Abstract
Purpose
Stargardt macular dystrophy (STGD) results in early central vision loss. We sought to explain the genetic cause of STGD in a cohort of 88 patients from three different cultural backgrounds.
Methods
Next Generation Sequencing using a novel capture panel was used to search for disease causing mutations. Unsolved patients were clinically re-examined and tested for copy number variations (CNVs) as well as intronic mutations.
Results
We determined the cause of disease in 67% of our patients. Our analysis identified 35 novel ABCA4 alleles. Eleven patients had mutations in genes not previously reported to cause STGD. Finally, 45% of our unsolved patients had single deleterious mutations in ABCA4, a recessive disease gene. No likely pathogenic CNVs were identified.
Conclusions
This study expands our knowledge of STGD by identifying dozens of novel STGD causing alleles. The frequency of patients with single mutations in ABCA4 is higher than controls, indicating these mutations contribute to disease. Eleven patients were explained by mutations outside ABCA4 underlining the need to genotype all retinal disease genes to maximize genetic diagnostic rates. Few ABCA4 mutations were observed in our French Canadian patients. This population may contain an unidentified founder mutation. Our results indicate that CNVs are unlikely to be a major cause of STGD.
Keywords: Vision research, Clinical genetics, Macular degeneration, Diagnosis, Copy-number Variations
BACKGROUND
Stargardt macular dystrophy (STGD) is the most common form of juvenile macular dystrophy affecting approximately one in ten thousand individuals worldwide 1. STGD consists of childhood visual acuity loss, central scotomas on visual field testing and an atrophic maculopathy. The sub-retinal buildup of oily droplets called lipofuscin in the retinal pigment epithelium (RPE) cell layer disrupts photoreceptors, leading to photoreceptor death and retinal degeneration2.
This disease is particularly devastating because affected individuals lose central vision, which is necessary common tasks including reading, schooling, driving and recognizing faces. Most cases of STGD are caused by recessive 3. “STGD-like” autosomal dominant diseases can be caused by mutations in PROM14 and ELOVL45. Of these three genes, ABCA4 is by far the most frequent cause of disease and the only known cause of recessive STGD. Mutations in ABCA4 also cause the related disorders of Cone-Rod Dystrophy (CRD) and Retinitis Pigmentosa (RP)6.
Ongoing gene and drug therapy trials aim to treat retinal genetic disorders7. These trials have been largely successful, in part due to several unique features of the eye8. However, it is often unclear exactly which disorder is causing patient phenotypes. This is because of a large degree of similarity between different retinal diseases, and the broad range of phenotypes caused by mutations in a single gene. This makes genetic diagnosis a necessary first step towards personalized therapy.
Genotyping microarrays, such as the Asper Ophthalmics genomics chip, are typically used for genetic diagnosis of STGD and identify the cause of disease in 30-40% of patients9. By contrast, a small number of studies using next generation sequencing (NGS) display increased sensitivity of molecular diagnosis, largely due to their ability to detect novel disease alleles10. In order to maintain the accuracy of NGS while reducing cost, capture protocols have been developed that enrich for DNA of interest, allowing targeted sequencing.
Most cases of STGD are caused by recessive ABCA4 mutations. However, in many unsolved STGD patients only one disease causing ABCA4 mutation is detected10. Deep intronic variants, deletions that escape genetic detection or a currently unknown STGD gene may explain these cases. Our aim in this study is to utilize next generation sequencing to assess the spectrum of ABCA4 mutations in our patient cohort, and to examine patients for disease causing mutations in other retinal disease genes.
Here we present results from highly accurate capture panel sequencing of all known retinal disease genes, leading to an overall molecular diagnosis rate of 67% in our cohort consisting of 18 French Canadian, 39 other Canadian and 31 Chinese patients. Over half (53%) of disease was explained by homozygous or compound heterozygous mutations in ABCA4. From the 88 individuals examined in this study, we identified 35 novel ABCA4 mutations. While results for Chinese and Canadian cohorts were similar, our French Canadian cohort had a statistically significantly reduced solving rate, indicating that unidentified are mutations enriched in this population. Finally, we identified causative mutations currently only associated with other macular dystrophies, not clinical STGD. This may be due an incorrect initial clinical diagnosis or genetic heterogeneity of disease. Either way this emphasizes the importance of sequencing all known retinal disease genes when performing molecular diagnosis of STGD.
MATERIAL AND METHODS
Two cohorts of patients were examined. The first STGD cohort was from China recruited by Dr. Ruifang Sui, while the second contained individuals recruited in Canada by Dr. Robert Koenekoop.
Clinical evaluation – Ruifang Sui
Probands and other family members were ascertained primarily at Peking Union Medical College Hospital (PUMCH, Beijing, China). Medical and family histories were recorded. Detailed ophthalmologic examinations, including visual acuities, color vision test (pseudoisochromatic plates and D-15 color plates), slit lamp biomicroscopy, tonometry and dilated ophthalmoscopy were conducted. Macular structure was examined with optical coherence tomography (OCT) (3D OCT-2000 Spectral Domain; Topcon, Tokyo, Japan). Auto fluorescence images (HRA 1; Heidelberg Engineering, Heidelberg, Germany) were obtained. Full-field ERGs were performed (RetiPort ERG system, Roland Consult, Wiesbaden, Germany) in selected patients. The method was performed in concordance with the International Society for Clinical Electrophysiology of Vision standard protocol (ISCEV).
Diagnosis of STGD was based on the clinical manifestations. Written informed consents were obtained from participants or their guardians. Genomic DNA was isolated from peripheral leukocytes using a commercial kit (QIAamp Blood Midi; Qiagen, Hilden, Germany) according to manufacturer's protocol. This study was approved by the Institutional Review Board of PUMCH and adhered to the tenets of the Declaration of Helsinki and the Guidance on Sample Collection of Human Genetic Diseases by the Ministry of Public Health of China.
Clinical evaluation – Robert Koenekoop
All patients were seen at Montreal Children's Hospital McGill Ocular Genetics clinic (MOGL) and informed consent was obtained. All patients underwent a detailed history and pedigree analysis and detailed eye examinations, including best corrected visual acuities by projected Snellen charts, near vision, slit lamp biomicroscopy and dilated retinal exams. In vivo retinal imaging was performed by the Heidelberg OCT (Heidelberg Engineering), followed by fundus auto-fluorescence (FAF) and began in 2009, thus some patients did not receive this testing until after their molecular diagnosis was confirmed. Kinetic fields were measured by Goldmann perimetry. In selected patients we performed ISCEV standard ERGs and multifocal ERGs (Diagnosys, Boston USA). Clinical diagnosis of STGD was made when the patient, usually a child, developed central visual acuity loss with an atrophic maculopathy with or without flecks. Peripheral blood was collected in lavender top (EDTA) tubes for DNA extraction.
Clinical evaluation – Comparison
The clinician authors of this study actively collaborated to control for differences in diagnosis between the cohorts. This involved both clinicians reviewing and coming to a consensus on clinical data in some cases where the diagnosis was in question. Both clinicians used largely identical visual acuity tests, slit lamp biomicroscopy, dilated ophthalmoscopy and OCT in all patients when generating a clinical diagnosis. However, despite our effort to maintain consistent criteria, slight differences did exist between the two cohorts. Specifically, ERGs were performed in all patients in the Chinese cohort but only in selected patients in the Canadian cohort, while FAF was performed on all Canadian patients but only some Chinese patients.
Capture sequencing and data analysis
Patient DNA was extracted from peripheral blood using standard techniques. A pre-capture library was generated11, then captured on a custom capture panel designed using Agilent Sureselect targeting all known retinal disease genes (Table S1). Resultant DNA was bar-coded and prepared, then shotgun sequenced on an Illumina GIIx machine (http://www.illumina.com/systems/genome_analyzer_iix.ilmn). Reads were aligned to hg19 using BWA 12. Recalibration and realignment were performed using GATK 13. Samtools14 was used to sort and index the resultant bam files. SNPs and Indels were called using Atlas SNP and Atlas Indel, respectively15.
These variants were filtered and annotated using a suite of software. First, variants frequency > .5% in the 1 000 genomes project builds 2010 & 201116, NHLBI GO Exome Sequencing Project (ESP) cohort 17, or an internal control database at the human genome sequencing center were filtered out. Second, variants that occurred at a frequency of >10% in our patient cohort were filtered out, as these are likely systematic errors. Third, variants were annotated using ANNOVAR 18. Variants not affecting protein sequence or splicing were removed. Finally, variants were annotated with predictions from SIFT 19, POLYPHEN2 20, and various other annotation programs using dbNSFP version 2.0b4 21. Final results were verified by hand on up to date online tools for HGMD 22, SIFT and POLYPHEN2, accessed 2/17/2014.
Sanger verification and intronic sequencing
All reported causative mutations were confirmed by Sanger sequencing using standard techniques. Where possible segregation was performed. All patients that remained unsolved after capture sequencing were tested for causative intronic mutations. Specifically, we tested for the seven intronic mutations from 23, as well as sequencing retinal specific exons reported in 24. Primers and Sanger sequencing tracks are available upon request.
Molecular diagnosis
If at least 2 variants were observed in ABCA4 that passed filtering criteria, the variants were considered as candidate disease causing mutations. In all cases where a novel ABCA4 mutation was observed, no other set of mutations in the patients was observed that is known to be sufficient to cause retinal disease. Patients whose disease is unexplained by ABCA4 variants were examined for mutations known to cause other retinal diseases.
Copy number variation analysis
Copy number variation (CNV) analysis was performed using a custom-designed Comparative Genomic Hybridization (CGH) microarray (Agilent Technologies, Inc). The high density 8x60K array covers known STGD genes and genes associated with age-related macular dystrophy. The designed probe density was >50 oligos per kb. The digestion, labeling, and hybridization processes were performed according to the manufactures’ instructions. Array slides were scanned with the Agilent G2565 Microarray Scanner. Resulting images were quantified using Agilent Feature Extraction software and analyzed utilizing Agilent genomic Workbench (http://www.genomics.agilent.com).
RESULTS
We designed and tested new capture sequencing reagents targeting all known exons of all known retinal disease genes (at time of design, 12/13/2012). Our panel was designed using Agilent SureSelect and captures 213 known retinal disease genes. All genes included and a justification for their relevance to retinal disease can be found in the supplemental data.
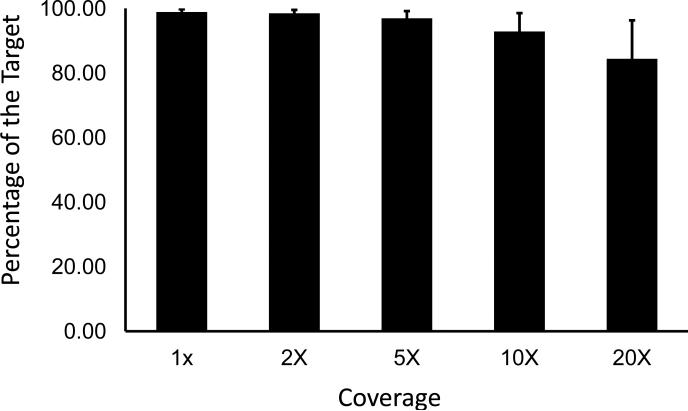
This capture panel was applied to 88 patients with an original diagnosis of STGD, including 57 patients examined at the Montreal Children's Hospital in Montreal, Canada and 31 patients examined at Peking Union Medical College Hospital in China. Rigorous quality control was performed, examining the total number of reads, percent of reads mapping to the target, coverage in the target region and number of variants observed before and after filtering. Experiments with poor data quality were repeated. On average, 98.86% of the target was covered with 92.86% of the target having coverage >10×, which is sufficient to call heterozygous mutations25 (Figure 1).
Figure 1.
High quality sequencing results were obtained, with consistently high coverage across the targeted region. Almost the entire target was covered sufficiently to call SNPs. Error bars display one standard deviation.
After verifying the quality of our sequencing results, we examined our patients for pathogenic mutations in ABCA4. All mutations were Sanger verified. In addition, patients whose disease could not be explained by capture sequencing alone were checked for known disease causing intronic mutations in ABCA4. Three patients (patient 27, 28, and 52) had mutation V1 from Braun et al 201323. Patient 28 also had an observed novel exonic hit and is thus considered solved. No mutations at the positions of V2-V7 from Braun et al 2013 were observed. No predicted pathogenic mutations were found in the retinal specific exons from Farkas et al 201324.
STGD in 48 patients (55%) was explained by recessive ABCA4 mutations (supplemental table S2). Only 22 patients could be solved using previously known STGD causing mutations. However, we identified 35 novel mutations in ABCA4 contributing to the diagnosis of 25 STGD patients. This contained 10 novel mutations leading to amino acid substitutions already known to cause disease and mutations known to cause diseases other than STGD. In addition, we identified 13 novel nonsense mutations. The remaining 12 novel mutations are well justified, and novel mutations were either completely absent or extremely rare in controls.
Interestingly, while we found disease causing ABCA4 mutations in 61% (19/31) and 59% (23/39) of our Chinese and Canadian cohorts, respectively, only 33% (6/18) of our French Canadian cohort was resolved by leveraging ABCA4 mutations. This result is statistically significant (P<.05 vs. Chinese and Canadian cohorts using Fisher's exact test). This indicates that one or more unidentified founder STGD causing mutations may exist in this French Canadian population, resulting in our low rate of molecular diagnosis. Founder mutation(s) could occur in ABCA4 exons, introns, or in other retinal disease genes. Alternatively, the French Canadian population may have a different mutation spectrum than other cohorts. The FC population did not show a statistically significant increase in unsolved patients with a single observed mutation in ABCA4. This was due to a lack of power, as only 8 FC patients are unsolved. The remaining 4 FC patients have disease likely caused by mutations in other retinal disease genes (Table 1).
Table 1.
Disease in 11 patients explained by mutations outside ABCA4
| ID / Cohort | Gene | NM# | Genotype | cDNA | Protein | Justification |
|---|---|---|---|---|---|---|
| 13 / Can | PRPH2 | NM_000322 | Heterozygous | c.37 C>T | p.(R13W) | Known (RP)34 |
| 55 / FC | CRB1 | NM_001193640 | Compound | c.635 G>A | p.(C212Y) | SIFT, PolyP |
| Heterozygous | c.2507 G>A | p.(C836Y) | Known AA (RP)35 | |||
| 57 / FC | PRPH2 | NM_000322 | Heterozygous | c.499 G>A | p.(G167S) | Known (PD) 26,36 |
| 61 / Can | DMD | NM_004010 | Homozygous | c.2458 C>T | p.(R820C) | SIFT, PolyP, H |
| 62 / Can | USH2A | NM_206933 | Compound | c.5953 G>A | p.(E1985K) | PolyP |
| Heterozygous | c.2802 T>G | p.(C934W ) | Known (RP) 37 | |||
| 69 / Can | BEST1 | M_004183 | Homozygous | c.830 C>T | p.(T277M) | SIFT, PolyP |
| 70 / Can | CDH23 | NM_001171930 | Homozygous | c.2263 C>T | p.(H755Y) | Known (USH1) 38-40 |
| 80 / FC | CRB1 | NM_001193640 | Compound | c.3350 G>C | p.(C1117S) | SIFT, PolyP |
| Heterozygous | c.493_501del | 165_167del | O | |||
| 96 / Can | CFH | NM_000186 | Homozygous | c.101 C>G | p.(T34R) | SIFT |
| 105 / FC | PROM1 | NM_006017 | Heterozygous | c.980 A>G | p.(N327S) | STGD |
| 145 / Chi | PROM1 | NM_006017 | Compound | c.2466 G>A | p.(M822I) | SIFT, STGD |
| Heterozygous | c.277-1 G>A | Splicing | Splice, STGD |
Patients who were clinically examined following the molecular diagnosis are in bold. Under Justification, “Known AA” indicates that the amino acid substitution is known to cause disease while “Known” indicates this specific DNA change is known to cause disease. The disease the mutation was associated with follows in parenthesis. RP stands for Retinitis Pigmentosa; PD stands for Pattern Dystrophy, USH1 stands for Usher Syndrome Type 1. FC = French Canadian, Can= other Canadian, Chi = Chinese. All novel mutations had a frequency <0.002 in a control cohort of 6,500 individuals (ESP6500). For novel mutations, use the following key: Sift = Predicted damaging by SIFT. PolyP = Predicted damaging by Polyphen 2. Splice = Predicted splice site loss mutation. STGD = This gene is known to cause Stargardt-like phenotypes. O=Overlaps with known disease causing missense and non-frameshift deletion mutations.
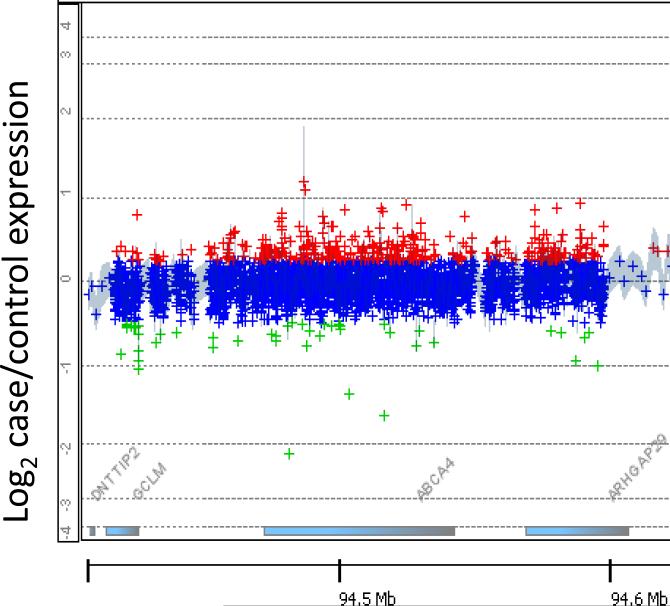
Surprisingly, 13/29(45%) unsolved patients had a single observed likely pathogenic mutation in ABCA4 and no observed second ABCA4 mutation, which is significantly higher than the expected rate in the general population (5%). This effect had no significant variation between cohorts. To investigate whether copy number variations in ABCA4 could explain some of these unsolved cases, we performed chromosomal analysis on the 12 patients with a single heterozygous hit in ABCA4. No likely deleterious copy number variations were observed (fig 2). Those patients that were not solved following thorough analysis of ABCA4 were checked for mutations in other retinal disease genes based on capture sequence data. Eleven patients (13%) were solved invoking retinal disease genes not previously linked to STGD (table 1), bringing the total number of molecularly diagnosed patients to 59 (67%). Ten novel candidate disease causing mutations outside of ABCA4 were observed (table 1).
Figure 2.
Representative CGH microarray from a patient with one hit in ABCA4. Despite a very high probe density, no significant CNVs were found in any of the 12 patients with one mutation in ABCA4
We wished to determine if patients with mutations outside ABCA4 were observed because of an initial clinical misdiagnosis, or whether mutations in these 9 genes can cause STGD-like phenotypes. To this end, two patients harboring mutations outside ABCA4 were re-examined in detail. Clinically, these subjects (Fig.3-4) had characteristic STGD visual acuity loss and with an atrophic maculopathy and with retinal flecks and central scotomas on Goldmann visual fields. Both patients had been seen by several ophthalmologists, retinal specialists or pediatric ophthalmologists and the consensus clinical diagnosis was STGD. Upon molecular testing we first ruled out ABCA4 mutations then identified two likely pathogenic mutations in the Bestrophin-1 (BEST1) and Crumbs homolog 1 (CRB1) genes, respectively.
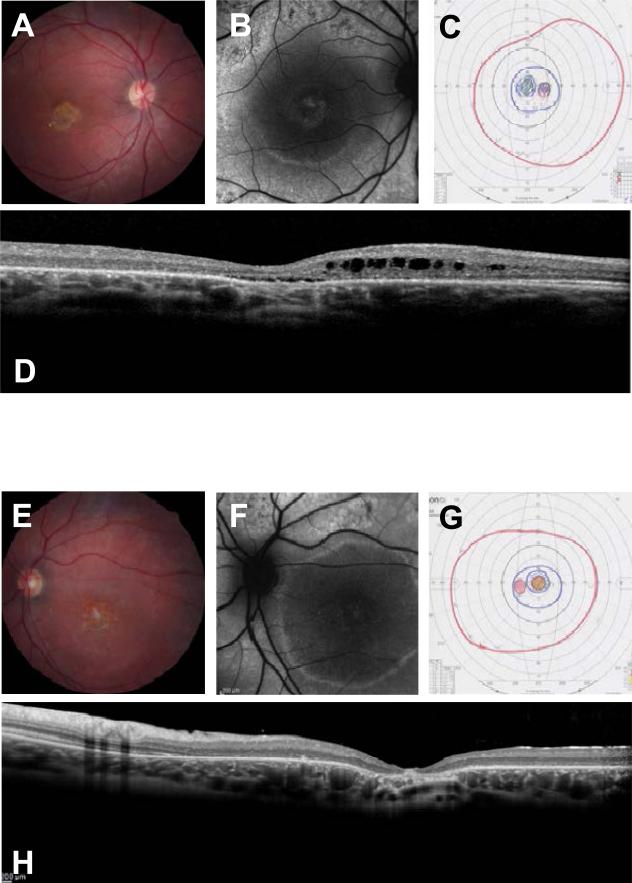
Figure 3.
A,E) Fundus photographs of patient 69 show a bilateral atrophic maculopathy with yellow retinal flecks while the retinal vasculature and optic discs are within normal limits. B,F) Fundus auto-fluorescence images show an incomplete rim of hyper-fluorescence in the right macular area and a complete hyper-fluorescent ring in left macula. C,G) GVF shows central scotoma in both eyes. D,H) OCT studies show loss of the IS/OS junctions, a disorganized external limiting membrane and outer nuclear layer, thinning of the outer nuclear layer in the fovea and sub-foveal edema. There were marked cystic spaces in the inner nuclear layer.
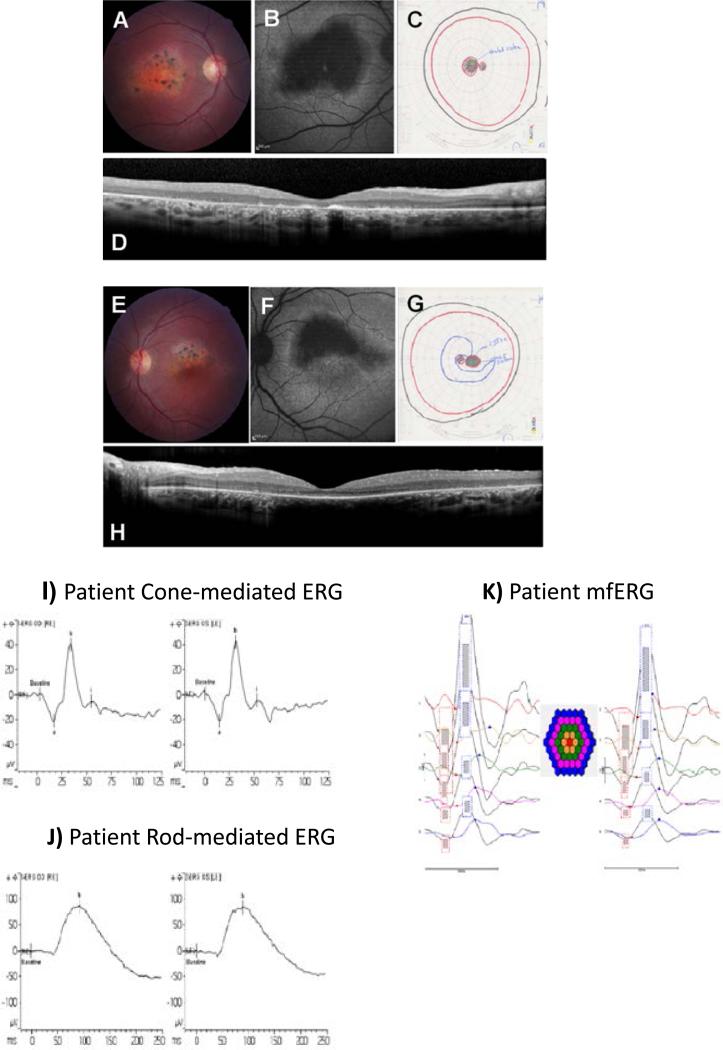
Figure 4.
A,E) Fundus photographs of patient 55 show an atrophic maculopathy involving the fovea in the OD with clumps of pigmentation in the macular area. The OS shows an atrophic maculopathy sparing the inferior half of fovea. Retinal vessels and optic discs are within normal limits. B,F) Fundus auto-fluorescence images show macular atrophy appears dark black involving the fovea in the OD and in the OS inferior half of fovea is spared. C,G) Goldmann Visual Fields show an absolute central scotoma OU. D,H) OCT shows bilateral loss of IS/OS junctions and decreased retinal thickness. Accumulation of auto-fluorescent photoreceptor debris in the foveal area is seen in the OD. I) Cone mediated ERG a-waves are within normal amplitude limits (OU) and peak times are delayed (0D) and within normal limits (OS). The b-waves are within the lower limits of normal amplitude (OU) with peak times that are delayed (0D) and within normal limits (OS). J) The rod-mediated ERG b-wave is within normal amplitude and peak time limits (OU). K) mfERGs are attenuated in amplitude at all eccentricities.
Clinical phenotypes of two unusual patients with unexpected clinical re-diagnoses
Subject 69 with the homozygous BEST1 mutation is a 33 year old female from the Mohawk nation of Montreal, Quebec and presented with progressive decrease in central vision since early childhood. Her best corrected visual acuity (BCVA) was 20/70 in OD and 20/400 in OS. Her problem started at 13 years of age. Her parents were nonconsanguineous, but from a small and ancient original Canadian community. The proband's brother also lost central vision in his 20's. The proband had a peripheral iridectomy for glaucoma in both eyes. Clinically, the patient's ocular phenotype was STGD (diagnosed by three retinal specialists) but her molecular diagnosis was autosomal recessive bestrophinopathy as she carries homozygous BEST1 mutations. Figure 3 shows fundus photographs, optical coherence tomography (OCT), fundus auto-fluorescence images (FAF), and Goldmann visual fields (GVF) which were performed after the molecular diagnosis. The patients phenotype consists of an atrophic macular lesion, subfoveal and parafoveal retinal edema, loss of the IS/OS junction in the macula, a perifoveal hyper fluorescent ring on FAF and central scotomas on GVF.
Subject 55 is a 23 year old French-Canadian female from Quebec who presented with progressive central vision loss at age 5 and has compound heterozygous mutations in CRB1. Her BCVA was 20/400 in OD and 20/50 in OS. She denied nyctalopia. Her parents were also from Quebec and non-consanguineous, although it is well-known the Quebec population has strong founder effects. Her clinical phenotype was STGD with characteristic atrophic maculopathy and retinal flecks. Retinal vasculature and peripheral retina were within normal limits in both eyes. Molecular testing results showed that the proband is carrying compound heterozygous CRB1 mutations: p.Cys212Tyr / p.Cys948Tyr and c.635G>A / c.2843G>A. The C212Y mutation in CRB1 is a novel splicing mutation, leading to a loss of that exon and a frame shift in the CRB1 protein, while the C948T mutation was previously reported to cause RP.
After genotyping, an ERG was performed at age 23 which was essentially normal for both rod and cone function. It showed that the rod-mediated ERG b-wave was within normal amplitude and peak time limits (OU) (Fig. 4). The rod-cone mediated ERG a- and b-wave amplitudes were within normal limits (OU), a-wave peak times were within normal limits (OU) while b-wave peak times were significantly delayed (OU). The cone mediated ERG a-waves were within normal amplitudes in OU and b-waves within lower limits of normal amplitude. This is a very unusual ERG for a patient with CRB1 mutations. Figure 4 shows patient fundus photographs, OCT, FAF, GVF, focal electroretinogram (fERG) and multi-focal electroretinogram (mfERG). This illustrates an atrophic maculopathy, a large central FAF hypo-fluorescence, loss of IS/OS junctions in the macula on OCT and central scotomas on GVF. The ERG is significant for a patient with CRB1 mutations in that it is essentially intact.
The other patient with CRB1 mutations (Patient 80, see Table 1) presented with visual acuity loss in the left eye at 8 years old and visual acuity loss in the right eye at age 45. We observed Bull`s Eye maculopathy with retinal flecks in both eyes, with 20/50 visual acuity in the right eye, with counting fingers in the left eye and an essentially normal full field cone and rod ERG. The patient tested normal for Goldmann visual fields (GVF). Based on this data, the patient was diagnosed with STGD. Fundus autofluorescence (FA) showed central hypo-fluorescence, and optical coherence tomography (OCT) showed severe foveal thinning. In conclusion, the two patients with CRB1 mutations have similar phenotypes, both of which fall within the standard clinical definition of STGD disease. In addition, it is interesting to note that this is not the first report of CRB1 mutations causing disease in a patient originally diagnosed with STGD. In accord with our patients, this patient was found to have more severe degeneration in one eye, along with severe central vision impairment occurring at age 37, though unlike our patients no fundus flecks were observed26. Taken together, these data suggest a novel phenotype associated with CRB1 mutations.
DISCUSSION
This study demonstrates the ability of capture sequencing to increase the rate of molecular diagnosis in a cohort of clinically diagnosed STGD patients, a vital first step towards therapy and personalized medicine. We identified the molecular cause of disease in 59 out of our 88 patients, and achieving a rate of 67%. The increase in accuracy we obtained over traditional methods is due to three features of our capture sequencing protocol, which allowed for a comprehensive analysis of the ABCA4 locus and, when this failed to identify the cause of disease, other retinal disease genes. First, because we sequenced the entire coding region of ABCA4, we were able to identify novel STGD causing mutations. This feature contributed to the diagnosis of 25(28%) of our patients. Second, our capture technique allowed us to identify mutations in other known retinal disease genes, allowing for correction in cases of unclear or misdiagnosis and allowing us to identify novel phenotype-genotype correlations. This feature has contributed to the diagnosis of 11(13%) of our patients. Third, we tested for recently identified intronic mutations in ABCA4 and 3 such mutations in our patient cohort. The candidate novel mutations identified by this study are well supported.
Numerous other recent papers have noted that there is much overlap between genes causing different forms of retinal disease 27-29. These recent findings support the conclusion that there is no simple connection between the gene in which a mutation occurs and retinal phenotype of a patient, making strict categorization of genetic disease complicated. Further, this observation indicates a thorough description of any patients’ disease should include both genetic and clinical information. The high percentage of patients harboring novel alleles and mutations in genes not previously associated with STGD also demonstrates the strength of NGS as a molecular diagnostic tool. All novel mutations and all mutations outside of ABCA4 would have been missed by a standard diagnostic genotyping array, reducing our molecular diagnosis rate by 40%. In addition, our approach identified interesting phenotype-genotype correlations.
In particular, two patients were diagnosed by multiple retinal and pediatric ophthalmology specialists with STGD but had disease causing mutations outside ABCA4. These patients were re-examined clinically. The genetic testing allowed us to refine their diagnosis and re-classify them into new clinical diagnostic categories. Patient 69 was diagnosed with STGD but was found to have autosomal bestrophinopathy due to recessive BEST1 mutations. Ocular phenotypes associated with mutations in BEST1 include retinitis pigmentosa, rod-cone dystrophy and bestrophinopathy30, changing the differential diagnosis of this patient.
In the second case, we found CRB1 mutations in a child with maculopathy, central scotomas and an essentially robust and normal ERG (patient 55). CRB1 mutations are known to cause severe diffuse retinal degeneration with absent or severely diminished ERGs including LCA, juvenile RP, and CRD. To our knowledge this is the first case of a retinal dystrophy caused by CRB1 mutation with a normal ERG, as usually CRB1 mutations lead to severe RP or LCA with extinguished or severely diminished ERGs. There are certain specific retinal findings (including (PPRPE and nummular pigment) which indicate CRB1 mutations are causative , yet these were not found in this patient. Largely similar results were observed in a second patient with CRB1 mutations. This data demonstrates a novel CRB1 phenotype. Given that overlapping phenotypes are caused by mutations in different genes and many genes cause a spectrum of phenotypic outcomes, the current best way to determine the genetic cause of disease is through capture sequencing.
The enrichment for patients with a single hit in the recessive disease gene ABCA4 indicates that ABCA4 is likely involved in the disease of many of our undiagnosed patients. Exonic regions are well covered by our method, and despite examining a number of patients with high density aCGH, no copy number variations were observed. This leads to the exciting possibility that novel types of mechanisms (regulatory mutations, cryptic splice site formation, or digenic effects) are contributing to the genetic cause of disease in our patients. Indeed, a recent study has identified numerous altered transcripts in ABCA4 as a result of splicing modifications and synonymous variants 23. Alternatively, these ABCA4 mutations may act in a di-genic or multi-genic fashion to cause disease.
Comparison of our three cohorts showed surprising similarity between the Chinese and Canadian populations. However, a paucity of disease causing ABCA4 mutations was observed in our French Canadian cohort. This effect is statistically significant, and raises the possibility of currently unknown founder mutation(s) in this cohort, which may occur in the exons or introns of ABCA4, or in another gene. Quebec patients from French-Canadian origin are well known to come from a gene pool with the potential for founder effects, as today's 8 million people are descendants of 2500 French fore-fathers, mostly from Northern France. Further studies targeting French Canadian STGD patients are thus recommended to elucidate the reason for this missing inheritance.
Several recent studies have also performed NGS based genetic diagnosis of STGD patients10,31-33. The central conclusions of these studies are that ABCA4 holds additional pathogenic mutations that are missed during exon sequencing and that NGS significantly improves the accuracy of genetic diagnosis. Given the increase in accuracy of capture sequencing over array based diagnosis, health care institutions should adopt this new technology whenever possible. Unfortunately, several obstacles remain, including methodologies to handle variants of unknown significance and standardized pipelines and metrics to ensure repeatability and reliability of data. None-the-less, the increase in accuracy afforded by capture sequencing will make it an invaluable tool in the development of personalized medicine. This study represents a first step towards the treatment for two thirds of our patients by identifying the genetic cause of their disease, giving them the potential participate in developing genetic therapy techniques.
Supplementary Material
Acknowledgements
We sincerely thank all patients, parents and families involved in this study. We would like to thank Dr. James R. Lupski and Dr. Phillip Michael Boone for their assistance with the microarray analysis. J.Z. is supported by a training fellowship from the Keck Center of the Gulf Coast Consortia, on the NLM Training Program in Biomedical Informatics (NLM Grant No. T15LM007093). R.C. is supported by grants from Retinal Research Foundation, Foundation Fighting Blindness (BR-GE-0613-0618-BCM) and the National Eye Institute (R01EY022356, R01EY018571). R.KK. is supported by Foundation Fighting Blindness Canada, CIHR, FRSQ, and NIH. R.S. is supported by the Ministry of Human Resource and Social Security of the People's Republic of China and the Foundation Fighting Blindness USA (CD-CL-0808-0470-PUMCH). F.W. is supported by a pre-doctoral fellowship: The Burroughs Welcome Trust Fund, The Houston Laboratory and Population Sciences Training Program in Gene Environment Interaction.
REFERENCES
- 1.Burke TR, Tsang SH. Allelic and phenotypic heterogeneity in ABCA4 mutations. Ophthalmic genetics. 2011;32(3):165–174. doi: 10.3109/13816810.2011.565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Progress in lipid research. 2010;49(4):476–492. doi: 10.1016/j.plipres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan J, Gerber S, Larget-Piet D, et al. A gene for Stargardt's disease (fundus flavimaculatus) maps to the short arm of chromosome 1. Nature genetics. 1993;5(3):308–311. doi: 10.1038/ng1193-308. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Chen Y, Lillo C, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. The Journal of clinical investigation. 2008;118(8):2908–2916. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang K, Kniazeva M, Han M, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nature genetics. 2001;27(1):89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
- 6.Kjellstrom U. Association between genotype and phenotype in families with mutations in the ABCA4 gene. Molecular vision. 2014;20:89–104. [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Science translational medicine. 2012;4(120):120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaneveld J, Wang F, Wang X, Chen R. Dawn of ocular gene therapy: implications for molecular diagnosis in retinal disease. Science China. Life sciences. 2013;56(2):125–133. doi: 10.1007/s11427-013-4443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernest PJ, Boon CJ, Klevering BJ, Hoefsloot LH, Hoyng CB. Outcome of ABCA4 microarray screening in routine clinical practice. Molecular vision. 2009;15:2841–2847. [PMC free article] [PubMed] [Google Scholar]
- 10.Fujinami K, Zernant J, Chana RK, et al. ABCA4 gene screening by next-generation sequencing in a British cohort. Investigative ophthalmology & visual science. 2013;54(10):6662–6674. doi: 10.1167/iovs.13-12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenekoop RK, Wang H, Majewski J, et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nature genetics. 2012;44(9):1035–1039. doi: 10.1038/ng.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics (Oxford, England) 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics (Oxford, England) 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, Wan Z, Coarfa C, et al. A SNP discovery method to assess variant allele probability from next-generation resequencing data. Genome research. 2010;20(2):273–280. doi: 10.1101/gr.096388.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peloso GM, Auer PL, Bis JC, et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. American journal of human genetics. 2014;94(2):223–232. doi: 10.1016/j.ajhg.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic acids research. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols. 2009;4(7):1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 20.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Human mutation. 2011;32(8):894–899. doi: 10.1002/humu.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenson PD, Ball EV, Mort M, et al. Human Gene Mutation Database (HGMD): 2003 update. Human mutation. 2003;21(6):577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 23.Braun TA, Mullins RF, Wagner AH, et al. Non-exomic and synonymous variants in ABCA4 are an important cause of Stargardt disease. Human molecular genetics. 2013;22(25):5136–5145. doi: 10.1093/hmg/ddt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farkas MH, Grant GR, White JA, Sousa ME, Consugar MB, Pierce EA. Transcriptome analyses of the human retina identify unprecedented transcript diversity and 3.5 Mb of novel transcribed sequence via significant alternative splicing and novel genes. BMC genomics. 2013;14:486. doi: 10.1186/1471-2164-14-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulonen AM, Ellonen P, Almusa H, et al. Comparison of solution-based exome capture methods for next generation sequencing. Genome biology. 2011;12(9):R94. doi: 10.1186/gb-2011-12-9-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strom SP, Gao YQ, Martinez A, et al. Molecular diagnosis of putative Stargardt Disease probands by exome sequencing. BMC medical genetics. 2012;13:67. doi: 10.1186/1471-2350-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Wang H, Sun V, et al. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. Journal of medical genetics. 2013;50(10):674–688. doi: 10.1136/jmedgenet-2013-101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Wang H, Tuan HF, et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Human genetics. 2014;133(3):331–345. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Q, Wang F, Wang H, et al. Next-generation sequencing-based molecular diagnosis of a Chinese patient cohort with autosomal recessive retinitis pigmentosa. Investigative ophthalmology & visual science. 2013;54(6):4158–4166. doi: 10.1167/iovs.13-11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boon CJ, van den Born LI, Visser L, et al. Autosomal recessive bestrophinopathy: differential diagnosis and treatment options. Ophthalmology. 2013;120(4):809–820. doi: 10.1016/j.ophtha.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Ge X, Shi W, et al. Molecular diagnosis of putative Stargardt disease by capture next generation sequencing. PloS one. 2014;9(4):e95528. doi: 10.1371/journal.pone.0095528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zernant J, Xie YA, Ayuso C, et al. Analysis of the ABCA4 genomic locus in Stargardt disease. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin X, Qu LH, Meng XH, Xu HW, Yin ZQ. Detecting genetic variations in hereditary retinal dystrophies with next-generation sequencing technology. Molecular vision. 2014;20:553–560. [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson SG, Cideciyan AV, Kemp CM, Sheffield VC, Stone EM. Photoreceptor function in heterozygotes with insertion or deletion mutations in the RDS gene. Investigative ophthalmology & visual science. 1996;37(8):1662–1674. [PubMed] [Google Scholar]
- 35.Henderson RH, Mackay DS, Li Z, et al. Phenotypic variability in patients with retinal dystrophies due to mutations in CRB1. The British journal of ophthalmology. 2011;95(6):811–817. doi: 10.1136/bjo.2010.186882. [DOI] [PubMed] [Google Scholar]
- 36.Glockle N, Kohl S, Mohr J, et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. European journal of human genetics : EJHG. 2014;22(1):99–104. doi: 10.1038/ejhg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, Dai H, Lu T, Zhang X, Dong B, Li Y. Seven novel mutations in the long isoform of the USH2A gene in Chinese families with nonsyndromic retinitis pigmentosa and Usher syndrome Type II. Molecular vision. 2011;17:1537–1552. [PMC free article] [PubMed] [Google Scholar]
- 38.Oshima A, Jaijo T, Aller E, et al. Mutation profile of the CDH23 gene in 56 probands with Usher syndrome type I. Human mutation. 2008;29(6):E37–46. doi: 10.1002/humu.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell CJ, Dinwiddie DL, Miller NA, et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Science translational medicine. 2011;3(65):65ra64. doi: 10.1126/scitranslmed.3001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg JS, Adams M, Nassar N, et al. An informatics approach to analyzing the incidentalome. Genetics in medicine : official journal of the American College of Medical Genetics. 2013;15(1):36–44. doi: 10.1038/gim.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.