Abstract

Using the technique described in this report, the presence or absence of sperm in spermathecae of female Aedes aegypti is detectable without dissection. Spermathecae of a lightly anesthetized female can be visualized by phase contrast microscopy through the distended abdomen, after the intersegmental membranes are stretched by ventral placement of a glass cover slip. Most females recovered after the procedure were capable of subsequent reproductive activities. Albeit tedious, this technique preserves the female alive for subsequent experiments or observations. Its extension to other mosquito species, or other Diptera and insects, will depend on spermathecal and sperm visibility through the distended abdomen.
Keywords: Insemination status, live, Aedes aegypti, new technique
1. Introduction
Insemination status in female mosquitoes is typically assessed by dissections. Traditionally, the female is anesthetized and her genitalia with internal organs, including the spermathecae, are removed with forceps or needles under a dissecting microscope. Sperm, if present, are seen as a mass of threads in the spermathecae (Rosay, 1969). This traditional technique is very useful and reliable for checking presence or absence of sperm, e.g., to assess the timing of sexual receptivity after female emergence (Gwadz & Craig, 1968; Lounibos et al., 1996; O’Meara and Lounibos, 1981) or of mating after mark-release-recapture field experiments (Lounibos et al., 1998; Reisen and Aslamkhan, 1979). However, the procedure is fatal to the female, which cannot be kept alive for subsequent experiments or observations.
Females of most insects store and maintain sperm internally in the spermathecae (Gullan and Cranston, 2010; Simmons, 2001). Sperm in the spermathecae remain viable for prolonged periods of time, e.g., several years in the case of honey bees (Collins et al., 2004; Snodgrass, 1956) and ants (Tschinkel, 1987; Wheeler, 1960), and around one or two months in mosquitoes (Christophers, 1960). Most insect species have a single spermatheca (Gullan and Cranston, 2010; McAlpine et al., 1981), but the number of spermathecae varies from one to three among mosquito species (Yuval, 2006). Aedes aegypti females have three spermathecae (Christophers, 1960). Harbach and Knight (1980) prefer the term ‘spermathecal capsules’ for the reservoirs at the ends of the spermathecal ducts, which for simplicity herein we call spermathecae.
The new technique described here that allows detection of sperm in spermathecae of live insects, specifically in A. aegypti females, is facilitated by the distensibility of the female’s abdomen. Each segment of the abdomen of an adult mosquito is connected laterally by a pleural membrane, which continues unbroken throughout the length of the abdomen (Christophers, 1901; Harbach, 2014). The intersegmental membrane connects the tergum and sternum of adjacent abdominal segments (Harbach and Knight, 1980). The elasticity of these membranes allows the abdomen to become distended when the female takes a blood meal or when she is gravid and full of eggs (Christophers, 1960; Harbach, 2014).
2. Materials and Methods
2.1 Source of Aedes aegypti
Aedes aegypti were F5 progeny from collections in Key West, Florida. Mosquitoes were reared and maintained in an insectary at 27°C, and 70 % RH, and 14L:10D day length as described elsewhere (Bargielowski et al., 2013).
2.2 Sperm check
Each A. aegypti female was placed individually in a small cardboard container and lightly anesthetized with chloroform for approximately 10 seconds. Under a dissecting microscope, each female was placed ventral surface up on a glass slide, and the distal segments of the abdomen were placed in a drop of water to facilitate abdominal distention and to create a fluid medium for microscopic observations.
A square glass cover slip (15 × 15 mm), was lowered gently onto the abdomen, covering the VI– VIII segments and the genitalia (Fig. 1). The pressure from the cover slip stretched the pleural and intersegmental membranes. After the cover slip was positioned on the female’s abdomen, spermathecae were observed under a phase contrast compound microscope, using magnifications from 40 to 200x. In inseminated females, masses of motile spermatozoa were seen within the spermathecae (Fig. 2, video). In uninseminated females, the spermathecae appeared empty (Fig. 3). After checking for sperm, a few drops of water were applied with a pipette to the edges of the cover slip to facilitate its removal with fine jeweler’s forceps. Females were immediately returned to the individual containers and usually recovered from the procedure in 30–60 minutes. If the first sperm check was inconclusive, because abdominal distention was inadequate and/or spermathecae were not observed clearly, the procedure was repeated two or three times.
Fig. 1.
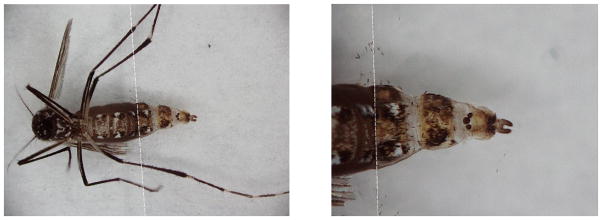
Glass cover slip lowered onto the distal segments of the abdomen, covering the VI, VII and VIII abdominal segments and the genitalia. Dotted line marks the edge of the glass coverslip.
Fig. 2.
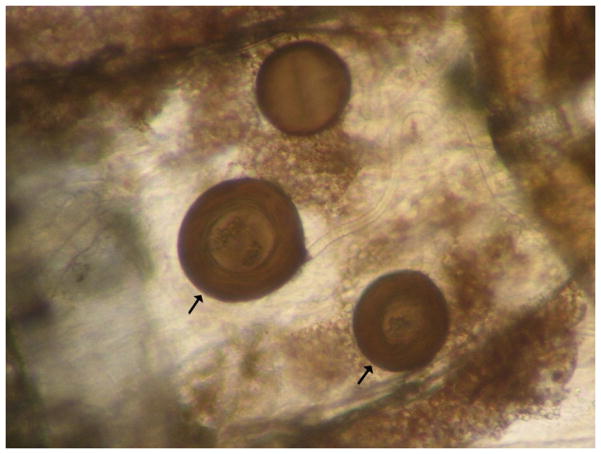
The three spermathecae of A. aegypti, one large, and two smaller and lateral, are seen through the extended abdomen. In this photo, the two spermathecae that cointain sperm are pointed by arrows.
Fig. 3.
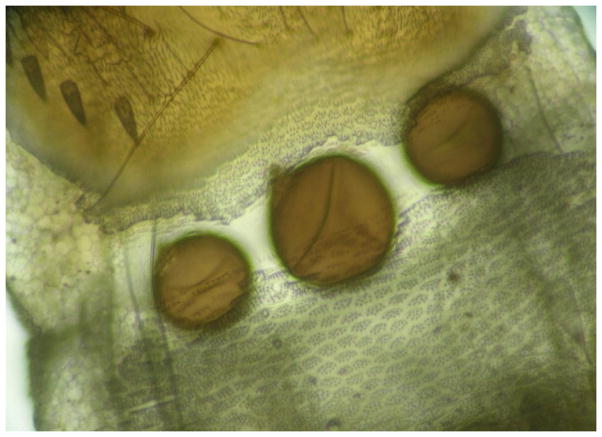
Three spermathecae without sperm seen through the cover slip.
2.3. Validation of the technique
Two experiments were performed. In the first, the tester received in individual containers 60 females, whose histories were known only to a second party. The samples consisted of A. aegypti females that had been exposed for two weeks to conspecific males or two week old virgin females. Each female was checked for insemination, as described previously. In the second experiment, sperm detection was performed on A. aegypti females that had been exposed previously for three weeks to A. albopictus males. Under these conditions, approximately 50% of A. aegypti females from Key West are inseminated by A. albopictus (Bargielowski et al., 2013). After spermathecal observation on 97 live females, each was dissected by traditional, lethal techniques to compare results between the two procedures. We included this interspecific cross for the validation of the technique, because of other ongoing work in our laboratory (e.g., Bargielowski et al., 2013) and because this cross provided blind samples of females, which might have been mated or not, from the same cage.
2.4. Survivorship
An experiment was performed to assess female survivorship after the procedure. To obtain specimens for a live sperm check, 150 A. aegypti females (Key West) and 150 A. albopictus males (F5, Vero Beach, FL), were exposed to one another for three weeks in a cage. After spermathecal detections of live females, each was confined individually in a small cardboard container and observed every 24 hours for three days. A female was considered healthy if she was able to stand upright and fly. As controls, A. aegypti females exposed to the same conditions without spermathecal observations, were transferred directly to the cardboard containers and survivorship was assessed. All females had ad libitum access to a sugar water solution.
2.5. Mating capacity
An experiment was performed to determine mating capacity after the procedure. The tester received in individual containers four replicates of 20 females each, whose histories were known only to a second party. The replicates included A. aegypti that had been exposed for three weeks to conspecific males or three week old virgin females. Spermathecal observations on live females were performed. After females diagnosed to be virgin recovered from the procedure, each was exposed for 24 hours in an individual cage to two A. aegypti males. As controls, virgin A. aegypti females without spermathecal observations were exposed to two A. aegypti males under the same conditions. Four control replicates of 10 females were done. After 24 hours of exposure to males, traditional spermathecal dissections were done to confirm if females had been inseminated. All females had ad libitum access to a sugar water solution.
3. Results and discussion
Experiments performed to validate the technique were completed successfully on the majority of the females. In the first experiment, the procedure was performed on 60 A. aegypti females, of which the presence or absence of sperm was resolved in 59, as 33 inseminated and 26 non-inseminated, for 100% accuracy. In the one unresolvable female, spermathecae were not observable because the terminal abdominal segments were twisted and the abdomen did not distend normally. In the second experiment, the procedure was performed on 97 A. aegypti females exposed to A. albopictus males, of which the presence or absence of sperm was resolved in 94, for 98.9% accuracy (50 females positive for sperm in spermathecae and 43 females negative), as confirmed with the traditional dissecting technique. The assessment was incorrect (false negative) in one female (1.1%). For the three live females whose spermathecae were not observable, abdomens did not distend. The live observation of the only misdiagnosed female was probably inaccurate because she had relatively few sperm in only one of the small spermathecae. This mistake is not likely to occur in intraspecific matings, in which usually the big and one or two of the small spermathecae have sperm (Clements, 1999; Jones and Wheeler, 1965). In our observations we have noticed that in interspecific matings between A. aegypti females and A. albopictus males, females tend to store less sperm in the spermathecae, and patterns of spermathecal use differ from intraspecific matings (Tripet et al., 2011). We validated the technique and performed all our experiments with chloroform, however we anesthetized a few females using carbon dioxide. Under carbon dioxide anesthesia we obtained good abdominal distention and motile sperm were observable in spermathecae.
In the experiments performed to assess survivorship and mating capacity most females remained healthy after the procedure. In the experiment in which survivorship was tested, between 88 and 96% of the females subjected to the procedure survived with no apparent negative impacts for at least three days afterwards (Table 1). The rest of the females either died or were not able to stand upright and fly. Kaplan-Meier survival analysis showed no significant difference in survivorship among the three classes: controls, inseminated and uninseminated females (Log Rank test: X2 = 3.68 df =2, p = 0.159).
Table 1.
Percentage of healthy females after sperm check
| Female status (n) | Percentage of healthy females after sperm check | ||
|---|---|---|---|
| After 24 hours | After 48 hours | After 72 hours | |
| Inseminated females (n=52) | 96.1 | 94.2 | 94.2 |
| Uninseminated females (n=56) | 89.28 | 87.5 | 87.5 |
| Control (n=20) | 100 | 100 | 100 |
Although no significant differences were detected, the survivorship was a little lower in the uninseminated females, possibly because when spermathecae looked empty we repeated the procedure and the added manipulations may lead to a higher mortality. As we were applying the technique, we noticed that it is usually easier and faster to confirm presence of sperm in spermathecae rather than absence, as once sperm are observed the assessment is complete. If the spermathecae appear empty, it is recommended to repeat the procedure using a bigger glass cover slip (18×18 mm to 25×25 mm), which facilitates distention of the abdomen and provides a better view. We use a smaller cover slip for initial observations because spermathecae with sperm may rupture easier than empty ones. When spermathecae are empty, breakage is unlikely. We also note that permanent access of caged females to sugar water facilitates improved abdominal distention.
In the experiment in which mating capacity was tested, most virgin females were capable of subsequent mating. After spermathecal observations were completed on live females, each uninseminated female (n=40) was exposed to two conspecific males. Approximately, 90 (± 14.1 SD) % of the females (n=36), survived and were able to stand upright and fly after their exposure to males. Of these, an average of 94.7 (± 6.1 SD) % mated successfully as evidenced by the presence of sperm in spermathecae. In the controls, 97.5 (±5 SD) % of the females were inseminated.
To date we have successfully applied this technique only on A. aegypti females. Its use on other mosquito species, or other Diptera and insects, will depend on spermathecal and sperm visibility through the distended abdomen, female body size and sclerotization, size of fat bodies and distensibility of the intersegmental and pleural membranes. This technique could be useful for studies that perform other procedures (after assessing insemination) on the body of the mosquito, e.g., experiments that examine the effect of mating status on body cuticle traits, such as cuticular hydrocarbons (Polerstock et al., 2002; Wagoner et al., 2014). In such laboratory studies, as it is necessary to keep the mosquito body for further procedures, it is usually assumed that females are mated if they have been exposed to males. With the new technique, if sperm presence is checked in live females, it will be possible to confirm insemination status, keeping the intact body. Also, it would be feasible to study the effect of mating status on chemical traits of field-collected mosquitoes. Detection of insemination status in live females could also be valuable in behavioral studies, e.g., experiments that assess the effect of insemination on locomotor activity (Jones and Gubbins, 1978; Lima-Camara et al., 2014). In such studies, insemination status is usually verified through dissections at the end of the experiment. This new technique would allow determination of insemination status in live females before performing behavioral tests.
This technique could have potential use in colonization and rearing of mosquito species in the laboratory, particularly those that are difficult to colonize or when few adults are available to start a colony. For example, in species, such as Mansonia annulata, that have low adult emergence rates in the laboratory (Samung et al., 2006), it would be particularly useful to check if mating is taking place without losing adult females. Also, in experiments in which mated females are required for subsequent observations, e.g., oviposition and vertical transmission of arboviruses (Buckner et al., 2013), our new technique could be potentially useful. Most females that recovered after our procedures were capable of subsequent reproductive activities, but for purposes of colonization or arbovirus studies, it would be important to assess blood feeding and egg laying capacities, after the in vivo sperm check.
The few supplies and equipment needed are usually found in entomology laboratories. However, the technique may be considered as tedious, needing to be performed with care on each female, and sometimes it is necessary to relocate the cover slip several times on the mosquito’s abdomen until a good view of the spermathecae is obtained. Thus, it does not provide the quick assessment that is obtained with the conventional technique in which the spermathecae are dissected away from other tissues. Our intention is not to compare this new technique with the traditional dissecting technique, as the two have different applications. This new technique allows confirmation of insemination status in live A. aegypti females, a procedure that had not been available previously.
Supplementary Material
Highlights.
Presence or absence of sperm in spermathecae is discernible without dissection.
Spermathecae are viewed by phase contrast microscopy through the distended abdomen.
Most females are not adversely affected by the procedure.
Acknowledgments
We are very grateful to Jorge Rey and George O’Meara for their valuable comments in an early version of the manuscript; Irka Bargielowski for her help in the validation of the technique; and Naoya Nishimura for providing blind samples for the mating capacity experiment. This research was supported by U.S. National Institutes of Health Grant R21 AI095780 (to L.P.L.) and a Colciencias (Colombia) graduate fellowship (to M.C.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Bargielowski IE, Lounibos LP, Carrasquilla MC. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2888–92. doi: 10.1073/pnas.1219599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner EA, Alto BW, Lounibos LP. Vertical transmission of Key West Dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes from Florida. Journal of Medical Entomology. 2013;50:1291–1297. doi: 10.1603/me13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers SR. Reports to the Malaria Committee of the Royal Society. 1901. Anatomy and Histology of the Adult Female Mosquito. 4th Series. [Google Scholar]
- Christophers SR. Its Life History, Bionomics and Structure. Cambridge University Press; 1960. Aedes aegypti (L.) The Yellow Fever Mosquito. [Google Scholar]
- Clements AN. The Biology of Mosquitoes, Volume 2, Sensory Reception and Behaviour. CABI publishing; Wallingford, UK: 1999. [Google Scholar]
- Collins AU, Williams V, Evans JD. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Molecular Biology. 2004;13:141–146. doi: 10.1111/j.0962-1075.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Gullan PJ, Cranston PS. The Insects: An Outline of Entomology. 4. Wiley-Blackwell, John Wiley & Sons, Ltd; West Sussex, UK: 2010. Reproduction; pp. 121–150. [Google Scholar]
- Gwadz RW, Craig GB., Jr Sexual receptivity in female Aedes aegypti. Mosquito News. 1968;28:586–593. [Google Scholar]
- Harbach RE. [accessed on July 23 2014];Mosquito Taxonomic Inventory. 2014 http://mosquito-taxonomic-inventory.info/
- Harbach RE, Knight KL. Taxonomist’s Glossary of Mosquito Anatomy. Plexus Publishing Inc; Marlton, New Jersey: 1980. [Google Scholar]
- Jones MDR, Gubbins SJ. Changes in the circadian flight activity of the mosquito Anopheles gambiae in relation to insemination, feeding and oviposition. Physiological Entomology. 1978;3:213–220. [Google Scholar]
- Jones JC, Wheeler RE. Studies on spermathecal filling in Aedes aegypti (Linnaeus). I. Description. Biological Bulletin. 1965;129:134–150. doi: 10.2307/1539731. [DOI] [PubMed] [Google Scholar]
- Lima-Camara TN, Pereira Lima JB, Vieira Bruno R, Peixoto AA. Effects of insemination and blood-feeding on locomotor activity of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) females under laboratory conditions. Parasites & Vectors. 2014;7:304. doi: 10.1186/1756-3305-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Esther RL, Duzak D, Martin EA. Body size, sexual receptivity and cannibalism in relation to protandry among Toxohynchites mosquitoes. Oikos. 1996;77:309–316. [Google Scholar]
- Lounibos LP, Lima DC, Lourenço-de-Oliveira R. Prompt mating of released Anopheles darlingi in western Amazonian Brazil. Journal of the American Mosquito Control Association. 1998;14:210–3. [PubMed] [Google Scholar]
- McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM. Manual of Nearctic Diptera. Vol. 1. Agriculture Canada; 1981. p. 27. Coordinators. Monograph. [Google Scholar]
- O’Meara GF, Lounibos LP. Reproductive maturation in the pitcher-plant mosquito, Wyeomyia smithii. Physiological Entomology. 1981;6:437–443. [Google Scholar]
- Polerstock AR, Eigenbrode SD, Klowden MJ. Mating alters the cuticular hydrocarbons of female Anopheles gambiae sensu stricto and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:545–552. doi: 10.1603/0022-2585-39.3.545. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Aslamkhan M. A release-recapture experiment with the malaria vector, Anopheles stephensi Liston, with observations on dispersal, survivorship, population size, gonotrophic rhythm and mating behavior. Annals of Tropical Medicine and Parasitology. 1979;73:251–269. doi: 10.1080/00034983.1979.11687255. [DOI] [PubMed] [Google Scholar]
- Rosay B. Anatomical indicators for assessing age of mosquitoes: Dissection techniques and field application of methods. Mosquito News. 1969;29:419– 423. [Google Scholar]
- Samung Y, Palakul K, Apiwathnasorn C, Prummongkol S, Asavanich A, Leemingsawat S. Laboratory colonization of Mansonia mosquitoes with an emphasis on Ma. annulata and Ma. bonneae. The Southeast Asian Journal of Tropical Medicine and Public Health. 2006;37:656–661. [PubMed] [Google Scholar]
- Simmons LW. Sperm Competition and its Evolutionary Consequences in Insects. Princeton University Press; New Jersey: 2001. [Google Scholar]
- Snodgrass RE. Anatomy of the Honey Bee. Comstock Publishing Associates; New York: 1956. [Google Scholar]
- Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser E. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. American Journal of Tropical Medicine and Hygiene. 2011;85:265–270. doi: 10.4269/ajtmh.2011.10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel WR. Fire ant queen longevity and age: estimation by sperm depletion. Annals of the Entomological Society of America. 1987;80:263–266. [Google Scholar]
- Wagoner KM, Lehmann T, Huestis DL, Ehrmann BM, Cech NB, Wasserberg G. Identification of morphological and chemical markers of dry- and wet-season conditions in female Anopheles gambiae mosquitoes. Parasites & Vectors. 2014;7:294. doi: 10.1186/1756-3305-7-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler WM. Ants: Their Structure, Development and Behavior. 3. Columbia University Press; New York: 1960. [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Annual Review of Entomology. 2006;51:413–40. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.