Abstract
The intracarotid amobarbital or Wada procedure is a component of the presurgical evaluation for refractory epilepsy, during which monitoring the onset and offset of transient anesthetic effects is critical. In this study, we characterized changes of 8 QEEG measures during 26 Wada tests, which included alpha, beta, theta, and delta powers, alpha/delta power ratio (ADR), beta/delta power ratio (BDR), median amplitude-integrated EEG (aEEG) and 90% spectral edge frequency (SEF90), and correlated them with contralateral hemiplegia. We found that on the side of injection, delta and theta powers, ADR, BDR, and SEF90 peaked within 1 min after injection of 70-150 mg amobarbital or 4-7 mg methohexital. When contralateral arm strength returned to 3/5, delta power and aEEG decayed on average 24% and 19%, respectively, for amobarbital, similar to that of methohexital (27% and 18%). Since delta power resolution most closely mirrored that of the hemiplegia and aEEG had the highest signal/noise ratio (SNR), these QEEG values appear to be the best measures for decay of anesthetic effects. Increase in alpha power persisted longest, and therefore may be the best measure of late residual anesthetic effects.
Keywords: intracarotid amobarbital procedure (IAP), methohexital, compressed spectral array (CSA), hemiplegia, human
Introduction
The Wada test (Wada and Rasmussen, 1960), also known as intracarotid amobarbital procedure (IAP), involves delivery of anesthetics, usually sodium amobarbital (brand name Amytal), or other drugs (Buchtel et al., 2002; Magee et al., 2012; Patel et al., 2011) including methohexital (brand name Brevital), through a percutaneously inserted catheter placed into the iliac artery and threaded up into the internal carotid arteries, typically by an interventional neuroradiologist (Acharya and Dinner, 1997; Blume et al., 1973). While the injected hemisphere is anesthetized, hopefully with minimal effects on the contralateral side, language and memory tests are conducted to predict the possible risk of amnesia and language deficit following temporal lobe resection. It is critical to determine the initiation, termination and depth of anesthetic effects in the injected hemisphere (Peters et al., 2012). The most common monitoring technique is a series of contralateral arm strength exams (Wada and Rasmussen, 1960), which defines the start and end time of a valid Wada test based on the times at which contralateral hemiplegia occurs and contralateral hemiparesis begins to recover (Buchtel et al., 2002; Kim et al., 2007; Mikati et al., 2009; Takayama et al., 2004). However this technique has limitations. Arm strength must be tested intermittently with patient cooperation (Blume et al., 1973), which distracts patients from memory and language tests. There is no universal standard regarding how often arm strength should be tested. Arm strength scoring is subjective and does not provide continuous data. Visual interpretation of online EEG slow wave activity is another common technique with the same purpose (Blume et al., 1973; Rausch et al., 1984) that provides continuous data (Peters et al., 2012). However, interpretation of EEG slowing has low inter-observer agreement, with a reported kappa score of 0.1 (Abend et al., 2011). Thus, this technique is also subject to inaccuracy. Delta-to-theta transition time was an EEG landmark frequently reported in our center during Wada tests, but its variability and relationship to the end time of a valid Wada test has not been reported.
The limitations of these techniques have existed for over half a century since the introduction of the Wada procedure and may have compromised their accuracy (Acharya and Dinner, 1997). While its popularity has declined over the last decade due to invasiveness, inaccuracy and emergence of non-invasive alternatives, the Wada test remains a useful, and commonly used presurgical evaluation tool (Baxendale, 2009; Roberts, 2011; Sharan et al., 2011). QEEG analysis eliminates subjectivity of visual EEG reviews, potentially revealing subtle, gradual and long-term changes (Akman et al., 2011; Stuart et al., 2010) that may improve accuracy of Wada results. Thus far, changes of QEEG measures have not been characterized to define start and end time of valid language and memory tests or to indicate full recovery of anesthetic effects. We postulate that QEEG measures are sensitive and stable indicators of cerebral anesthetization, and are useful in defining the start and end of valid memory and language, as well as the time of total recovery from anesthetization. To test this hypothesis, changes of 8 QEEG measures evoked by injection of either amobarbital or methohexital during Wada procedures were retrospectively characterized and then correlated with contralateral arm strength. These QEEG measures included those previously reported, including delta, theta, alpha and beta frequency powers (Bouwer et al., 1993; Douw et al., 2009), and those related to EEG slowing, such as the ratios of alpha to delta and beta to theta powers (Morita et al., 2011), as well as those useful in monitoring anesthetic effects, such as SEF90 and aEEG (McKeever et al., 2012). Trends in QEEG changes evoked by both drugs were compared and effects of drug cross-filling were investigated.
Methods
Subjects and Wada tests
Following IRB approval, Wada EEGs and reports between January 2006 and July 2011 at the Columbia University campus of New York Presbyterian Hospital were reviewed. Among 57 available Wada EEGs, 26 were selected for further digital analysis; the remaining records were excluded due to prominent artifacts in more than half of the record between 5 min before and 10 min after injections evaluated by visual examination (n=24), multiple doses during each injection (n=4, all injected with amobarbital) since multiple peak QEEG changes and prolonged time course from multiple doses at various intervals not suitable to be combined in analysis of QEEG change parameters for single does injections, or unsuccessful injections without adequate contralateral hemiplegia (n=3). Among the 26 studies selected, patients’ age ranged 19-59 years old (34±13, mean±SD); 13 patients were injected with 75-150 mg (111.5±19.0) amobarbital; 13 patients were injected with 4-7 mg (5.2±0.8) methohexital. Data from 2 injections (one on each side) were analyzed for each patient, with inter-injection intervals ranging 16.9-32.0 min (24.6±3.6). In 4 methohexital cases, a repeat injection was performed to complete language and memory tests. In these cases, the first injection was selected for analysis if meeting 2 criteria: 1) inducing complete hemiplegia and 2) with acceptable EEG quality. If the second injection met these criteria but the first one did not, the second injection was selected. Contralateral and repeated injections were performed at least 5 min after the EEG returned to baseline. Cross-filling during injection was graded by radiologists according to angiography on a 0-3 scale, 0 representing no cross-filling and 3 representing maximal cross-filling.(Castillo et al., 2000) We compared cases with significant cross-filling (2.5 or 3 in the ACA region) and none (0 for all regions) to study the effects of cross-filling on QEEG. After each Wada injection, neurologists evaluated contralateral hemiplegia intermittently by scoring arm strength on a scale of 0/5 to 5/5, with 0/5 representing complete loss of arm strength and 5/5 representing full arm strength.
EEG acquisition
Ag/AgCl EEG electrodes were placed according to international 10-20 system, affixed to the scalp with Ten20 conductive paste (Weaver and Company, Aurora, CO), and further secured by wrapping the entire head with gauze. A ground electrode was also placed between the left or right central and parietal electrodes. Impedances were kept below 5kΩ. EEGs were recorded at a sampling rate of 256/sec using Xltek hardware (Model EMU40 amplifiers), and reviewed with Neuroworks software (version 6.1.0 build 892; Natus Medical Inc., San Carlos, CA).
QEEG analysis
All QEEG measures were calculated using Magic Marker functions in InsightII Software (Version 11, Persyst Development Cooperation, Prescott, AZ). EEG power values were calculated from bipolar montages in the left (F3-C3 C3-P3 P3-O1 F7-T7 T7-P7 P7-O1) and right (F4-C4 C4-P4 P4-O2 F8-T8 T8-P8 P8-O2) hemispheres via fast Fourier transformation (Scheuer and Wilson, 2004) using the following parameters: 1 sec/window, 4 windows/epoch without overlap. Resulting power values had frequency resolution of 1 Hz; each power value was calculated from 4 sec of recording. Maximal frequency for power analysis was 64 Hz (at least 4 data points needed to calculate frequency). Power values were then combined into 4 frequency bands: delta power <4 Hz, theta power 4-7 Hz, alpha power 8-13 Hz, and beta power 14-30 Hz, and were then logarithmically transformed in order to approximate normal data distribution. The aEEG refers to median aEEG values of 4 sec recordings, and was calculated with frequency range of 2-20 Hz, higher pass filter 70 Hz, notch filter 60 Hz and time constant of 0.16 sec. SEF90, a QEEG value useful in assessing the relative increase in lower frequency activity (typically delta and theta) and/or decrease in higher frequency activity (typically alpha and beta) during anesthesia (McKeever et al., 2012), is the frequency below which 90% of 1 Hz to 30 Hz total EEG power was contained. All QEEG values were normalized using the median value 0-5 min before an injection as control. SNR was calculated by dividing the maximal change after each injection by the standard deviation of control values.
Statistical analysis
Statistical tests between QEEG measures, including 1-way or 2-way ANOVA and Bonferroni post-test analysis (for normally distributed values), Friedman test followed by Dunn’s Multiple Comparison Test (for non-normally distributed values), and KS normality test (normal distribution was defined as p>0.1), were performed using GraphPad Prism 6 software (La Jolla, CA). Significant changes were defined as p<0.05. Logarithmic transformation and power histogram were performed using Matlab software (Version R2001b, MathWorks Inc., Natick, MA). Time course parameters, including peak changes from baseline (0-5 min before injections), time to peak changes and 10-90% decay times (time for effects to decrease from 90% to 10% of the peak value) were analyzed using Clampfit 10 software (Molecular Devices, LLC. Sunnyvale, CA). Nonlinear curve fit for averaged QEEG time course was performed with Origin Lab 9.0 software (OriginLab Corporation, Northampton, MA) using a bi-Gaussian model (built-in function of the software). The curve fitting results with adjusted R-Square >0.9 and reduced Chi-Sqr <0.05 (measures for goodness of fit, with perfect fit defined as R-Square approaching 1 and Chi-Sqr approaching 0) were considered significant and were plotted in Figure 3.
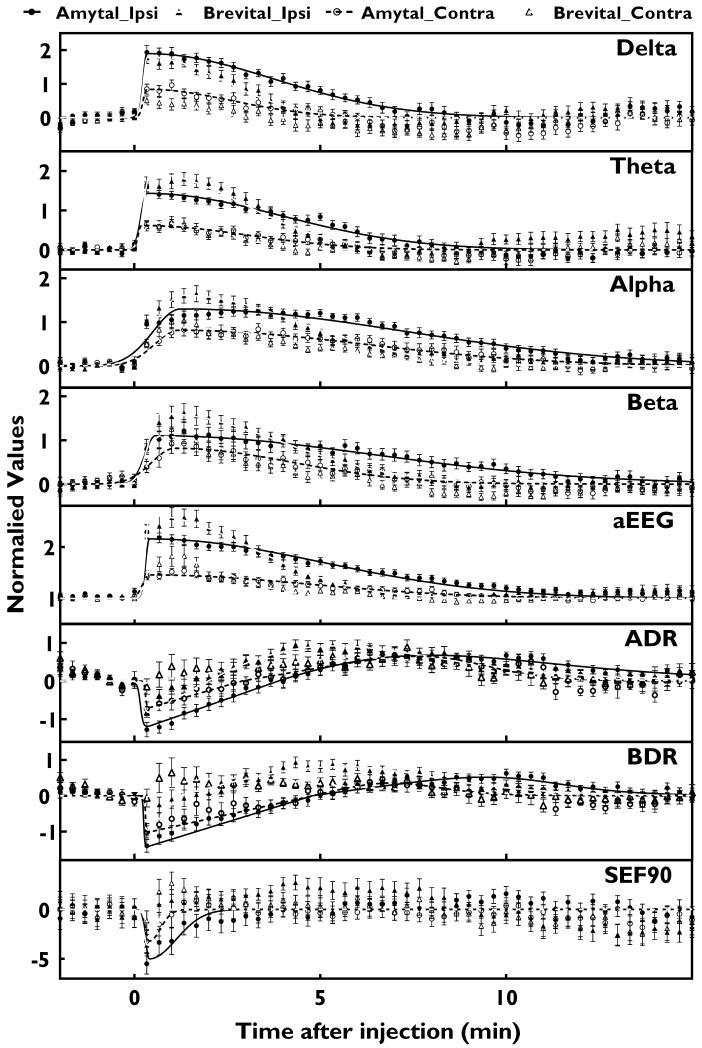
Figure 3.
Changes in QEEG measures on ipsilateral (closed data points) and contralateral (open data points) hemispheres of the brain evoked by amobarbital (circle) and methohexital (triangle) injections. Data points and error bars represent average and s.e.m. of power values in 20 sec segments of EEG calculated from 26 injections from 13 patients. For delta, theta, alpha, beta, ADR, and BDR, values are normalized logarithmic power values using average values within 1 min before each injection as controls. For aEEG, values were percentage of control period. SEF90 values represent frequency change in Hz from control. The solid lines (ipsilateral) and dashed lines (contralateral) are non-linearly fit curves after amobarbital (black) and methohexital (gray) injections.
Results
Visual assessment of Wada EEG changes
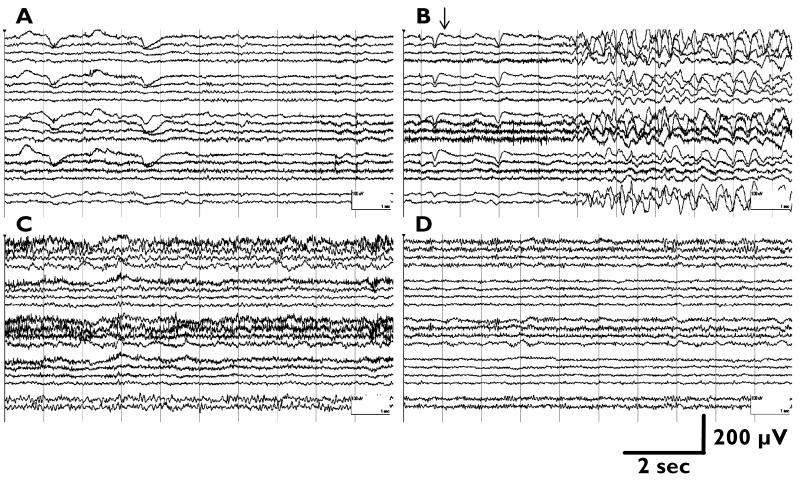
Per EEG reports, visual exams of ipsilateral Wada EEGs showed that injections of methohexital and amobarbital increased delta activity after 1 to 10 sec (mean±SD, 3.6±1.6 sec), with delta activity transitioning to theta activity at 4 to 232 sec (84±51 sec) and 20 to 266 sec (134±82 sec) respectively, and returned to baseline at 167 to 574 sec (283±107 sec) and 120 to 840 sec (507±211 sec). Figure 1 shows a typical example of EEG changes after injection.
Figure 1.
An example of Wada EEG in a surgical candidate with left mesial temporal epilepsy. A) Baseline recording, showing left temporal slowing. B) After a left-sided 100 mg amobarbital injection (arrow), increases in bilateral (left more prominent than right) delta and theta, as well as faster frequency (alpha and beta) activity were noted. C) At 2.3 min after injection, more prominent alpha and beta activity and less prominent delta and theta activity was noted than initially. D) At 7 min after injection, EEG returns to baseline with residual left-sided alpha/beta activity. Longitudinal bipolar montage was shown; EEG traces in each panel (start from the top) represent Fp1/F3, F3/C3, C3/P3, P3/O1, (gap), Fp2/F4, F4/C4, C4/P4, P4/O2, F7/P7, P7/O1, (gap), Fp1/F7, F7/T7, T7/P7, P7/O1, (gap), Fp2/F8, F8/T8, T8/P8, P8/O2, (gap), Fz/Cz, Cz/Pz.
Time course of QEEG changes evoked by Wada injections
Significant changes in all QEEG measures ipsilateral to side of injection started within the first minute post injection for both drugs (Fig. 2 & 3). QEEG parameters, including peak changes, time to peak changes, and 10-90% decay time were first calculated from QEEG trends of each injection, then averaged and summarized in Table 1.
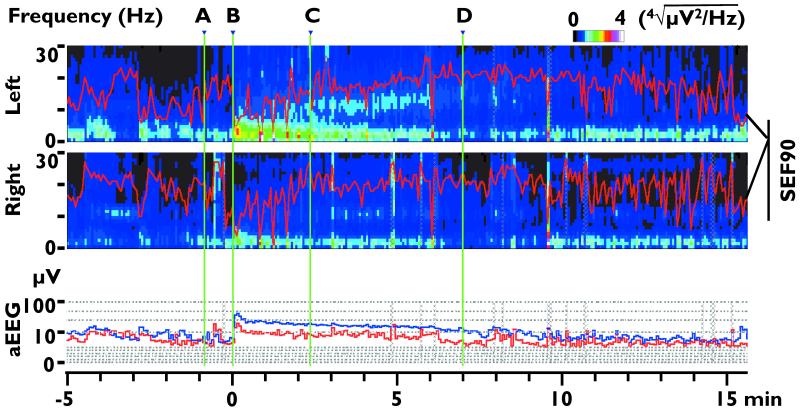
Figure 2.
Exemplary QEEG corresponding to Figure 1. The upper 2 panels show the left and right compressed spectrum (CSA), and 90% spectral edge frequency (SEF90, red lines overlapped on each spectrum). In order to better illustrate the spectrum in the higher frequency range, the power values (color-coded legend above the 1st panel) in the spectrum represent the 4th root of the actual power value. Lower panel shows aEEG median values of both sides (blue line: left; red line: right). The green vertical lines labeled A-D correspond to time points of panels in Figure 1. The vertical axis of aEEG is at 0-10 μV range on a linear scale and at 10-100 μV range on a logarithmic scale.
TABLE 1. Changes of ipsilateral QEEG values evoked by amobarbital or methohexital injections during Wada testing. (Values are average ± s.e.m, n=26).
| Drug | Delta | Theta | Alpha | Beta | aEEG | ADR | BDR | SEF90 | |
|---|---|---|---|---|---|---|---|---|---|
|
Peak
Change * |
Amo- barbital |
2.34 ±0.11 |
1.83 ±0.09 |
1.67 ±0.12 |
1.69 ±0.20 |
1.52 ±0.12 |
−1.66 ±0.14 |
−1.91 ±0.16 |
−6.08 ±0.88 |
|
| |||||||||
| Metho- hexital |
1.92 ±0.13 |
1.97 ±0.15 |
1.99 ±0.14 |
2.20 ±0.19 |
1.93 ±0.18 |
−1.19 ±0.12 |
−1.38 ±0.13 |
−4.83 ±0.85 |
|
|
| |||||||||
|
Time to
peak change (s) |
Amo- barbital |
29.69 ±4.48 |
44.23 ±12.39 |
164.00 ±18.69 |
97.54 ±11.27 |
46.92 ±12.07 |
22.15 ±3.01 |
30.62 ±6.17 |
55.54 ±13.70 |
|
| |||||||||
| Metho- hexital |
38.46 ±5.40 |
91.54 ±11.04 |
141.08 ±14.50 |
99.08 ±10.13 |
83.08 ±10.62 |
20.77 ±3.08 |
33.54 ±10.00 |
66.00 ±15.73 |
|
|
| |||||||||
|
10-90%
Decay Time (s) |
Amo- barbital |
253.22 ±18.14 |
312.46 ±23.63 |
318.16 ±20.77 |
329.31 ±19.68 |
402.98 ±27.80 |
87.32 ±9.71 |
94.28 ±14.49 |
113.44 ±23.58 |
|
| |||||||||
| Metho- hexital |
149.95 ±8.75 |
198.70 ±13.84 |
253.02 ±16.02 |
274.66 ±18.84 |
227.75 ±17.35 |
49.65 ±9.90 |
39.09 ±8.83 |
56.42 ±11.93 |
|
|
| |||||||||
| SNR | Amo- barbital |
5.63 ±0.46 |
6.02 ±0.47 |
5.80 ±0.55 |
5.76 ±0.97 |
11.02 ±1.13 |
4.49 ±0.57 |
4.95 ±0.57 |
2.50 ±0.45 |
|
| |||||||||
| Metho- hexital |
4.92 ±0.37 |
7.17 ±0.63 |
11.06 ±1.41 |
8.69 ±1.59 |
21.60 ±2.40 |
3.73 ±0.29 |
3.49 ±0.31 |
2.03 ±0.34 |
|
Peak Change: For Delta, Theta, Alpha, Beta, ADR, and BDR, values are ratio of logarithms of peak values versus control values. Negative peak changes represent troughs. For aEEG, values are the ratio of peak value divided by control values. For SEF90, values are decreases in Hz compared to controls. All control values were median values calculated from recordings 0-5 min before injections.
In order for QEEG time course to be better analyzed with minimal recording noise and inter-injection variations, median values of every 5 data points were extracted (sampling rate became one data point every 20 sec) and the resultant trends from all injections for each drug were averaged based on time points before or after injections (Fig. 3), then curve-fit with a bi-Gaussian model (Yu and Peng, 2010). When compared to non-averaged data in Table 1, this averaged data showed smaller peak changes and longer decay times in all amplitude-related EEG measures with similar time to peak changes (Table 2).
TABLE 2. Averaged changes of ipsilateral amplitude-related QEEG measures during Wada testing based on injection times of amobarbital or methohexital. (Values are comparable to Table 1, Note that s.e.m. was not applicable to time to peak changes and decay times since calculated from one trace, i.e. the averaged trace).
| Drug | Delta | Theta | Alpha | Beta | aEEG | |
|---|---|---|---|---|---|---|
| Peak Change | Amobarbital | 1.93± 0.20 | 1.60± 0.13 | 1.26± 0.11 | 1.21± 0.19 | 1.29± 0.14 |
|
| ||||||
| Methohexital | 1.77± 0.17 | 1.79± 0.15 | 1.65± 0.18 | 1.63± 0.21 | 1.57± 0.19 | |
|
| ||||||
|
Time to peak
change (s) |
Amobarbital | 20 | 20 | 200 | 80 | 20 |
|
| ||||||
| Methohexital | 20 | 80 | 100 | 80 | 60 | |
|
| ||||||
|
10-90% Decay
Time (s) |
Amobarbital | 344.7 | 365.2 | 611.4 | 597.0 | 470.9 |
|
| ||||||
| Methohexital | 206.6 | 251.1 | 324.3 | 325.5 | 279.9 | |
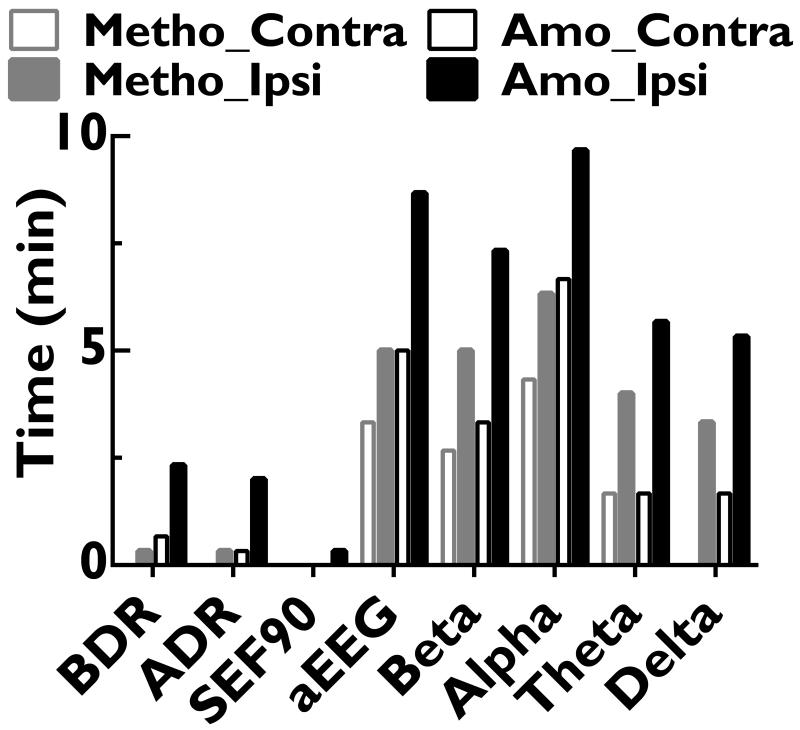
For the QEEG measures that are non-amplitude related, the logarithms of ADR and BDR first decreased within 1 min and then increased to above baseline, peaking at 5-10 min (antipeak) before falling back to baseline within 11 min (Fig. 3 and Table 1). SEF90 also immediately decreased, with peak changes at ~1 min, and shorter 10-90% decay time than other QEEG values (Table 1). Curve-fitting was not performed for ADR, BDR or SEF90 due to lack of curve-fitting models with significant goodness of fit. Significant QEEG changes (Fig 5), which may be impeded by higher baseline EEG noise levels, lasted longest for alpha power (9.7 and 6.4 min for amobarbital and methohexital respectively), followed by aEEG (8.7 and 5.0 min) and beta power (7.3 and 5.0 min).
Figure 5.
Persistence of QEEG changes. Statistically significant QEEG changes persisted longer after amobarbital (Amo) injections (black) than after methohexital (Metho) injections (gray), and were more prominent on the ipsilateral (Ipsi) side of injections (closed columns) than on the contralateral (Contra) side (open columns).
Wada injections also evoked significant changes in all contralateral QEEG values (Fig. 2 & 3). Compared to the ipsilateral QEEG changes, contralateral peak changes for delta, theta, alpha, beta, aEEG, ADR, BDR and SEF90 (mean±s.e.m, n=52) were 71.1%±3.5%, 70.2%±3.3%, 63.4%±3.3%, 69.0%±3.6%, 65.2%±3.7%, 76.7%±5.2%, 79.6%±5.7%, 64.8%±10.3% smaller, respectively.
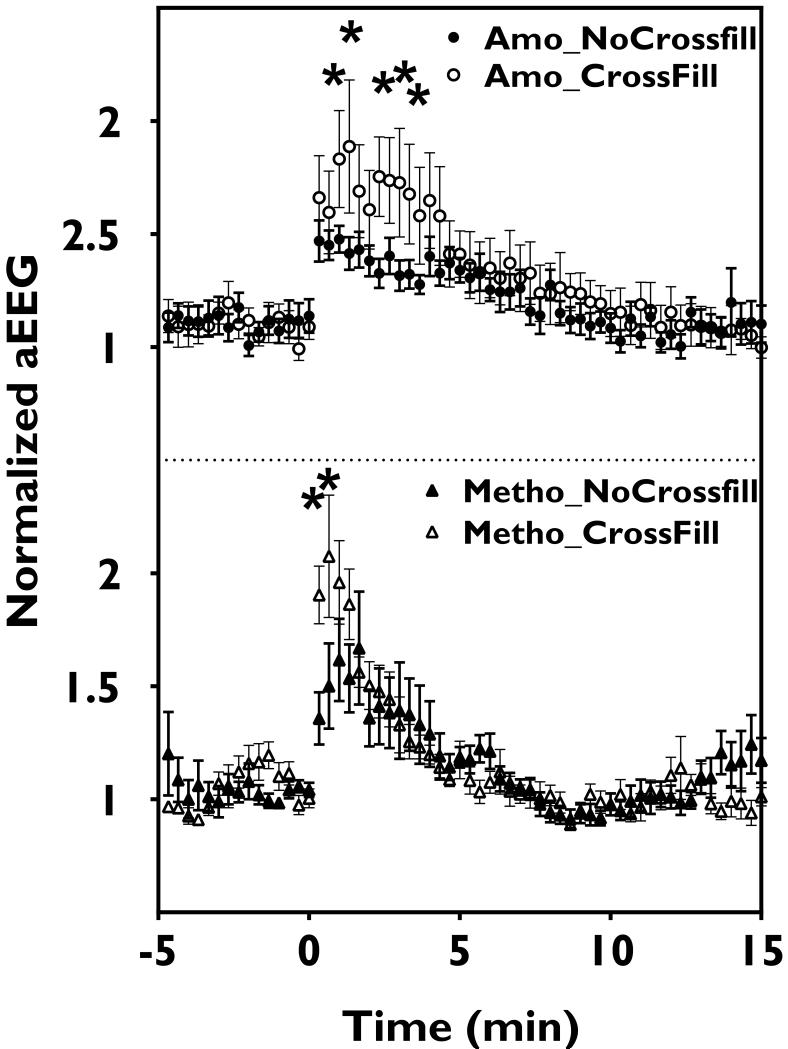
QEEG detects cross-filling effects and differences in injection drugs
During 1-4 min post injection, contralateral QEEG after injections with extensive (≥grade 2.5, n=7) cross-filling in the ACA region had significantly more prominent aEEG (for methohexital and amobarbital, Fig. 6) and theta (for methohexital only, data not shown) changes than those without detectable cross-filing (grade 0, n=12). Cross-fillings in MCA and PCA regions for all injections were graded ≤1. Compared to amobarbital, the 10-90% decay times for delta, theta power and aEEG changes with methohexital were shorter (Table 1). This difference was not observed in other QEEG measures. Amobarbital injections also yielded more persistent significant changes in all QEEG measures (Fig 5).
Figure 6.
Cross-filling effects on QEEG. More prominent aEEG changes were seen 1-4 min after amobarbital (Amo) or 1-2 min after methohexital (Metho) injections in cases with ≥2.5 grade cross-filling (Crossfill) compared to those without prominent cross-filling (NoCrossfill). Data points and error bars represent averages and s.e.m.; * represents significant changes.
Time course of arm strength and its relation to visual EEG interpretations and QEEG measures
Contralateral arm strength was reported 3-10 times (5.3±1.9, mean ± SD) at intervals of 18-138 (44±28) sec after each injection. Total contralateral arm strength loss (T0/5) occurred at 33±30 sec and 30±32 sec, with recovery to 3/5 arm strength (T3/5) at 2.4±0.9 and 3.0±1.7 min, and to 5/5 arm strength (T5/5) at 3.6±1.2 and 7.1±6.0 min for methohexital and amobarbital injections, respectively (Fig. 4). The 10-90% decay time for arm strength recovery was 2.2±0.5 min (methohexital) and 4.1±3.0 min (amobarbital). All electroencephalographers noted induction of delta activity after injection, followed by transitions to faster frequencies (theta, then beta and alpha), but only time of delta-to-theta transition was reported, at 1.4±0.8 min (n=24) for methohexital and at 2.2±1.4 min (n=10) for amobarbital. Although significant contralateral EEG changes were found, ipsilateral arm strength was not affected by any Wada injections, including injections with ≥2.5 grade cross-filling in one of the communicating arteries.
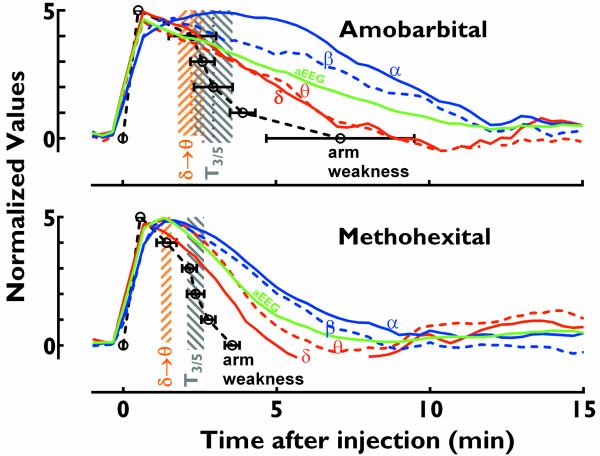
Figure 4.
Clinical correlation of QEEG values. Correlation of amplitude-normalized average ipsilateral QEEG trends for delta (σ, red solid line), theta (zθ, red dashed line), aEEG (green line), beta (β, blue dashed line), and alpha (α, blue solid line) with arm weakness (black open circles: average values; error bars, s.e.m.) in response to amobarbital (upper panel) and methohexital (lower panel) injections. Delta-to-theta transition time (σ θ, brown shadow) is earlier than the arm strength of 3/5 (gray shadow). QEEG curves were derived from moving averages of 3 data points in Figure 3. Values on Y-axis denoted relative effects, with 0 being baseline and 5 being maximal effect.
Compared to averaged QEEG peak parameters (Fig 4), T0/5 was not significantly different from the peak times of delta, theta, aEEG, ADR, BDR, or SEF90 for amobarbital, and the peak times of delta, aEEG, ADR, BDR, or SEF90 for methohexital, but was significantly earlier than the peak times of alpha and beta for both injection drugs. Based on averaged QEEG changes (Fig 3), T3/5 occurred when delta decayed 24% and aEEG decayed 19% for amobarbital, and 27% (delta) and 18% (aEEG) for methohexital. T5/5, the time when arm strength returned to baseline, occurred when delta and aEEG decayed 91% and 60% for amobarbital and 57% and 40% for methohexital, respectively (Fig 4).
Noise and variability of arm strength tests, visual EEG interpretation and QEEG measures
The influence of noise on each QEEG measurement was evaluated using a signal/noise ratio (SNR). The ipsilateral SNR was highest for aEEG, followed by delta and theta power, and lowest for SEF90 (Table 1). Subtracting contralateral values from ipsilateral values did not significantly increase SNR. Systematic error of QEEG due to recording noise and human error from arm strength exams were compared based on coefficients of variation (CVs) among injections. The CVs of amplitude-related QEEG measures ranged from 0.3-0.4 for 10-90% decay times, significantly lower than those of QEEG peak times (0.5-1.4), arm strength scores (0.3-1.1), and visually-determined delta-to-theta transition time (0.6). No differences in SNR or CVs were found between amobarbital and methohexital injections.
Analysis of excluded studies
Among 57 patients’ Wada EEGs reviewed in this study, nearly half (24 cases, 18 amobarbital and 6 methohexital) were excluded due to prominent artifacts related to patient’s agitation and other movements, as well as inadequate scalp electrode fixation. QEEGs calculated from these 46 injections (2 cases only had one injection due to atherosclerosis or fibromuscular dysplasia of the internal carotid artery) failed to show significant changes in QEEG measures after drug injection (data not shown), either due to high noise level of control recording that was close to the level of maximal QEEG changes (2 injections), or related to artifact contamination of drug-evoked QEEG changes preventing evaluation of peak and decay parameters (15 injections), or both (29 injections). All these tests had proper drug-filling on angiography and adequate hemiparesis on exam. Injection drug doses (5.0±0.1 mg, n=11 for methohexital and 110.1±19.1 mg, n=35 for amobarbital) were not significantly different (p>0.1 student t tests) from the injections included in this study.
For the 4 cases excluded from this study due to additional drug doses on the same side, additional peak changes corresponding to the second doses, delayed return to EEG baseline and more prolonged hemiplegia were noted. Unfortunately, artifacts prevented calculation of QEEG peak changes and the time retuning to baseline in these cases.
For the 3 cases excluded from this study due to incomplete hemiplegia even with repeated injections on the same side, all showed adequate drug-filling visualized by angiography and brain lesions on the side of injection. Among these cases, two were unable to cooperate with arm strength tests during the Wada test. Although injections evoked prominent delta activity in EEG segments with less artifact, prominent artifact prevented analysis of QEEG peak and decay values. A third case had prominent delta activity at baseline on the side of injection and baseline arm weakness due to a large area of encephalomalacia, for which neither visual EEG examination nor QEEG analysis showed any significant changes after injections. The injection to the other side also failed due to patient’s inability to cooperate.
Discussion
To summarize, the important ipsilateral EEG and contralateral hemiplegia markers after Wada drug injections in this study are as follows: 1) Within 10 sec, beginning of delta activity was observed in raw EEG. 2) At around 30 sec, or within 1 min, changes in delta and theta powers, ADR, BDR and SEF90 peaked, corresponding to contralateral arm plegia. Memory and language testing usually started at this time. 3) At 2.4 min (methohexital) and 3.0 min (amobarbital), contralateral arm strength recovered to 3/5. This corresponded to a 24-27% drop in delta power and an 18-19% drop in mean amplitude on aEEG, as well as maximal increase in alpha power. This is the time at which language and memory tests were considered less reliable and additional barbiturate injection doses were given (more often with methohexital due to faster recovery) to obtain additional reliable tests. 4) At 3.6 min (methohexital) and 7.1 min (amobarbital), contralateral arm strength returned to baseline while changes in delta, theta, alpha, beta powers and aEEG were still resolving. 5) At 4.7 min (methohexital) and 8.5 min (amobarbital), visual examination indicated EEG returned to baseline. 6) At 6.3 min (methohexital) and 9.7 min (amobarbital), the increase in alpha power, the longest lasting QEEG alteration, resolved to a level statistically identical to the noise level of control recording prior to injection. Contralateral Wada injection and memory recall tests should only start after this, to ensure no residual anesthetic effect from prior injection.
The best QEEG measure for Wada tests
All QEEG changes, with the exception of theta, alpha and beta powers, reached maximum when total contralateral arm strength loss occurred, and resolved more slowly than the weakness. The delta power recovery time was closest to the recovery time of contralateral arm strength (Fig 4), making delta power a better predictor of recovery from anesthetic effects. The aEEG values have the highest SNR, hence are most resistant to artifacts, with decay times between those of lower frequency powers (delta and theta) and higher frequency powers (alpha and beta). Therefore, aEEG may be a useful QEEG measure in identifying recovery from anesthetic effects. In addition, changes in alpha power had the slowest decay times and may be the most conservative and useful measure of full recovery and return to baseline EEG.
ADR and BDR may theoretically cancel noise that concurrently affects alpha, beta and delta power values since they are ratios. However, our data did not show higher SNRs for ADR and BDR than those for alpha, beta, or delta power. SEF90 had similar peak time as delta and theta values, but had the lowest SNR among all QEEG measures. In addition, significant ADR, BDR and SEF90 changes after injection returned to baseline earlier than motor function (before 3/5 strength returned). The data does not support the usefulness of ADR, BDR or SEF90 in Wada tests.
QEEG changes were sensitive to large amount of cross-filling and different injection drugs
The shorter anesthetic effects after methohexital compared to amobarbital injections, including briefer speech arrest, memory and motor deficits and EEG delta activity (Buchtel et al., 2002; Loddenkemper et al., 2009) are reproduced by our QEEG analysis. It is unlikely that this difference is a result of relatively higher amobarbital dosage since the difference in QEEG peak values between the two drugs was not statistically significant (Figure 3, Table 1). It is more likely due to methohexital’s more rapid central action and clearance (Balasubramaniam et al., 1970; Hudson et al., 1983).
The presence of significant, but less evident, contralateral QEEG changes, even in the absence of significant cross-filling observed by angiography, is consistent with prior reports (Ahern et al., 1995; Douw et al., 2009; Gotman et al., 1992; Hong et al., 2000). This suggests that contralateral EEG changes are not induced by direct perfusion of drugs from the ipsilateral injection, but rather by a transient functional disconnection from the ipsilateral hemisphere. In addition, we also found that extensive cross-filling in the ACA region was associated with greater contralateral changes in amplitude and theta power, confirming that QEEG measures can detect major cross-filing, and that aEEG and theta power appear to be the best potential indicators of contralateral anesthetic effects related to cross-filling.
Advantages of QEEG measures
Unlike serial arm strength exams, the QEEG measures can display continuous decay of anesthetic effects without disturbing language and memory tests, with more objectivity and accuracy, and can be monitored in real-time, using commercially available software. We found that recovery times of QEEG changes during Wada tests had smaller variance than those of arm strength scores, demonstrating that QEEG may be a more reliable technique. Controversies exist regarding the level of arm strength that correlates with the end time of valid memory and language (Acharya and Dinner, 1997). Although most authors reported 3/5 arm strength as the marker for the end of valid language and memory tests (Kim et al., 2007; Mikati et al., 2009; Takayama et al., 2004), some used 2/5 arm strength as a marker (Buchtel et al., 2002), while others did not mention this criterion (Kirsch et al., 2005; Rathore et al., 2013). QEEG analysis provides continuous and objective indicators of anesthetic effects, which warrants further exploration as a reliable, continuous, unobtrusively obtained biomarker of anesthetic effect. QEEG measures could also be used to decide when to give additional doses of drug and when to perform memory recall tests, especially for methohexital.
Limitation of using QEEG in Wada
A major limitation of Wada QEEG is its requirement of high quality, low artifact EEGs. For all cases excluded due to artifacts, the neurologists were able to read raw EEGs and reported approximate times of delta activity onset and when EEG returned to baseline, likely because some human experts can see delta activity even when contaminated by some artifacts, while the QEEG analysis is based on values derived from a few seconds of EEG segments (4 sec segments in this study) and is sensitive to artifacts. It is important to obtain good quality baseline EEG recording before Wada injections since the majority (67%) of cases excluded due to artifacts had prominent artifacts in the baseline recording. Beginning an injection after obtaining a few minutes of good quality control EEG recording is a practical way to improve QEEG analysis. Ways to minimize artifacts include securing EEG electrodes by experienced EEG technicians, using better fixatives such as collodion or electrode types such as subdermal wires (which also minimizes interference with the angiogram images), and minimizing patient anxiety and agitation with a pre-Wada procedure (Petersen, 1993; Rathore et al., 2013). Many mathematical methods may also be useful in conditioning EEG recordings to meet standards of QEEG analysis. These include new artifact rejection or removal algorithms of EEG software, independent component analysis (ICA), principal component analysis (PCA), regression-based myogenic correction and wavelet transform (Jung et al., 2000; McMenamin et al., 2009; Zima et al., 2012). Further investigation is required to evaluate whether these methods eliminate artifacts without altering QEEG values. On the other hand, the artifact-sensitive nature of QEEG may also as an artifact monitor for Wada EEG. Impressions from visual review of raw EEG with prominent artifacts may be inaccurate when artifacts reach a certain degree, and QEEG is an objective way to set a standard on the amount of artifacts tolerable for a valid Wada studies and agreement among EEG experts may be required to set this criterion.
We conclude that Wada QEEG provides detailed, accurate and objective information to cerebral functional changes that could not be obtained otherwise, and we propose that clinical use of QEEG could improve the accuracy and reliability of Wada testing when used in combination with visual EEG assessment and arm strength exams. Future studies will focus on correlating QEEG changes with injection-induced, quantifiable neurological and cognitive changes, evaluating kinetics of other QEEG measures such as rhythmicity, coherence, and application of real-time QEEG parameters in Wada tests.
Acknowledgement
We acknowledge Drs. Mark Scheuer, Catherine Schevon and Nicolas Gaspard for reading the manuscript and providing valuable comments and suggestions. This publication was supported, in part, by the National Cancer Institute, National Institutes of Health, through Grant Number KM1 CA156709 (MJH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Past meeting presentation: Part of this study was presented in Society of Neuroscience Annual Meeting in Nov, 2011 in Washington DC, and in American Clinical Neurophysiology Society Annual meeting in Feb, 2012 in San Antonio, TX.
Disclosure
None of the authors has any conflict of interest to disclose.
References
- Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol. 2011;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya JN, Dinner DS. Use of the intracarotid amobarbital procedure in the evaluation of memory. J Clin Neurophysiol. 1997;14:311–325. doi: 10.1097/00004691-199707000-00004. [DOI] [PubMed] [Google Scholar]
- Ahern GL, Labiner DM, Talwar D, et al. Quantitative analysis of the electroencephalogram in the intracarotid amobarbital procedure: Ii. Coherence analysis. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 1995;12:285–290. doi: 10.1097/00004691-199505010-00006. [DOI] [PubMed] [Google Scholar]
- Akman CI, Micic V, Thompson A, et al. Seizure detection using digital trend analysis: Factors affecting utility. Epilepsy Res. 2011;93:66–72. doi: 10.1016/j.eplepsyres.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam K, Lucas SB, Mawer GE, et al. The kinetics of amylobarbitone metabolism in healthy men and women. British journal of pharmacology. 1970;39:564–572. doi: 10.1111/j.1476-5381.1970.tb10364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale S. The wada test. Curr Opin Neurol. 2009;22:185–189. doi: 10.1097/WCO.0b013e328328f32e. [DOI] [PubMed] [Google Scholar]
- Blume WT, Grabow JD, Darley FL, et al. Intracarotid amobarbital test of language and memory before temporal lobectomy for seizure control. Neurology. 1973;23:812–819. doi: 10.1212/wnl.23.8.812. [DOI] [PubMed] [Google Scholar]
- Bouwer MS, Jones-Gotman M, Gotman J. Duration of sodium amytal effect: Behavioral and eeg measures. Epilepsia. 1993;34:61–68. doi: 10.1111/j.1528-1157.1993.tb02376.x. [DOI] [PubMed] [Google Scholar]
- Buchtel HA, Passaro EA, Selwa LM, et al. Sodium methohexital (brevital) as an anesthetic in the wada test. Epilepsia. 2002;43:1056–1061. doi: 10.1046/j.1528-1157.2002.00902.x. [DOI] [PubMed] [Google Scholar]
- Castillo M, Mukherji SK, McCartney WH. Cerebral amobarbital sodium distribution during wada testing: Utility of digital subtraction angiography and single-photon emission tomography. Neuroradiology. 2000;42:814–817. doi: 10.1007/s002340000438. [DOI] [PubMed] [Google Scholar]
- Douw L, Baayen JC, Klein M, et al. Functional connectivity in the brain before and during intra-arterial amobarbital injection (wada test) Neuroimage. 2009;46:584–588. doi: 10.1016/j.neuroimage.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Gotman J, Bouwer MS, Jones-Gotman M. Intracranial eeg study of brain structures affected by internal carotid injection of amobarbital. Neurology. 1992;42:2136–2143. doi: 10.1212/wnl.42.11.2136. [DOI] [PubMed] [Google Scholar]
- Hong SB, Kim KW, Seo DW, et al. Contralateral eeg slowing and amobarbital distribution in wada test: An intracarotid spect study. Epilepsia. 2000;41:207–212. doi: 10.1111/j.1528-1157.2000.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Hudson RJ, Stanski DR, Burch PG. Pharmacokinetics of methohexital and thiopental in surgical patients. Anesthesiology. 1983;59:215–219. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- Kim JH, Joo EY, Han SJ, et al. Can pentobarbital replace amobarbital in the wada test? Epilepsy Behav. 2007;11:378–383. doi: 10.1016/j.yebeh.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Kirsch HE, Walker JA, Winstanley FS, et al. Limitations of wada memory asymmetry as a predictor of outcomes after temporal lobectomy. Neurology. 2005;65:676–680. doi: 10.1212/01.wnl.0000174440.31387.65. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Moddel G, Dinner DS, et al. Language assessment in wada test: Comparison of methohexital and amobarbital. Seizure: the journal of the British Epilepsy Association. 2009;18:656–659. doi: 10.1016/j.seizure.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Magee JA, Pender NP, Abrahams S, et al. A comparison of propofol and amobarbital for use in the wada test. Seizure: the journal of the British Epilepsy Association. 2012;21:399–401. doi: 10.1016/j.seizure.2012.02.001. [DOI] [PubMed] [Google Scholar]
- McKeever S, Johnston L, Davidson AJ. An observational study exploring amplitude-integrated electroencephalogram and spectral edge frequency during paediatric anaesthesia. Anaesthesia and intensive care. 2012;40:275–284. doi: 10.1177/0310057X1204000210. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, et al. Validation of regression-based myogenic correction techniques for scalp and source-localized eeg. Psychophysiology. 2009;46:578–592. doi: 10.1111/j.1469-8986.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Naasan G, Tarabay H, et al. Intracarotid propofol testing: A comparative study with amobarbital. Epilepsy & behavior: E&B. 2009;14:503–507. doi: 10.1016/j.yebeh.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Morita A, Kamei S, Mizutani T. Relationship between slowing of the eeg and cognitive impairment in parkinson disease. J Clin Neurophysiol. 2011;28:384–387. doi: 10.1097/WNP.0b013e3182273211. [DOI] [PubMed] [Google Scholar]
- Patel A, Wordell C, Szarlej D. Alternatives to sodium amobarbital in the wada test. Ann Pharmacother. 2011;45:395–401. doi: 10.1345/aph.1P476. [DOI] [PubMed] [Google Scholar]
- Peters JM, Tomas-Fernandez M, van Putten MJ, et al. Behavioral measures and eeg monitoring using the brain symmetry index during the wada test in children. Epilepsy & behavior: E&B. 2012;23:247–253. doi: 10.1016/j.yebeh.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Petersen RCS FW, Jack CR. Intracarotid amobarbital testing. In: Wyllie E, editor. The treatment of epilepsy: Principles and practice. Lea & Febiger; Philadelphia: 1993. pp. 1051–1061. [Google Scholar]
- Rathore C, Kesavadas C, Sarma SP, et al. Usefulness of wada test in predicting seizure outcome following anterior temporal lobectomy. Epilepsy Res. 2013;107:279–285. doi: 10.1016/j.eplepsyres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Rausch R, Fedio P, Ary CM, et al. Resumption of behavior following intracarotid sodium amobarbital injection. Ann Neurol. 1984;15:31–35. doi: 10.1002/ana.410150106. [DOI] [PubMed] [Google Scholar]
- Roberts DW. Is there still a role for language-wada testing? World Neurosurg. 2011;75:425–427. doi: 10.1016/j.wneu.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Scheuer ML, Wilson SB. Data analysis for continuous eeg monitoring in the icu: Seeing the forest and the trees. J Clin Neurophysiol. 2004;21:353–378. [PubMed] [Google Scholar]
- Sharan A, Ooi YC, Langfitt J, et al. Intracarotid amobarbital procedure for epilepsy surgery. Epilepsy & behavior: E&B. 2011;20:209–213. doi: 10.1016/j.yebeh.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Stuart RM, Waziri A, Weintraub D, et al. Intracortical eeg for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2010;13:355–358. doi: 10.1007/s12028-010-9414-6. [DOI] [PubMed] [Google Scholar]
- Takayama M, Miyamoto S, Ikeda A, et al. Intracarotid propofol test for speech and memory dominance in man. Neurology. 2004;63:510–515. doi: 10.1212/01.wnl.0000133199.65776.18. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance - experimental and clinical observations. Journal of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Yu T, Peng H. Quantification and deconvolution of asymmetric lc-ms peaks using the bi-gaussian mixture model and statistical model selection. BMC Bioinformatics. 2010;11:559. doi: 10.1186/1471-2105-11-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima M, Tichavsky P, Paul K, et al. Robust removal of short-duration artifacts in long neonatal eeg recordings using wavelet-enhanced ica and adaptive combining of tentative reconstructions. Physiological measurement. 2012;33:N39–49. doi: 10.1088/0967-3334/33/8/N39. [DOI] [PubMed] [Google Scholar]