Abstract
Objective
To summarize the available published randomized controlled trial data regarding timing of progesterone supplementation during the luteal phase of patients undergoing ART.
Design
A systematic review.
Setting
Not applicable.
Patient(s)
Undergoing in vitro fertilization.
Intervention(s)
Different starting times of progesterone for luteal support.
Main Outcome Measure(s)
Clinical pregnancy and live birth.
Results
Five randomized controlled trials were identified that met inclusion criteria with a total of 872 patients. A planned meta-analysis was not performed due to a high degree of clinical heterogeneity in regards to the timing, dose, and route of progesterone. Two studies compared progesterone initiated before oocyte retrieval versus the day of oocyte retrieval and pregnancy rates were 5–12% higher when starting progesterone on the day of oocyte retrieval. One study compared starting progesterone on post retrieval day 6 versus day 3, reporting a 16% decrease in pregnancy in the day 6 group. Trials comparing progesterone start times on the day of oocyte retrieval versus two or three days post retrieval showed no significant differences in pregnancy.
Conclusions
There appears to be a window for progesterone start time between the evening of oocyte retrieval and day 3 after oocyte retrieval. While some studies have suggested a potential benefit in delaying vaginal progesterone start time to 2 days after oocyte retrieval, this review could not find randomized controlled trials to adequately assess this. Further randomized clinical trials are needed to better define progesterone start time for luteal support after ART.
Keywords: Progesterone, luteal support, in vitro fertilization
Introduction
The rise of progesterone in the luteal phase during natural human reproduction is exquisitely timed to embryo development. The luteinizing hormone surge induces oocyte maturation, ovulation, and progesterone production from the corpus luteum. Progesterone hormone action produces endometrial changes in gene expression, histologic appearance, and structural arrangements which lead to an endometrium receptive for implantation five to six days after ovulation (1). Pulsatile pituitary LH and eventually hCG from the implanted pregnancy stimulate corpus luteal progesterone (1, 2) which is necessary for maintenance of the pregnancy until placental progesterone production is adequate. Pituitary down-regulation by GnRH analogues in Assisted Reproductive Technologies (ART) results in a dysfunctional luteal phase for some patients. Exogenous progesterone administration has been used successfully in IVF to overcome this deficiency. Failure to use luteal phase progesterone results in low pregnancy rates between 0–18% (3).
While it is clear that exogenous luteal support improves the rates of successful implantation and early pregnancy in ART, there has been significant debate and research regarding timing, dose, and routes of progesterone administration (4, 5, 6). In regards to the timing of progesterone initiation, there is endogenous progesterone production from the corpus luteum after hCG triggering that persists until 5–6 days post oocyte retrieval (3,7). Therefore it is likely that progesterone supplementation should be initiated prior to day 5–6, but it is not clear how early should progesterone be initiated prior to the fall of endogenous progesterone. It has also been proposed that early progesterone administration may be of benefit for embryo transfer via the smooth muscle relaxing effect of progesterone on the uterus (8). Conversely, ART cycles may be associated with advancement of the endometrium leading to embryo-endometrial asynchrony and implantation failure (9) and too early administration of progesterone may further expand this asynchrony (10). These data suggest a window of progesterone initiation in ART cycles in which embryo-endometrial synchrony and exogenous luteal phase support can be optimized.
This systematic review was performed to summarize the available published randomized controlled trial data regarding timing of starting progesterone supplementation during the luteal phase of patients undergoing ART.
Methods
Study Design
This study was a systematic review of the effect of day of progesterone initiation for luteal support in ART cycles. This study was performed in accordance with PRISMA guidelines. All aspects of the systematic review were decided before the literature search and no post hoc changes were made. The study was IRB exempt and the authors had no conflicts of interest.
Literature Search
Literature searches were conducted to retrieve randomized controlled trials comparing different starting times for luteal phase exogenous progesterone support in ART cycles. Databases searched included PubMed and Embase. Additional literature searches were performed on the references from identified studies. The searches were performed in English, were executed in January 2014, and searched the databases from January 1, 1990 through December 31, 2013. Searches utilized keywords and specific database indexing terminology when available (search strategy is in detail in Supplemental Addendum).
Study Selection
Criteria for inclusion in the study were established prior to the literature search. Inclusion was limited to studies that were published randomized controlled trials, compared different starting point of progesterone, and study participants who were infertile or subfertile. Any type of exogenous progesterone was allowed, including intra-muscular and vaginal administration. Any type of autologous fresh ART cycle was included. Exclusion criteria included frozen embryo transfers, non-randomization, studies in which all arms of the trial initiated progesterone at the same time point, and data published as abstract only, meeting proceeding, book chapter, or review article. The studies were screened independently in parallel by two investigators (MTC and MJH) and there were no disagreements in the studies identified for inclusion. The search strategy yielded 709 publications after to duplication removal. Studies identified from the references of other papers added an additional 4 studies for a total of 713 studies after duplication removal (Supplemental Fig 1). The 713 abstracts were reviewed and 699 records were excluded during this review for failure to meet inclusion criteria based on data presented in the abstract, leaving 14 full text papers that were evaluated for inclusion and exclusion criteria. Of these, 5 papers met full inclusion criteria. One study was excluded as it evaluated 17-hydroxyprogesterone for suppression of uterine contractions, but otherwise had the same luteal support for both arms (11). Other studies were excluded when full text evaluation demonstrated that the studies compared different progesterone regimens with the same progesterone initiation times in all arms (12–18). One study was excluded in fresh donor recipients where the recipient endometrium was timed with the donor (23). Study quality and the potential for bias within each study was also ascertained, specifically evaluating for randomization method, concealment of allocation, blinding of providers and patients, and flow of patients through the randomization, treatment, and outcome stages.
Data Collection
Data were extracted in sequence by three authors (MTC, JMS, and MJH). Outcomes data (clinical pregnancy, live birth, and miscarriage) were extracted from the source papers in the form of 2×2 or 2×3 tables based on intent-to-treat results. When intent-to-treat results were not reported, data was extracted from the provided per-protocol results. Continuous data were extracted in the form of mean, standard deviation, and population size. Additional extracted data included: author, year of publication, journal, country of origin, randomization method, sample size, number of patients randomized, number of cycles performed, method of ovulation induction, type of progesterone support, duration of progesterone support, method of ovulation triggering, trial registry, and the reporting of conflicts of interest. A priori primary outcome was live birth and secondary outcomes were clinical pregnancy and miscarriage. Data were collected for per patient outcomes. No post hoc analyses were performed after data collection.
Meta-analysis
A meta-analysis of the data was planned to compare starting points of progesterone in fresh ART cycles. However, the studies had a high degree of clinical heterogeneity in regards to the timing, dose, and route of progesterone. One study was in donor oocytes with fresh time recipients, but the recipients did not receive ovarian stimulation or hCG trigger, making their luteal phase significantly different that the other five studies. Finally, Sohn et al. allowed multiple cycles per patients and had variation in progesterone doses between the groups. Based on these factors it was determined that the data was of insufficient quality to justify meta-analysis.
Results
Studies Included for Systematic Review
A total of 713 abstracts were identified, 14 full text articles were reviewed, and from these 5 trials met full inclusion criteria (Supplemental Figure 1) (8, 19–23) The 5 trials comprised 872 patients undergoing 1,010 cycles, with only one study allowing multiple cycles per patient (19). All 5 studies described inclusion criteria consistent with a general IVF patient population and were in fresh autologous IVF patients (8, 19–22) (Table 1). Four of the studies utilized a long GnRH agonist protocol for pituitary down-regulation (8, 19, 21–22) and one study utilized multiple pituitary protocols (20). All studies utilized either rFSH or hMG. Ovulation triggering was performed with either 5,000 units or 10,000 units of hCG in all studies, except for one study that did not specify the dose (19). One of the included studies utilized intramuscular progesterone (19) and the other five studies utilized vaginal progesterone. All of the studies used different protocols of progesterone type, dose, starting and stopping times (Table 1). Primary outcomes data for each study was summarized in Table 2.
Table 1.
Study characteristics of trials meeting inclusion in the systematic review.
| Authors | Country of study | Patients | Cycle Type | Ovarian stimulation | Progesterone Type | Randomization Groups (by initiation of P) | P regimen | Ovulation triggering |
|---|---|---|---|---|---|---|---|---|
| Sohn et al. 1999 | USA | General IVF-no exclusion reported | Fresh augolous IVF | Long GnRH agonist with hMG and/or FSH. | Progesterone 12.5 mg IM then 25mg IM Progesterone 25 mg IM | Group A: 12.5mg dose 12 hours before OR and the evening of OR then 25mg dose Group B: evening of OR |
Daily through first trimester | hCG (amount not stated) |
| Williams et al. 2001 | USA | General IVF-no exclusion reported | Fresh augolous IVF | Long GnRH agonist, luteal GnRH agonist stop, or GnRH agonist micro-dose flare. rFSH 150–450 IU daily. | Prometrium, 200mg vaginally TID | Group A: morning of the 3rd day after OR Group B: morning of the 6th day after OR |
Daily until 10 weeks gestation | 10,000 units hCG |
| Fanchin et al. 2001 | France | General IVF-excluded patients with abnormal uterus | Fresh augolous IVF | Long GnRH agonist. rFSH 225 IU FSH for 5 days, then flexible dosing | Crinone 8% vaginally | Group A: immediately after OR Group B: evening of ET |
Daily until pregnancy ruled out | 10,000 units hCG |
| Baruffi et al. 2003 | Brazil | General IVF-no exclusion reported | Fresh augolous IVF | Long GnRH agonist. rFSH 150–300 IU daily. | Utrogestan, 400 mg vaginally | Group A: evening of ORGroup B: evening of ET | Not stated | 5,000-10,000 units hCG |
| Mochtar et al. 2006 | Netherlands | General IVF-no exclusion reported | Fresh augolous IVF | Long GnRH agonist. rFSH, hMG, or hpFSH. | Micronized progesterone 400 mg vaginally BID | Group A: evening of hCG administration Group B: evening after OR Group C: evening 3 days after OR |
Daily until 18 days post OR | 10,000 units hCG |
Table 2.
Primary pregnancy outcomes in the 5 included randomized controlled trials reported on a per patient basis
| Study | Initiation of P in relation to OR (Day 0) | Patients (n) | Implantation rate | P value | Biochemical pregnancy | P value | Clinical pregnancy | P value | Live Birth | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Sohn et al. 1999 | 12 hrs before | 158 (cycles) | NR | NR | NR | NR | 12.9% | 0.011 | NR | NR |
| Day 0 | 156 (cycles) | NR | NR | 24.6% | NR | |||||
| Williams et al. 2001 | Day 3 | 59 | 27% | NS | NR | NR | 61.0% | 0.05 | NR | NR |
| Day 6 | 67 | 20% | NR | 44.8% | NR | |||||
| Fanchin et al. 2001 | Day 0 | 43 | 18% | NR | NR | NR | 42.0% | 0.26 | NR | NR |
| Day 2 | 41 | 12% | NR | 29.0% | NR | |||||
| Baruffi et al. 2003 | Day 0 | 51 | 12.6% | 0.98 | NR | NR | 27.4% | 1.00 | NR | NR |
| Day 2 | 52 | 13.4% | NR | 28.8% | NR | |||||
| Mochtar et al. 2006 | 36 hrs before | 130 | NR | NR | 25.4% | NR | 23.1% | 0.56 | 20.0% | NR |
| Day 0 | 128 | NR | 30.5% | 28.1% | 21.1% | |||||
| Day 3 | 127 | NR | 32.3% | 29.1% | 20.5% |
Assessment of Bias Risk
None of the trials documented allocation concealment, blinding of the physicians or patients, or blinding of outcomes data (Supplemental Table 1). Reporting of the randomization process was only clearly reported in two of the studies. Only Mochtar et al. adequately reported on the flow of patients through the study, utilized and intent to treat analysis, and was at low risk of incomplete data reporting (22). The remaining studies either partially or completely failed to adequately report patient flow and these studies analyzed their data on a per protocol basis or unclear basis. There was no pharmaceutical support disclosed in any of the trials. Funnel plots were not utilized due to the low number of studies assessing the same comparisons.
None of the studies demonstrated baseline differences between the two randomized groups in regards to age, fertility diagnoses, or duration of infertility. One study reported a statistical difference in the number of supernumerary embryos for freezing between the two randomized groups (Day 3 progesterone group: 1.3 embryos for freezing versus Day 6 progesterone group: 2.7 embryos for freezing, P=0.01) (20). Supernumerary embryos have been associated with an increased likelihood of pregnancy (24).
Comparison of Live Birth
Only Mochtar et al. reported live birth rates (22). They found no difference in live birth between patients randomized to receive progesterone 36 hours prior to oocyte retrieval (20.0%), the evening of oocyte retrieval (21.1%), or day 3 after oocyte retrieval (20.5%). However, this study was not powered to detect a difference in live birth rates. There was insufficient reporting of live birth in other trials to use this as the primary outcome.
Comparison of Clinical Pregnancy
All five studies reported clinical pregnancy as a primary outcome. The definition of clinical pregnancy was heterogeneous between the studies, ranging from undefined to defined as either a gestational sac in the uterus or to a fetus in the uterus with cardiac activity. Clinical pregnancy rates ranged from 12.9% to 61.0% in the studies (Figure 1). Only two studies reported statistically significant differences in clinical pregnancy between the groups. Sohn et al. reported a lower clinical pregnancy rate in patients starting progesterone 12 hours prior to oocyte retrieval compared to those starting progesterone the evening of oocyte retrieval (12 hours prior to retrieval: 12.9% versus evening of retrieval: 24.6%, P=0.01)(19). Williams et al. reported a lower pregnancy rate in fresh autologous patients starting progesterone on day 6 after oocyte retrieval compared to day 3 after oocyte retrieval (6 days after retrieval: 44.8% versus 3 days after retrieval: 61.0%, P=0.05) (20). There were three studies that compared clinical pregnancy in patients starting progesterone the evening of oocyte retrieval versus two days after and 3 days after oocyte retrieval (8, 22,23). None of these studies reported significant differences in pregnancy rates between the groups.
Figure 1.
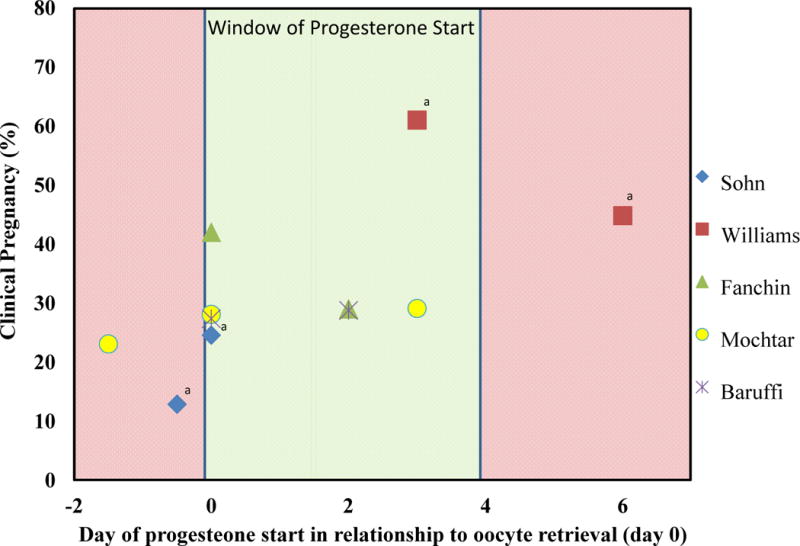
Window of progesterone initiation. Graphic representation of clinical pregnancy rates on the y axis and day of progesterone initiation on the x axis. Markers represent the 6 different RCTs results. The red shaded area represents time points with potential lowered pregnancy rates if progesterone is started. The green shaded area represents the window of progesterone start times based on the available randomized controlled data.
aResults reported as statistically significant in the primary studies.
Comparison of Miscarriage
None of the included studies reported miscarriage as an outcome.
Subgroup analysis
An a priori subgroup analysis was planned to compared IM and vaginal routes of progesterone. It has been proposed that vaginal progesterone results in more rapid uterine uptake of the hormone and may advance the endometrium more rapidly than the IM route (10). Thus the timing of progesterone initiation may be affected by the route of progesterone administration. However, only one study evaluated IM progesterone and adequate comparisons could not be made.
Discussion
The results of this systematic review suggest that the timing of luteal progesterone support initiation can affect the likelihood of pregnancy. Studies performed on luteal support initiation before oocyte retrieval versus day of oocyte retrieval suggest a potential decreased likelihood of pregnancy if progesterone was initiated before oocyte retrieval (Figure 1). When progesterone was initiated on the evening of oocyte retrieval versus days 1–3 after oocyte retrieval, studies found no difference in clinical pregnancy. One study investigated progesterone initiation on day 3 or 6 after oocyte retrieval and reported a decreased likelihood of pregnancy on day 6 initiation. These results suggest a window between the evening of oocyte retrieval and day 3 after retrieval as the ideal time for initiation of progesterone.
Multiple factors affecting progesterone timing and serum levels during the luteal phase in ART cycles have been proposed. These include: endometrial advancement from premature progesterone release, disruption of granulosa cells during oocyte retrieval, pituitary down-regulation or blockade of GnRH receptors, hypothalamic suppression of GnRH, method of oocyte maturation induction, and differing routes of progesterone administration.
Over the past 5 years, data from several large retrospective studies have demonstrated that even subtle early rises in progesterone effect pregnancy rates. Bosch et al. and Xu et al. combined to examine over 14,000 cycles; both were able to show that progesterone levels over 1.5ng/ml on the day of hCG trigger decreased pregnancy rates (9, 27). Numerous additional studies have also supported this work (28–32). Micro array studies evaluating expression of genes and RNA involved in endometrial receptivity and implantation have demonstrated dysregulation of genes and proteins when exposed to premature elevation in progesterone (33– 35). While it is clear that subtle premature rises in progesterone affect pregnancy rates by advancing the endometrium, it is unclear if modulating progesterone initiation can mitigate this risk.
Disruption of the granulosa cell mass during oocyte retrieval has been posited as an explanation for the shortened luteal phase in ART cycles. However, data have shown that endogenous progesterone levels are much higher after oocyte aspiration in ART cycles when compared to natural cycles. Natural mid luteal progesterone levels are typically around 15ng/ml (36,37). Mid-luteal progesterone levels following hCG trigger and follicle aspiration are much higher, ranging from 30–80 ng/ml (38,39). Furthermore, data from Haas et al. have demonstrated good luteal progesterone levels with hCG support alone. This would suggest that the corpus luteum functions well in response to LH receptor activation (26). These data strongly suggest that oocyte retrieval does not affect endogenous progesterone or timing of supplementation.
Long GnRH agonist protocols to suppress premature ovulation are commonly used in ART cycles. Constant GnRH agonist exposure results in down-regulating the pituitary GnRH receptor and de-coupling post receptor signaling mechanisms (40–42). Long GnRH agonist protocols still have suppressed LH levels 9 days after the agonist was discontinued (43,44). This decreased LH pulsatility does not allow for adequate progesterone to be produced by the corpus luteum. However, hCG for final oocyte maturation continues to stimulate the corpus luteum after retrieval. This stimulation ends around day 5 or 6 and this may explain the outcomes in the Williams et al. study. Patients that started luteal support on day 6 had lower pregnancy rates. These individual factors play a role in the endogenous levels of progesterone and suggest a window for when luteal support is needed (Figure 1).
Several routes of progesterone have been studied for luteal support including oral, vaginal, and intramuscular (IM). Oral route provides significantly less bioavailability due to the liver first pass effect and have been shown to be inferior to IM progesterone (45,46). This has left significant debate over the comparison between vaginal and IM routes. Cicinelli et al. demonstrated higher serum progesterone levels with IM administration versus vaginal administration (29.4 versus 4.8 ng/ml), however IM progesterone had lower levels of endometrial progesterone (0.43 versus 1.05 ng/ml) (47). The debate over the route of progesterone administration has led to a related discussion of progesterone timing.
Propst and coworkers randomized women undergoing IVF to IM or vaginal progesterone on the day after oocyte retrieval. The vaginal arm had a decreased likelihood of clinical pregnancy and live birth (48). The same group, in a subsequent randomized controlled trial, delayed the initiation of vaginal progesterone until 48 hours after oocyte retrieval but kept IM progesterone at 24 hours post retrieval. Live births were similar in each group (10). Taking all this data together, this would suggest a first pass effect with the uterus; resulting in quicker peak levels in the endometrium and more rapidly advancing the implantation window. This is further supported by a prospective nonrandomized trial. When vaginal progesterone was initiated 2 days after oocyte retrieval, higher pregnancy rates were obtained with vaginal as compared to IM progesterone (49).
Summarizing the data on these various factors that affect endogenous and exogenous progesterone levels reveals numerous variables modulating progesterone during the window of implantation in ART cycle, the most important of which are the method of oocyte maturation triggering and the timing of progesterone supplementation. In the natural menstrual cycle, progesterone levels rise slightly before ovulation, continue to rise over the next several days, and peak at 7 days after ovulation (50). In the unsupplemented ART cycle with hCG triggering, progesterone levels initially rise from the luteal effects of hCG, then fall to very low levels, only to once again rise if hCG from the pregnancy rescues the corpus luteum (3) (Figure 2). In rLH or GnRH agonist trigger protocols, the initial fall of progesterone from the corpus luteum occurs even more rapidly (3). This creates a window during which exogenous progesterone must be administered to keep progesterone over a threshold of 80–100 nmol/L (25), bridging progesterone production of the corpus luteum between the triggering stimulation and the hCG stimulation of the pregnancy (Figure 2).
Figure 2.
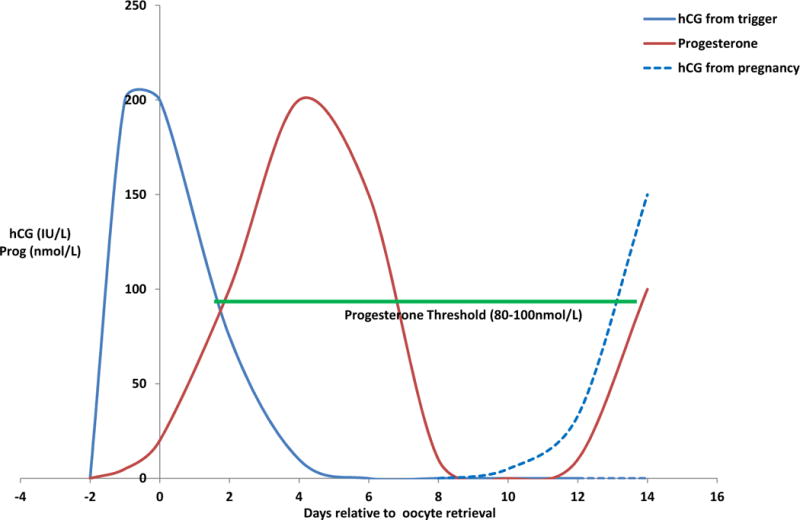
Summary of hCG and progesterone levels from the time of hCG trigger until early pregnancy during ART. After hCG trigger (day -2), hCG levels rapidly rise to around 200 IU/L at the time of oocyte retrieval (day 0) and are then cleared by around day 5 after retrieval (Beckers et al., 2003). Progesterone levels follow more slowly, as granulosa cells become luteinized, and peak around day 5 after retrieval and rapidly drop there after (Beckers et al., 2000). This creates several days during which endogenous progesterone levels lack hCG stimulation and require supplementation to remain over the threshold of 80–100 nmol/L to maintain pregnancy (Andersen and Andersen, 2014).
It is important to point out that the majority of these studies in this present review involved long GnRH agonist protocols with hCG triggering in fresh autologous ART cycles. This impacts the luteal phase in several distinct ways which were reviewed. The hCG trigger results in a higher levels and a longer duration of endogenous progesterone release as compared to a GnRH agonist or rLH trigger (3, 51). Fresh autologous patients will have initial endogenous progesterone production following hCG trigger whereas donor recipient patients utilizing an artificial cycle will not have this endogenous production. For these reasons, the results of this meta-analysis should be interpreted primarily in autologous fresh IVF with long GnRH agonist protocols and hCG triggering.
There are limitations on the data reviewed in this paper. First there were only 5 studies that met inclusion criteria, limiting the volume of evidence available for analysis. Secondly, there was significant clinical heterogeneity throughout all five studies with differences in progesterone preparation, dose, and timing between the studies, making metal-analysis not possible. Thus, the results of the studies should be interpreted with caution. While meta-analysis data synthesis can utilize random effects models to account for some heterogeneity between studies, the studies in this review varied so greatly in their clinical protocols that it was felt inappropriate to attempt to synthesize the outcomes statistically. For example, the 5 trials studied 6 different initiation times of progesterone supplementation, making it inappropriate to attempt to statistically combine the effects of progesterone initiation into meaningful data. The majority of the randomized trials did not adequately report on allocation, concealment, and blinding which could introduce potential bias. Open-label trials may be subject to potential bias due to physician and patient awareness of treatment allocation (52) and there is meta-epidemiologic evidence to suggest that unclear allocation concealment or lack or blinding may cause overoptimistic estimates of treatment effects (63). For these reasons, the heterogeneity and limited data indicate that the results of this systematic review should be interpreted with caution.
In conclusion, this data from this systematic review suggests that starting progesterone the day before oocyte retrieval or waiting until day 6 post retrieval may result in lower pregnancy rates. There appears to be a window for progesterone start time between the evening after oocyte retrieval and day 3 after oocyte retrieval. While some studies have suggested a potential benefit in delaying vaginal progesterone start time to 2 days after oocyte retrieval, this review could not find adequate randomized controlled trials to adequately assess this. It remains unclear if pregnancy rates can be improved by delaying the progesterone initiation until the end of this progesterone window to avoid endometrial advancement. Further randomized clinical trials are needed to better define progesterone start time for luteal support, particularly for vaginal progesterone which may more rapidly advance the endometrium.
Supplementary Material
Acknowledgments
MJH would like to thank John Bauer PhD. for his many helpful discussions on this topic.
Financial Support: This work was supported, in part, by the Program in Reproductive and Adult Endocrinology, NICHD, NIH, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Disclaimer: The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of Health and Human Services, Department of Defense, or the U. S. Government.
References
- 1.Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358:155–65. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smitz J, Devroey P, Van Steirteghem AC. Endocrinology in luteal phase and implantation. Br Med Bull. 1990;46:709–19. doi: 10.1093/oxfordjournals.bmb.a072426. [DOI] [PubMed] [Google Scholar]
- 3.Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88:4186–92. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- 4.Hill MJ, Whitcomb BW, Lewis TD, Wu M, Terry N, DeCherney AH, et al. Progesterone luteal support after ovulation induction and intrauterine insemination: a systematic review and meta-analysis. Fertil Steril. 2013;100:1373–80. doi: 10.1016/j.fertnstert.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2011;(10):CD009154. doi: 10.1002/14651858.CD009154.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Soliman S, Daya S, Collins J, Hughes EG. The role of luteal phase support in infertility treatment: a meta-analysis of randomized trials. Fertil Steril. 1994;61:1068–76. doi: 10.1016/s0015-0282(16)56758-2. [DOI] [PubMed] [Google Scholar]
- 7.Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, et al. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH-agonists and HMG in a desensitization or flare-up protocol. Hum Reprod. 1992;7:1225–9. doi: 10.1093/oxfordjournals.humrep.a137831. [DOI] [PubMed] [Google Scholar]
- 8.Fanchin R, Righini C, de Ziegler D, Olivennes F, Ledée N, Frydman R. Effects of vaginal progesterone administration on uterine contractility at the time of embryo transfer. Fertil Steril. 2001;75:1136–40. doi: 10.1016/s0015-0282(01)01787-3. [DOI] [PubMed] [Google Scholar]
- 9.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 10.Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization-embryo transfer cycles: a prospective randomized study. Fertil Steril. 2010;94:2596–9. doi: 10.1016/j.fertnstert.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Musa A, Usta I, Nassar A, Hajami F, Hannoun A. Effect of 17alpha-hydroxyprogesterone caproate before embryo transfer on the outcome of in vitro fertilization and embryo transfer: a randomized trial. Fertil Steril. 2008;89:1098–102. doi: 10.1016/j.fertnstert.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Doody KJ, Schnell VL, Foulk RA, Miller CE, Kolb BA, Blake EJ, et al. Endometrin for luteal phase support in a randomized, controlled, open-label, prospective in-vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertil Steril. 2009;91:1012–7. doi: 10.1016/j.fertnstert.2008.01.069. [DOI] [PubMed] [Google Scholar]
- 13.Ghanem ME, Sadek EE, Elboghdady LA, Helal AS, Gamal A, Eldiasty A, et al. The effect of luteal phase support protocol on cycle outcome and luteal phase hormone profile in long agonist protocol intracytoplasmic sperm injection cycles: a randomized clinical trial. Fertil Steril. 2009;92:486–93. doi: 10.1016/j.fertnstert.2008.07.1717. [DOI] [PubMed] [Google Scholar]
- 14.Kleinstein J, Group LPS. Efficacy and tolerability of vaginal progesterone capsules (Utrogest 200) compared with progesterone gel (Crinone 8%) for luteal phase support during assisted reproduction. Fertil Steril. 2005;83:1641–9. doi: 10.1016/j.fertnstert.2004.11.073. [DOI] [PubMed] [Google Scholar]
- 15.Mui Lam P, Chun Cheung M, Ping Cheung L, Ingrid Lok H, John Haines C. Effects of early luteal-phase vaginal progesterone supplementation on the outcome of in vitro fertilization and embryo transfer. Gynecol Endocrinol. 2008;24:674–80. doi: 10.1080/09513590802360751. [DOI] [PubMed] [Google Scholar]
- 16.Miller CE, Zbella E, Webster BW, Doody KJ, Bush MR, Collins MG. Clinical comparison of ovarian stimulation and luteal support agents in patients undergoing GnRH antagonist IVF cycles. J Reprod Med. 2013;58:153–60. [PubMed] [Google Scholar]
- 17.Stadtmauer L, Silverberg KM, Ginsburg ES, Weiss H, Howard B. Progesterone vaginal ring versus vaginal gel for luteal support with in vitro fertilization: a randomized comparative study. Fertil Steril. 2013;99:1543–9. doi: 10.1016/j.fertnstert.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 18.Wang LJ, Huang FJ, Kung FT, Lin PY, Chang SY, Lan KC. Comparison of the efficacy of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support in blastocyst stage embryo transfers. Taiwan J Obstet Gynecol. 2009;48:375–9. doi: 10.1016/S1028-4559(09)60326-0. [DOI] [PubMed] [Google Scholar]
- 19.Sohn SH, Penzias AS, Emmi AM, Dubey AK, Layman LC, Reindollar RH, et al. Administration of progesterone before oocyte retrieval negatively affects the implantation rate. Fertil Steril. 1999;71:11–4. doi: 10.1016/s0015-0282(98)00404-x. [DOI] [PubMed] [Google Scholar]
- 20.Williams SC, Oehninger S, Gibbons WE, Van Cleave WC, Muasher SJ. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: a randomized, prospective study. Fertil Steril. 2001;76:1140–3. doi: 10.1016/s0015-0282(01)02914-4. [DOI] [PubMed] [Google Scholar]
- 21.Baruffi R, Mauri AL, Petersen CG, Felipe V, Franco JG. Effects of vaginal progesterone administration starting on the day of oocyte retrieval on pregnancy rates. J Assist Reprod Genet. 2003;20:517–20. doi: 10.1023/B:JARG.0000013653.54830.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochtar MH, Van Wely M, Van der Veen F. Timing luteal phase support in GnRH agonist down-regulated IVF/embryo transfer cycles. Hum Reprod. 2006;21:905–8. doi: 10.1093/humrep/dei437. [DOI] [PubMed] [Google Scholar]
- 23.Escribá MJ, Bellver J, Bosch E, Sánchez M, Pellicer A, Remohí J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: a randomized study. Fertil Steril. 2006;86:92–7. doi: 10.1016/j.fertnstert.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 24.Hill MJ, Richter KS, Heitmann RJ, Lewis TD, DeCherney AH, Graham JR, et al. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil Steril. 2013;99:1631–6. doi: 10.1016/j.fertnstert.2013.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yding Andersen C, Vilbour Andersen K. Improving the luteal phase after ovarian stimulation: reviewing new options. Reprod Biomed Online. 2014;28:552–9. doi: 10.1016/j.rbmo.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Haas J, Kedem A, Machtinger R, Dar S, Hourvitz A, Yerushalmi G, et al. HCG (1500IU) administration on day 3 after oocytes retrieval, following GnRH-agonist trigger for final follicular maturation, results in high sufficient mid luteal progesterone levels – a proof of concept. J Ovarian Res. 2014;7:35. doi: 10.1186/1757-2215-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–7.e1-4. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Kiliçdag EB, Haydardedeoglu B, Cok T, Hacivelioglu SO, Bagis T. Premature progesterone elevation impairs implantation and live birth rates in GnRH-agonist IVF/ICSI cycles. Arch Gynecol Obstet. 2010;281:747–52. doi: 10.1007/s00404-009-1248-0. [DOI] [PubMed] [Google Scholar]
- 29.Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol. 2012;13:464–70. doi: 10.2174/138920112799361927. [DOI] [PubMed] [Google Scholar]
- 30.Lahoud R, Kwik M, Ryan J, Al-Jefout M, Foley J, Illingworth P. Elevated progesterone in GnRH agonist down regulated in vitro fertilisation (IVFICSI) cycles reduces live birth rates but not embryo quality. Arch Gynecol Obstet. 2012;285:535–40. doi: 10.1007/s00404-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 31.Ochsenkühn R, Arzberger A, von Schönfeldt V, Gallwas J, Rogenhofer N, Crispin A, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril. 2012;98:347–54. doi: 10.1016/j.fertnstert.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Qiao J, Wang L, Zhen X, Lu Y. Serum progesterone concentration on day of HCG administration and IVF outcome. Reprod Biomed Online. 2008;16:627–31. doi: 10.1016/s1472-6483(10)60475-0. [DOI] [PubMed] [Google Scholar]
- 33.Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813–25. doi: 10.1093/humrep/der126. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Qiao J, Wang L, Li L, Zhen X, Liu P, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29. doi: 10.1186/1477-7827-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schuit F, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online. 2011;22:263–71. doi: 10.1016/j.rbmo.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Deichert U, Hackelöer BJ, Daume E. The sonographic and endocrinologic evaluation of the endometrium in the luteal phase. Hum Reprod. 1986;1:219–22. doi: 10.1093/oxfordjournals.humrep.a136388. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann F. Untersuchungen zur menschlichen Corpus luteum Funktion. In: Schirren C, Sermm K, editors. Fortschritte der Fertil. Grosse-Verlag; Berlin: pp. 30–34. [Google Scholar]
- 38.Humaidan P, Ejdrup Bredkjaer H, Westergaard LG, Yding Andersen C. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril. 2010;93:847–54. doi: 10.1016/j.fertnstert.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod. 2013;28:2511–21. doi: 10.1093/humrep/det249. [DOI] [PubMed] [Google Scholar]
- 40.Conn PM, Staley D, Harris C, Andrews WV, Gorospe WC, McArdle CA, et al. Mechanism of action of gonadotropin releasing hormone. Annu Rev Physiol. 1986;48:495–513. doi: 10.1146/annurev.ph.48.030186.002431. [DOI] [PubMed] [Google Scholar]
- 41.Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 42.Gordon K, Stegmann BJ. Clinical relevance for the fact that GnRH antagonists do not down-regulate the GnRH receptor. Hum Reprod. 2013;28:1144. doi: 10.1093/humrep/des469. [DOI] [PubMed] [Google Scholar]
- 43.Broekmans FJ, Hompes PG, Lambalk CB, Schoute E, Broeders A, Schoemaker J. Short term pituitary desensitization: effects of different doses of the gonadotrophin-releasing hormone agonist triptorelin. Hum Reprod. 1996;11:55–60. doi: 10.1093/oxfordjournals.humrep.a019034. [DOI] [PubMed] [Google Scholar]
- 44.Porcu E, Dal Prato L, Seracchioli R, Fabbri R, Longhi M, Flamigni C. Comparison between depot and standard release triptoreline in in vitro fertilization: pituitary sensitivity, luteal function, pregnancy outcome, and perinatal results. Fertil Steril. 1994;62:126–32. doi: 10.1016/s0015-0282(16)56827-7. [DOI] [PubMed] [Google Scholar]
- 45.Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update. 2000;6:139–48. doi: 10.1093/humupd/6.2.139. [DOI] [PubMed] [Google Scholar]
- 46.Hubayter ZR, Muasher SJ. Luteal supplementation in in vitro fertilization: more questions than answers. Fertil Steril. 2008;89:749–58. doi: 10.1016/j.fertnstert.2008.02.095. [DOI] [PubMed] [Google Scholar]
- 47.Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95:403–6. doi: 10.1016/s0029-7844(99)00542-6. [DOI] [PubMed] [Google Scholar]
- 48.Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization-embryo transfer cycles. Fertil Steril. 2001;76:1144–9. doi: 10.1016/s0015-0282(01)02872-2. [DOI] [PubMed] [Google Scholar]
- 49.Silverberg KM, Vaughn TC, Hansard LJ, Burger NZ, Minter T. Vaginal (Crinone 8%) gel vs. intramuscular progesterone in oil for luteal phase support in in vitro fertilization: a large prospective trial. Fertil Steril. 2012;97:344–8. doi: 10.1016/j.fertnstert.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Moghissi KS, Syner FN, Evans TN. A composite picture of the menstrual cycle. Am J Obstet Gynecol. 1972;114:405–18. doi: 10.1016/0002-9378(72)90617-5. [DOI] [PubMed] [Google Scholar]
- 51.Fatemi HM, Polyzos NP, van Vaerenbergh I, Bourgain C, Blockeel C, Alsbjerg B, et al. Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle-stimulating hormone-gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2013;100:742–7. doi: 10.1016/j.fertnstert.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 52.Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril. 2012;97:1108–14.e1. doi: 10.1016/j.fertnstert.2012.01.130. [DOI] [PubMed] [Google Scholar]
- 53.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–5. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu XR, Mu HQ, Shi Q, Xiao XQ, Qi HB. The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta-analysis. Reprod Biol Endocrinol. 2012;10:107. doi: 10.1186/1477-7827-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


