteins, such as Src homology-2 (SH2)-containing protein tyrosine phosphatase 2 (SHP2) and phosphatidylinositol 3-kinase (PI3K) regulatory subunit p85, thereby playing important roles in transducing RTKs-mediated signals into pathways with diversified biological functions. Here, we provide an up-to-date overview on the domain structure and biological functions of Gab1, the most intensively studied Gab family protein, in growth factor signaling and biological functions, with a special focus on angiogenesis.
Keywords: angiogenesis, endothelial cells, Gab1, receptor tyrosine kinase, tyrosine phosphorylation
1. Introduction
Growth factors and their associated receptor tyrosine kinases (RTKs) mediate a number of biological processes controlling cell-cycle progression, motility, survival, migration, metabolism, and differentiation[1-3]. Upon the engagement of the ligand on the cell-surface receptors, their intrinsic protein-tyrosine kinases are activated. Receptor tyrosine-phosphorylation creates docking sites for signal relaying proteins which contain Src-homology 2 (SH2) and phosphotyrosine-binding (PTB) domains[4]. These proteins fall into two general categories-enzymes and adaptors. Adaptor proteins, lacking the catalytic domain, can recruit one or more enzymes into signal transduction. The adaptor proteins Grb2-associated binders (Gab) are members of the insulin receptor substrate 1 (IRS1)-like multi-substrate docking adaptor protein family[5, 6], which possess a pleckstrin homology (PH) domain that can bind phosphatidylinositol lipids within biological membranes. These docking adaptor proteins also contain binding sites for SH3 domain-containing proteins and multiple tyrosine phosphorylation sites for recruitment of SH2 and PTB domain-containing proteins, which play important roles in the regulation of signal specificity, signal amplification and assembling multimeric signaling complexes[2, 4]. Gab genes encoding mammalian Gab1, Gab2, and Gab3, the Drosophila homolog Daughter of Sevenless (DOS), and the Caenorhabditis elegans homolog Suppressor of Clear (Soc1), define a family of docking adaptor proteins. Gab1 was originally identified as a Grb2 SH3-domain binding protein[7, 8]. Gab2 was isolated as a binding partner of the SH2 domain-containing protein tyrosine phosphatase (SHP2)[9]. Gab3 was discovered based on its sequence similarity with Gab1 and Gab2 within a large sequencing database[10]. Gab1 and Gab2 are expressed ubiquitously, while Gab3 is highly expressed in lymphoid tissue in particular. The Gab family proteins contain a PH domain in the amino-terminal region, as well as tyrosine-based motifs and proline-rich sequences (PXXP), which are potential binding sites for SH2 and SH3 domain containing proteins. Although the overall sequence identity among the Gab family is only 40-50%, the N-terminal PH domain, proline-rich motifs, and multiple potential tyrosyl and seryl/threonyl phosphorylation sites are conserved among Gab1, Gab2, and Gab3[5, 6] (Figure 1). However, each Gab protein also has unique structure in individual signal transduction.
Figure 1. Domain structures of the Gab superfamily of adaptors/scaffolding proteins.
The Gab family (Gab1, Gab2, and Gab3) is recruited by a wide variety of receptor tyrosine kinases (RTKs) and has multiple phosphorylation sites. Gab1, the most intensively-studied Gab family member, also contain serine and threonine phosphorylation sites, which negatively regulate HGF/Gab1 signaling. The Met-binding sequence (MBS, amino acid 487-499) within the Met-binding domain (MBD) in Gab1 is indicated with a red star. This specific MBS is absent in Gab2 and Gab3. P, proline-rich domain contains binding site for SH3 domain; motifs in red contain potential tyrosine phosphorylation sites for binding SHP-2 tyrosine phosphatase; motifs in blue contain potential tyrosine phosphorylation sites for binding PI3-K
Gab proteins can be recruited to activated RTKs through direct and indirect mechanisms. Direct mechanism has been described between Gab1 and c-Met, the receptor for hepatocyte growth factor (HGF)[8, 11-13]. Gab1 interacts with tyrosine-phosphorylated c-Met via the Met-binding domain (MBD, amino acids 450-532), which contains 13 essential amino acids (487-499) and is absent in Gab2 and Gab3[14-16]. Most RTKs recruit Gab1 indirectly via Grb2[5, 6]. Gab proteins harbor several proline-rich motifs which bind to Grb2 SH3 domain, while Grb2 contains an SH2 domain which targets the Grb2-Gab complex to receptors containing Grb2 SH2 domain binding sites[15]. It has been shown that indirect recruitment of Gab1 by c-Met is also physiologically important, since the mutation of Grb2 SH2 domain dramatically decreases the c-Met-Gab1 association[11, 17], thereby, blocking the HGF pathway.
2. Effector proteins involved in Gab1-mediated signal transduction
Gab1 is tyrosine-phosphorylated in response to many growth factors (including vascular endothelial growth factor (VEGF), HGF, nerve growth factor (NGF), platelet-derived growth factor (PDGF), EGF) and other stimuli [5, 6, 18], thereby propagating signals that are essential for cell proliferation, motility, and erythroblast development. Whereas, hyper-phosphorylation in serine and threonine of Gab1 (by PKC-α and PKC-β1) has been shown to negatively regulate HGF-induced biological responses which is critical for Gab1-induced signaling required for angiogenesis[19]. Gab2 is tyrosine-phosphorylated in response to cytokines IL-2, IL-3, IL-15, TPO, EPO, Kitl, M-CSF, Flt3l, and the stimulation of gp130, FcεRI, FcγR, and T and B antigen receptors [20]. To date, Gab3 is tyrosine-phosphorylated in response to M-CSF[10]. Our previous study showed that Gab1 was tyrosine-phosphorylated in endothelial cells (ECs) under mechanical stress such as fluid shear stress[21, 22]. These data show that Gab proteins act downstream of receptor tyrosine kinases, cytokine receptors, and possibly other receptor systems.
Gab proteins lack enzymatic activity but become rapidly phosphorylated on tyrosine residues, providing binding sites for multiple SH2 domain-containing proteins such as SHP2, phosphatidylinositol 3-kinase (PI3K) regulatory subunit p85, phospholipase C (PLC), Crk, and GC-GAP. Association of Gab1 with SHP2 and the p85 subunit of PI3K is considered to be essential for activation of extracellular signal-regulated kinase (ERK)1/2 and AKT, respectively. These interactions between Gab protein and effector molecules were found to be critical for transducing Gab-mediated signaling[5, 6, 20, 23].
Among the proteins bind to the Gab proteins, SHP2 has been shown to interact with all mammalian Gab proteins, as well as the Drosophila DOS and C. elegans Soc1, indicating that recruitment of SHP2 is a conserved feature that Gab family genes retained from C. elegans to mammalian systems[6]. Mutants of Gab family proteins incapable of binding SHP2 have been used to study the functional significance of the Gab-SHP2. Met-mediated morphogenesis, EGF-induced and fluid shear stress-induced MAPK signaling transduction, were blocked by overexpressing the Gab1 mutant unable to interact with SHP2. Accumulating evidence indicates that Gab1 Y627 and Y659 phosphorylation recruits and activates SHP2 phosphatase, which in turn activates MAPK signaling[15, 24, 25]. Moreover, DOS or Soc1 with all tyrosines mutated to phenylalanines, except those crucial for SHP2-binding, is sufficient to mediate RTKs signaling and to rescue the developmental lethality resulting from the loss-of-function mutations[26]. These results strongly demonstrated the physiological significance of Gab-SHP2 interaction.
Another well-studied effector protein of Gab family proteins is the PI3K p85-subunit. Mutations at the p85-binding sites of mammalian Gab1 and Gab2 resulted in defective signal transduction in many signaling systems[27-31]. The association between p85 and Gab1 or Gab2 is crucial in mediating the PI3K/Akt signaling pathway induced by a variety of stimuli. Overexpression of Gab1 wild type potentiates FGF-, VEGF-, and HGF-induced Akt activation, whereas overexpression of the p85-binding mutant of Gab1 results in decreased Akt activation[32]. This mutant also failed to convey the anti-apoptotic signaling in NGF stimulation[29]. These results suggest that the Gab–p85 association plays an important role in activating the PI3K/Akt pathway in mammalian cells. In addition, PIP3, the product of activated PI3K, binds to the PH domain of Gab proteins and further potentiates the activation of PI3K, forming a positive feedback loop to amplify the signals through the Gab proteins[33], which are important for signal specificity in certain systems.
Gab1 is essential for embryo survival, since Gab1−/− mice are not viable and only reach day 14 to 18 of gestation. Further analysis indicates that these mice have multiple developmental defects in heart, placenta, liver, spleen and muscle development[34]. The phenotype of Gab1 deficient mice showed similarity with those deficient of HGF, c-Met, PDGF, and EGF signaling pathways. In contrast, Gab2−/− and Gab3−/− mice can live to a normal age, with the exception that Gab2−/− mice have a defect in mast cell signaling[35, 36], while the loss of Gab3 does not result in noticeable developmental defects[36]. To better understand the tissue-specific role of Gab1, several studies have been carried out with cell type-specific (conditional) Gab1 knockout mice. For instance, liver-specific Gab1 knockout mice displayed a phenotype of defective liver regeneration triggered by partial hepatectomy surgery[37]. Very recently, Sun et al reported that cardiomyocyte-specific Gab1 knockout mice exhibited an increase in infarct size and a decrease in cardiac function after ischemia/reperfusion (I/R) injury[38], suggesting that Gab1 is also essential for cardioprotection against I/R oxidative injury. In addition, it has been reported that Gab1 and Gab2 may have the redundant roles for maintenance of cardiac function via neuregulin-1/ErbB signaling when using cardiomyocyte-specific Gab1/Gab2 double knockout mice[39]. Since there have been several excellent reviews on the biological functions of Gab proteins[5, 20, 40], in the next section, we will focus on the specific role of Gab1 in growth factor-mediated signaling and angiogenesis.
3. Gab1 and angiogenesis
In 2011, three independent groups (including our laboratory) simultaneously reported the crucial role of Gab1 in promoting postnatal angiogenesis using endothelial cell-specific Gab1 knockout (Gab1-ecKO) mice and hindlimb ischemia models[41-43] (Table 1). The Gab1-ecKO mice were viable, with no obvious defects on embryonic vasculogenesis and neonatal retinal angiogenesis, which indicate that Gab1 in the endothelium plays no crucial role during developmental vasculogenesis. All three groups consistently showed that Gab1-ecKO mice have severe defects in angiogenesis after hindlimb ischemia. Impaired blood flow recovery, low capillary density and necrotic limb were observed 2 weeks after the femoral artery ligation in Gab1-ecKO mice, while the WT control mice showed a time-dependent recovery of blood flow and increased capillary density in the gastrocnemius muscle[41-43]. Unlike Gab1-ecKO mice, no significant effects on angiogenesis were observed on conventional Gab2 knockout mice39.
Table 1.
Gab1 as a critical regulator of postnatal angiogenesis.
| Studies by | Animal Model | In vitro model | Signaling pathways involved |
|---|---|---|---|
| Zhao et al [41] | Gab1-ecKO+HLI | HGF | SHP2-ERK, PI3K-Akt |
| Shioyama et al [43] | Gab1-ecKO+HLI; Gab2-KO+HLI; VEGF, HGF gene transfer |
HGF; VEGF; FGF2 |
SHP2-ERK1/2-Egr1, SHP2-ERK5-KLF2; PI3K-Akt |
| Lu et al [42] | Gab1-ecKO+HLI | VEGF | SHP2-PKA-eNOS |
Abbreviations: Egr1, early growth response 1; eNOS, endothelial nitric oxide synthase; FGF2, fibroblast growth factor-2; Gab1-ecK0, endothelial cell-specific Gab1 knockout; HLI, hindlimb ischemia; HGF, hepatocyte growth factor; KLF2, kruppel-like factor 2; VEGF, vascular endothelial growth factor.
Although increased level of both VEGF and HGF, the potent pro-survival factors were observed in the ischemic hindlimb muscles. Zhao et al also reported a significant increase of apoptotic ECs in the gastrocnemius muscle from Gab1-ecKO mice in association with the low capillary density[41]. Furthermore, the viability of Gab1-deficient ECs remained low under the treatment of both growth factors (VEGF and HGF) in vitro, whereas wild-type cells are protected from apoptosis. One possible explanation might be that impaired PI3K/Akt signaling and activated caspase-3 in the absence of Gab1[41]. Shioyama et al showed that HGF specifically upregulates Krüppel-like factor 2 (KLF2) mRNA and protein expression in ECs overexpressing Gab1[43]. KLF2 functions as a potent anti-apoptotic factor, which acts, in part, through the activation endothelial nitric oxide synthase (eNOS), and mediates the Gab1-dependent cell survival signaling in ECs. Zhao et al also demonstrated that Gab1 is essential for HGF-induced ERK1/2 phosphorylation through SHP2 activation[41], while Shioyama et al showed that ERK5 is also activated downstream of Gab1-SHP2 after HGF stimulation[43]. In the third report, Lu et al revealed an important protein kinase A-dependent pathway for VEGF-induced eNOS activation and angiogenesis[42]. In addition to hindlimb ischemia-induced angiogenesis, Gab1 was also shown to be important for the tumor angiogenesis. Zhao et al. [41] demonstrated a significant low level of capillary density in tumors engrafted in the Gab1-ecKO mice as well as dramatically decreased tumor weight and volume. A logical follow-up question will be to address the mechanism of how Gab1 regulates the tumor angiogenesis, such as the potential role of Gab1 in matrix metalloproteinases (MMPs) activation and metastasis of tumor cells. Collectively, studies from three independent groups established the critical role of endothelial Gab1 in HGF-and VEGF-induced postnatal angiogenesis[41-43]. Taken together, Gab1 functions as a key molecule that regulates both VEGF- and HGF-mediated downstream signaling pathways involved in EC stabilization, proliferation, migration and survival which are crucial for angiogenic processes (Figure 2).
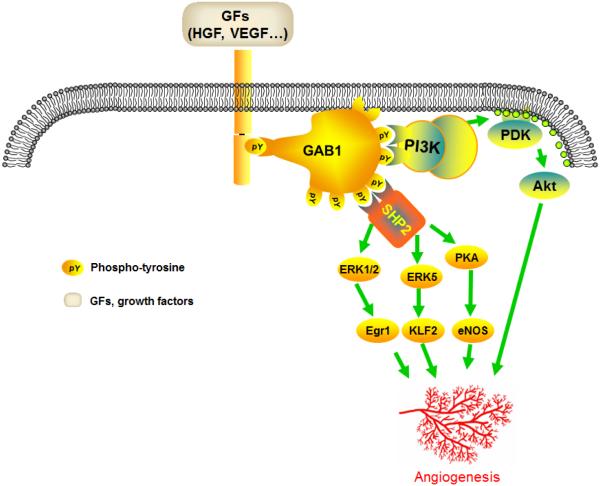
Figure 2. Schematic representation of the role of Gab1 in growth factor signaling and angiogenesis.
Growth factors, such as VEGF and HGF stimulate respective receptors on the ECs. Activation of the receptors is associated with phosphorylation of Gab1 and induction of signaling cascades dependent on association of Gab1 with either PI3K (represented by p110/p85 subunits) or the phosphatase SHP2. Downstream targets include Akt (downstream of PI3K), PKA/eNOS, ERK1/2/Egr1, and ERK5/KLF2 (all downstream of SHP2). These signaling pathways stimulate EC survival, proliferation, migration, stabilization, and tube formation, thereby contributing to angiogenesis. Collectively, Gab1 serves as the common regulator of ischemia-dependent angiogenesis.
Abbreviations: Egr1, early growth response 1; eNOS, endothelial nitric oxide synthase; FGF2, fibroblast growth factor-2; Gab1-ecKO, endothelial cell-specific Gab1 knockout; HGF; hepatocyte growth factor; HLI, hindlimb ischemia; KLF2, kruppel-like factor 2; VEGF, vascular endothelial growth factor
4. Future perspectives
Since the discovery of Gab docking proteins 18 years ago[7], it has become evident that these family of proteins extend beyond the original definition of docking proteins (as a platform for the assembly of multiple signaling branches) and play critical roles in a variety of pathophysiological processes[40]. Preventing protein-protein interactions involving Gab-family proteins and their associated effectors/adaptors will be a viable therapeutic strategy in diseases involving angiogenesis. One enduring question regarding the Gab1 signaling is: how is the specificity of each RTK achieved while they share the same downstream scaffolding adaptor Gab family proteins? Definitely, further studies on the Gab family members will allow us to understand more the complexity of the receptor-mediated signaling and ensuing biological functions.
Highlights.
Gab1 relays receptor tyrosine kinase-mediated signal transduction
Gab1 promotes endothelial cell survival, migration and tube formation
Gab1 is critical for HGF and VEGF induced postnatal angiogenesis
Acknowledgements
This work is supported by in part by the American Heart Association predoctoral fellowship (to W.W.) and the American Diabetes Association Basic Research Award 1-12-BS-92-R1 (to Z.G.J.), and the National Institutes of Health RO1 grants HL109502 and HL114570 (to Z.G.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb Perspect Biol. 2014;6(3) doi: 10.1101/cshperspect.a008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE. 2003;2003(191):RE12. doi: 10.1126/stke.2003.191.re12. [DOI] [PubMed] [Google Scholar]
- 5.Gu H, Neel BG. The "Gab" in signal transduction. Trends Cell Biol. 2003;13(3):122–30. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Rohrschneider LR. The gift of Gab. FEBS Lett. 2002;515(1-3):1–7. doi: 10.1016/s0014-5793(02)02425-0. [DOI] [PubMed] [Google Scholar]
- 7.Holgado-Madruga M, Emlet DR, Moscatello DK, et al. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379(6565):560–4. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 8.Weidner KM, Di Cesare S, Sachs M, et al. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384(6605):173–6. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 9.Gu H, Pratt JC, Burakoff SJ, et al. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2(6):729–40. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 10.Wolf I, Jenkins BJ, Liu Y, et al. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol Cell Biol. 2002;22(1):231–44. doi: 10.1128/MCB.22.1.231-244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen L, Holgado-Madruga M, Maroun C, et al. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272(33):20811–9. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 12.Aasrum M, Odegard J, Sandnes D, et al. The involvement of the docking protein Gab1 in mitogenic signalling induced by EGF and HGF in rat hepatocytes. Biochim Biophys Acta. 2013;1833(12):3286–94. doi: 10.1016/j.bbamcr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Barrow-McGee R, Kermorgant S. Met endosomal signalling: in the right place, at the right time. Int J Biochem Cell Biol. 2014;49:69–74. doi: 10.1016/j.biocel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Roshan B, Kjelsberg C, Spokes K, et al. Activated ERK2 interacts with and phosphorylates the docking protein GAB1. J Biol Chem. 1999;274(51):36362–8. doi: 10.1074/jbc.274.51.36362. [DOI] [PubMed] [Google Scholar]
- 15.Schaeper U, Gehring NH, Fuchs KP, et al. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149(7):1419–32. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lock LS, Royal I, Naujokas MA, et al. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem. 2000;275(40):31536–45. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 17.Bardelli A, Longati P, Gramaglia D, et al. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15(25):3103–11. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- 18.Lemarie CA, Lehoux S. The gift of Gab1 (Grb-2-associated binder 1) Arterioscler Thromb Vasc Biol. 2011;31(5):956–7. doi: 10.1161/ATVBAHA.111.225987. [DOI] [PubMed] [Google Scholar]
- 19.Gual P, Giordano S, Anguissola S, et al. Gab1 phosphorylation: a novel mechanism for negative regulation of HGF receptor signaling. Oncogene. 2001;20(2):156–66. doi: 10.1038/sj.onc.1204047. [DOI] [PubMed] [Google Scholar]
- 20.Wohrle FU, Daly RJ, Brummer T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal. 2009;7:22. doi: 10.1186/1478-811X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin ZG, Wong C, Wu J, et al. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate Akt and eNOS activation in endothelial cells. J Biol Chem. 2005;280(13):12305–9. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixit M, Loot AE, Mohamed A, et al. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res. 2005;97(12):1236–44. doi: 10.1161/01.RES.0000195611.59811.ab. [DOI] [PubMed] [Google Scholar]
- 23.Sarmay G, Angyal A, Kertesz A, et al. The multiple function of Grb2 associated binder (Gab) adaptor/scaffolding protein in immune cell signaling. Immunol Lett. 2006;104(1-2):76–82. doi: 10.1016/j.imlet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Sachs M, Brohmann H, Zechner D, et al. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol. 2000;150(6):1375–84. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunnick JM, Dorsey JF, Munoz-Antonia T, et al. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J Biol Chem. 2000;275(18):13842–8. doi: 10.1074/jbc.275.18.13842. [DOI] [PubMed] [Google Scholar]
- 26.Schutzman JL, Borland CZ, Newman JC, et al. The Caenorhabditis elegans EGL-15 signaling pathway implicates a DOS-like multisubstrate adaptor protein in fibroblast growth factor signal transduction. Mol Cell Biol. 2001;21(23):8104–16. doi: 10.1128/MCB.21.23.8104-8116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu H, Maeda H, Moon JJ, et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol. 2000;20(19):7109–20. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu H, Saito K, Klaman LD, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412(6843):186–90. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 29.Holgado-Madruga M, Moscatello DK, Emlet DR, et al. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci U S A. 1997;94(23):12419–24. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yart A, Laffargue M, Mayeux P, et al. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J Biol Chem. 2001;276(12):8856–64. doi: 10.1074/jbc.M006966200. [DOI] [PubMed] [Google Scholar]
- 31.Laffargue M, Raynal P, Yart A, et al. An epidermal growth factor receptor/Gab1 signaling pathway is required for activation of phosphoinositide 3- kinase by lysophosphatidic acid. J Biol Chem. 1999;274(46):32835–41. doi: 10.1074/jbc.274.46.32835. [DOI] [PubMed] [Google Scholar]
- 32.Ong SH, Hadari YR, Gotoh N, et al. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci U S A. 2001;98(11):6074–9. doi: 10.1073/pnas.111114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues GA, Falasca M, Zhang Z, et al. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20(4):1448–59. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh M, Yoshida Y, Nishida K, et al. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol. 2000;20(10):3695–704. doi: 10.1128/mcb.20.10.3695-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida K, Wang L, Morii E, et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood. 2002;99(5):1866–9. doi: 10.1182/blood.v99.5.1866. [DOI] [PubMed] [Google Scholar]
- 36.Seiffert M, Custodio JM, Wolf I, et al. Gab3-deficient mice exhibit normal development and hematopoiesis and are immunocompetent. Mol Cell Biol. 2003;23(7):2415–24. doi: 10.1128/MCB.23.7.2415-2424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bard-Chapeau EA, Yuan J, Droin N, et al. Concerted functions of Gab1 and Shp2 in liver regeneration and hepatoprotection. Mol Cell Biol. 2006;26(12):4664–74. doi: 10.1128/MCB.02253-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Chen C, Jiang B, et al. Grb2-associated binder 1 is essential for cardioprotection against ischemia/reperfusion injury. Basic Res Cardiol. 2014;109(4):420. doi: 10.1007/s00395-014-0420-2. [DOI] [PubMed] [Google Scholar]
- 39.Nakaoka Y, Nishida K, Narimatsu M, et al. Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling. J Clin Invest. 2007;117(7):1771–81. doi: 10.1172/JCI30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakaoka Y, Komuro I. Gab docking proteins in cardiovascular disease, cancer, and inflammation. Int J Inflam. 2013:141068. doi: 10.1155/2013/141068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J, Wang W, Ha CH, et al. Endothelial Grb2-associated binder 1 is crucial for postnatal angiogenesis. Arterioscler Thromb Vasc Biol. 2011;31(5):1016–23. doi: 10.1161/ATVBAHA.111.224493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y, Xiong Y, Huo Y, et al. Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway. Proc Natl Acad Sci U S A. 2011;108(7):2957–62. doi: 10.1073/pnas.1009395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shioyama W, Nakaoka Y, Higuchi K, et al. Docking protein Gab1 is an essential component of postnatal angiogenesis after ischemia via HGF/c-met signaling. Circ Res. 2011;108(6):664–75. doi: 10.1161/CIRCRESAHA.110.232223. [DOI] [PubMed] [Google Scholar]