Mu opioid receptors: function and dysfunction
Opioids are currently the most effective pain relieving pharmaceuticals. However, they are also rewarding and their repeated use can lead to dependence and addiction. In fact, addiction to opioid analgesics is a growing socioeconomic and health problem with potentially serious consequences documented by a rise in deaths due to overdose [1, 2]. A critical CNS locus for opioid reward is the ventral tegmental area (VTA, glossary, vide infra). Recent work indicates that there is great anatomical and pharmacological heterogeneity in VTA neurons and that there are numerous opioid synaptic actions within the VTA. Here we review the role VTA neurons play in opioid reward and reinforcement, and the synaptic and neural circuit mechanisms by which opioids control VTA neuronal activity.
How are we using the term reward?
Although there is broad consensus that addicting drugs produce ‘reward’, inconsistency in the use of the term is an impediment to progress in understanding how these drugs influence behavior [3]. The word ‘reward’ can be used as a noun (“rats will work for a reward”), a verb (“he intends to reward the winner”), or an adjective (a rewarding flavor). Furthermore, even when used as a noun, it has several distinct meanings: It can refer to the rewarding agent itself (e.g. a food or drug reward) or to the subjective hedonic feeling (i.e. pleasure). In behavioral psychology, it typically is used to denote a CNS process that increases the future probability of a behavioral response that has produced a beneficial outcome; a more precise term for this process is ‘positive reinforcement’. In this review, we focus on how the actions of mu opioid (MOP) receptor agonists in the VTA can produce positive reinforcement, a critical initial step leading to opioid addiction.
It is important to point out that positive reinforcement is not an elementary process; it consists of several inter-related processes occurring at different times (Figure 1) and each process is likely to require activation of a distinct and partially independent neural circuit. Disruption of any contributing circuit could impair positive reinforcement. For example, consider a rat that experiences a sensory cue immediately prior to approaching and pressing a lever, then enters a reward receptacle and consumes a sucrose pellet. If we then observe an increase in the probability of that behavior following the cue we can say that consuming the pellet has positively reinforced the ability of the cue to elicit the subsequent lever press, approach, receptacle entry and consumption of the pellet. For positive reinforcement to occur, the rat must have approached and consumed the pellet, determined that consuming the pellet was beneficial (the ‘benefit’ will depend in part on the animal’s motivational state (hunger, etc.) at the time of consumption), and remembered the sensory cue, the context, and the actions performed. At a minimum, this process includes signaling in circuits controlling motivation, attention/orientation, sensory discrimination, action selection, outcome assessment and working memory. Positive reinforcement likely requires changes in synaptic strength between neurons that result in a neural representation of the association between the outcome and the context, cue and action. It is these associations that are manifested as a change in response probability when the cue next occurs in the training context. There is compelling evidence that dopamine and opioids directly influence circuits that contribute to several different elements of positive reinforcement [3–11]. Although some VTA neurons including dopamine neurons encode reward prediction error, the downstream connections of these neurons have not been established. On the other hand, there is evidence that different VTA projections contribute to other functions. For example, VTA projections to the nucleus accumbens (NAc) contribute to encoding incentive salience while projections to the hippocampus promote spatial memory formation [12]. Because the neuronal mechanisms underlying the actions of opioids and dopamine may differ in each of these circuits, a complete understanding of their contributions to ’reward’ requires disentangling these functions and defining the circuits relevant to each.
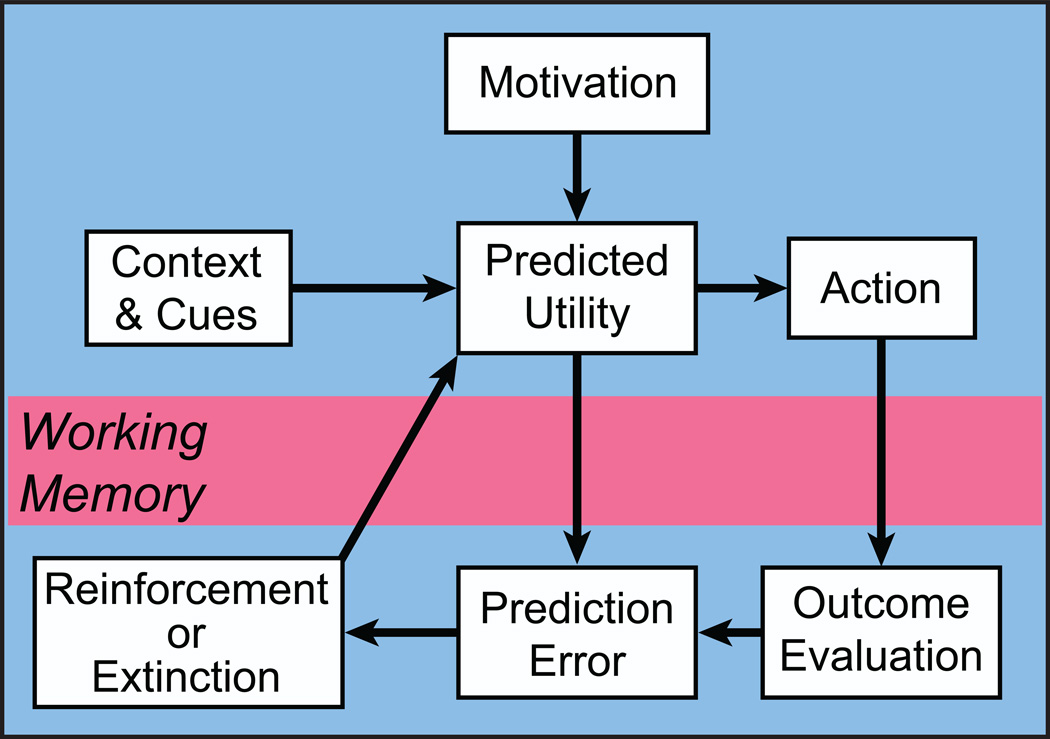
Figure 1.
Deconstruction of reward. Reward can be conceptualized as a teaching signal that promotes future actions that have been experienced as beneficial at specific times and places. The teaching signal includes several processes occurring at different times. Animals are subject to a variety of motivations for specific outcomes that improve their survival and reproductive success. Along with motivation, detection of contextual cues inform the animal about the current value (and cost) of actions. This information leads to a predicted outcome and an action is selected. The outcome of that action is then evaluated and compared to the predicted utility. If the outcome is better than predicted, i.e. a positive reward prediction error, subsequent utility predictions are greater and the likelihood of the action taken is increased in future under similar circumstances. Working memory is involved in two ways: first, to compare the predicted and actual outcome and second, to reinforce the actions and contextual cues leading to the outcome.
The VTA is a critical site for MOP receptor mediated reward
The most consistent and robust rewarding effects of opioids require a functional MOP receptor [13]. The significance of the VTA for MOP reward has been established by several lines of evidence. Specifically, conditioned place preference (CPP) produced by systemically administered MOP receptor agonists can be blocked by intra-VTA MOP receptor selective antagonists or genetic knockdown of MOP receptor [14, 15]. Microinjecting a MOP receptor antagonist into the VTA also accelerates IV heroin self-administration [16]. These observations do not prove that the systemic drug itself acts directly on receptors in the VTA; it could act at another CNS site that activates neurons that project to the VTA and release an endogenous MOP receptor agonist (e.g. enkephalin). However, the idea that the VTA is a critical site for the direct action of exogenous MOP receptor agonists is consistent with the observations that MOP receptor agonists are self-administered into the VTA in rats and mice [17, 18]. Other sites that are sufficient targets for morphine self-administration in mice include the NAc shell (but not NAc core or dorsal striatum), lateral and medial hypothalamus, amygdala and midbrain periaqueductal gray [19]. In addition, morphine produces CPP when injected directly into the VTA and rostral anterior NAc shell of the rat but ineffective at other sites such as medial frontal cortex, hippocampus, lateral nucleus of the amygdala, lateral hypothalamus, pedunculopontine tegmental nucleus, substantia nigra pars compacta (SNc), posterior hypothalamus, ventral palladium, or nucleus accumbens core or posterior shell [20–25]. Therefore a MOP receptor action in the VTA is sufficient to produce a positively reinforcing effect and VTA MOP receptors are necessary for the rewarding actions of systemically administered MOP receptor agonists.
Heterogeneity of VTA neurons: different neurotransmitters, distinct projection targets and afferent inputs
Early studies of VTA contributions to reward focused on the dopaminergic projection to the ventral striatum. However, different subsets of VTA dopamine neurons project to other CNS targets implicated in reward-relevant functions including: the amygdala, hippocampus, ventral pallidum, periaqueductal grey, bed nucleus of the stria terminalis, olfactory tubercle, locus coeruleus, and lateral habenula [26–29]. Furthermore, the properties of dopamine neurons vary based on their CNS projection targets [30–36]. In addition to dopamine neurons, the VTA has significant numbers of GABA and glutamate neurons that project to many of the same mesolimbic targets as the dopamine neurons [37, 38]. Importantly, the afferent connectivity of individual VTA neurons sorts by both neurotransmitter content and projection target (Figure 2). Similarly, the specific postsynaptic targets of VTA neuron terminals can differ within a single target. For example, VTA GABA neurons projecting to the NAc synapse predominantly onto cholinergic interneurons rather than medium spiny neurons [39]. In summary, the VTA encompasses different subsets of both dopamine and non-dopamine neurons that participate in distinct circuits that likely serve different behavioral functions.
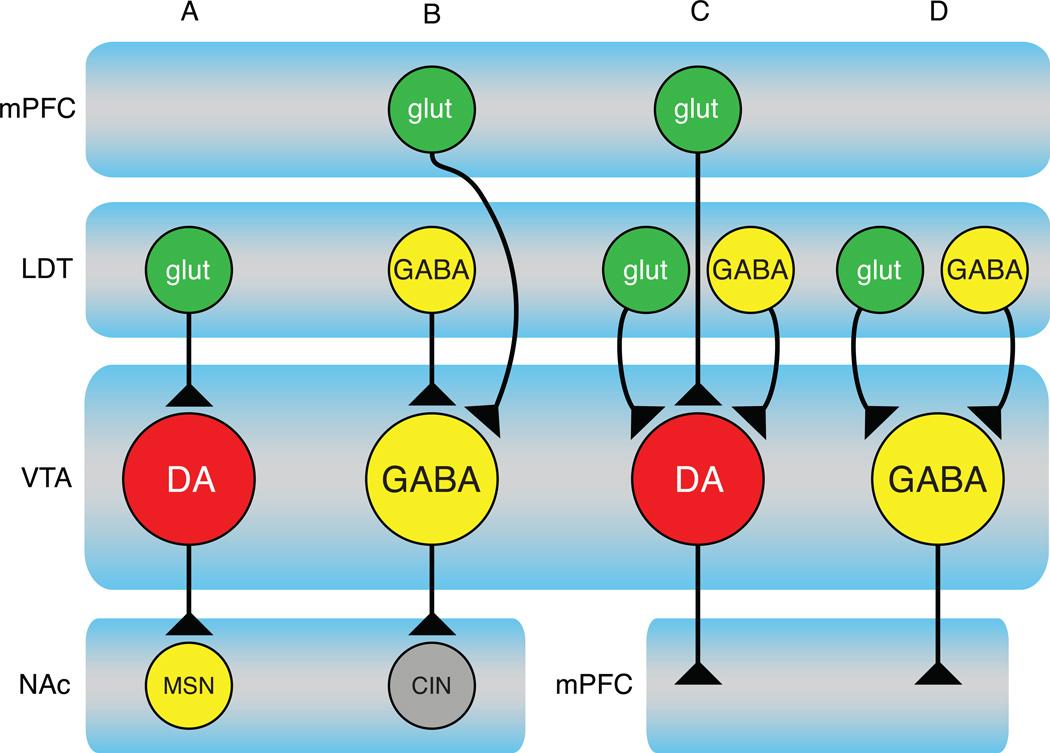
Figure 2.
Distinct circuits course through the VTA. A variety of studies demonstrate that the VTA receives inputs from and projects to many brain regions (see [26, 124] for review); researchers have determined only a small number of exact circuit connections to date. These studies have revealed that inputs to VTA neurons differ based on their neurotransmitter content and projection target. At least four distinct circuits have so far been identified:
A) A laterodorsal tegmental (LDT) glutamate input to VTA dopamine neurons projecting to NAc neurons, including medium spiny neurons (MSNs) [125].
B) A VTA GABA neuron projection specifically to NAc cholinergic interneurons (CIN) [39]. These VTA neurons receive inputs from mPFC and LDT [125, 126]. There is also evidence that these CINs can evoke release from NAc dopamine terminals via a presynaptic nicotinic cholinergic receptor [90].
C) A VTA dopamine neuron projection to mPFC receives glutamate inputs from mPFC and LDT and GABA inputs from the LDT [125]. It is unknown if these inputs converge onto all mPFC-projecting dopamine neurons.
D) A VTA GABAergic projection to mPFC receives both glutamate and GABA inputs from LDT [125]. It is important to point out that this figure underestimates the number of circuits running through the VTA. Importantly, it It does not illustrate the VTA glutamate neurons, which have a distinct pattern of projection targets, nor does it illustrate several other major targets of dopamine and GABA neurons (e.g. amygdala, hippocampus, BNST, olfactory tubercle, ventral pallidum and hypothalamus).
MOP receptor agonists activate a subset of VTA neurons including dopamine neurons
The VTA contains dense concentrations of both MOP receptors and endogenous opioid peptides [40–43]. Because dopamine neurons are clustered in this region and MOP receptor agonist injection in the VTA can produce positive reinforcement, early studies tested the possibility that MOP agonists activate dopamine neurons. Consistent with this idea, both systemic and VTA administration of MOP receptor agonists increase dopamine release in the ventral striatum [44–48]. In anesthetized animals, systemic or VTA injected morphine increases the firing rate of putative dopamine neurons [49–53]. These findings are consistent with ex vivo studies demonstrating activation of putative VTA dopamine neurons by bath application of the MOP receptor selective agonist DAMGO [54]. Taken together these data have been interpreted as firm support for the hypothesis that VTA reward depends upon activation of dopamine neurons. Kiyatkin and Rebec [52] replicated the observation that systemic heroin increases putative dopamine neuron discharge rates in anesthetized rats. However, in awake, drug naïve rats, passive injection of heroin decreased putative dopamine neuron firing. The effects of self-administered heroin was similar; the firing rate of VTA neurons dropped immediately following each self-administration event, slowly recovering and peaking just before the next self-administration [52, 55]. These results conflict with the dopamine model of opioid reward and highlight the importance of conducting recording experiments in awake behaving animals. There is, however, a major interpretational problem with all of these in vivo electrophysiological studies: the physiological and pharmacological criteria (e.g. dopamine D2 receptor inhibition, action potential duration, or firing pattern) used to identify VTA neurons as dopaminergic are unreliable [35, 56–58]; a definitive picture of the effect of MOP agonists on dopamine neurons will require a direct method of identification of neurotransmitter content in VTA neurons in awake behaving animals [e.g. 58].
Both dopaminergic and non-dopaminergic circuits can contribute to VTA opioid reward
Although there is widespread acceptance of the idea that a critical step in MOP reward is activation of midbrain dopamine neurons, the involvement of dopamine is more nuanced and variable. In fact, opioid reward can occur without normal dopamine function. For example, dopamine-depleted mice acquire morphine CPP [59]. One critical factor that determines the degree to which dopamine contributes to MOP reward is the state of the animal. This was studied by van der Kooy’s group who compared MOP CPP in rats that were either opioid naïve or opioid dependent (using either systemic [60] or intra-VTA microinjection of morphine [24]). In opioid naïve rats, morphine CPP is not blocked by systemic α-flupenthixol, a non-selective dopamine receptor antagonist. In contrast, this same dose of α-flupenthixol completely blocked morphine CPP in the opioid dependent rats. They observed the same pattern for systemic morphine CPP when injecting the same dopamine antagonist directly into the ventral striatum [61]. Food deprivation, social defeat stress, and intra-VTA BDNF also induce the same kind of ‘state dependent’ shift in VTA-dopamine reward circuit function [62–64]. It is important to point out in this regard that most studies of MOP receptor function in the VTA and of its role in behavior have been carried out in opioid naïve animals. Clearly, VTA MOP receptors can produce reward through a mechanism that does not require dopamine. Unfortunately, our knowledge of the non-dopaminergic VTA circuitry supporting MOP positive reinforcement is currently extremely limited.
Dopamine neuron firing can encode positive outcomes and produce positive reinforcement
Although some pharmacological manipulations that increase dopamine in the ventral striatum do not produce reward (Box 1), there is a body of evidence implicating dopamine in positive reinforcement. In vivo single unit recordings in both primate and rodents show that midbrain dopamine neurons encode beneficial outcomes [e.g. 7, 58]. More specifically, many dopaminergic neurons encode a signal consistent with the proposal that their firing reflects a reward prediction error. An encoded positive reward prediction error can act as a teaching signal and lead to positive reinforcement. Causal evidence that selective activation of dopamine neurons can produce positive reinforcement has recently been provided using rodents that express Cre recombinase under the tyrosine hydroxylase (TH) promoter (TH is currently the most reliable identifier of dopamine neurons in the VTA). In these rodents, expression of channel rhodopsin (ChR) can be selectively induced in VTA TH expressing neurons through local microinjection viruses with a Cre-inducible viral construct coding for ChR-2. These rodents learned to lever press to receive light activation of their VTA dopamine neurons [65, 66]. Furthermore, application of a burst pattern of light activation was capable of producing CPP, indicating that activity in VTA dopamine neurons is sufficient for positive reinforcement [67]. The sufficiency for positive reinforcement of precisely timed stimulation of dopamine neurons was recently demonstrated by Steinberg et al. [68], who were able to substitute optogenetic activation of rat VTA dopamine neurons for a ‘natural’ reward and significantly reduce extinction of learned approach behavior. Importantly, stimulation that occurred after a delay (thus degrading the temporal association of dopamine activation with the action that produced it) did not maintain responding. Clearly, there are conditions under which selective activation of TH expressing VTA neurons is sufficient to mediate positive reinforcement and mimic the effect of natural reward. This evidence is consistent with the idea that the timing of the dopamine signal in the relevant target site is instructive in the process of positive reinforcement.
Box 1: some pharmacological agents that increase NAc dopamine are not rewarding.
In general, drugs of abuse increase dopamine release in the NAc [99]. However, not all pharmacological manipulations that increase dopamine release in the NAc are rewarding. For instance, microinjecting delta opioid receptor agonists into the VTA increase dopamine release in the NAc but do not produce CPP [45, 100]. The same is true for glial cell-line derived neurotrophic factor [101, 102] and cholecystokinin [103–105]. Most strikingly, microinjecting a MOP receptor antagonist into the VTA increases dopamine levels in the NAc [106], and behaviorally produces a conditioned place aversion [107]. Furthermore, withdrawal from opioid treatment is quite aversive and is associated with an increase in NAc dopamine release [3]. On the other hand, dopamine antagonists in the NAc rarely produce aversion and inconsistently block psychostimulant reward [see 108, 109 for study summaries]. Together, these observations indicate that an increase in dopamine release in the NAc is not itself a reliable biomarker for reward.
While these studies strongly support a role for dopamine neurons in positive reinforcement, their interpretation must be informed by the fact that VTA TH expressing neurons can also release glutamate, GABA, and a variety of neuropeptides (Box 2). Another caveat to these experiments is that TH mRNA expression has been observed in neurons with varying levels of vesicular monoamine transporter expression, raising the possibility that some TH positive neurons may not release dopamine through a classical vesicular mechanism, if at all [69]. Understanding the contribution of these co-transmitters and modulators to opioid reward is an important area for future study.
Box 2: “dopamine” neurons co-release other neurotransmitters and neuromodulators.
Selective control of dopamine neurons, for example with optogenetics, provides an excellent opportunity to design experiments that test for causal links between dopamine neuron activity and behavioral outcomes. However, it is critical to note that stimulation of dopaminergic neurons likely releases more than dopamine. The most extensively studied of co-released signaling molecule is glutamate, which has been confirmed in VTA projections to the NAc, mPFC, and lateral habenula [110–113]. GABA release from dopamine neurons that project to the dorsal striatum and lateral habenula has also recently been reported [113–115]. Importantly, many peptides have been identified in dopaminergic neurons, including cholecystokinin [116–118], neurotensin [119], neurotrophin 3 [120], and BDNF [120]. CRF and CRF-binding protein, which appears to be required for some actions of CRF in the VTA, is also expressed by a subset of dopamine neurons [121, 122]. Consistent with the idea that these peptides can be released concurrently with dopamine, systemic morphine administration also increases CCK release in the NAc [123]. Any of these neurotransmitters or modulators may contribute to the behavioral outcome of “selectively” stimulating or inhibiting “dopamine” neurons.
How do MOP receptor agonists in the VTA excite dopamine neurons?
The most commonly reported direct synaptic actions of opioid agonists are inhibitory: either direct hyperpolarization of neurons through activation of somadendritic GIRKs (G-protein coupled receptor activated inwardly rectifying K+ channels) or inhibition of neurotransmitter release [70]. Because of this, the initial proposal for the mechanism of MOP excitation of VTA dopamine neurons was that it is indirect, through removal of tonic GABAergic inhibition [71]. In fact, opioid excitation through disinhibition was previously demonstrated in the hippocampus and other CNS sites [72]. Further, work in the neighboring substantia nigra (SN) supported the possibility of disinhibitory circuitry in the midbrain: SN pars compacta putative GABAergic neurons, but not dopamine neurons, are inhibited by MOP receptor agonists [73]. These studies set the stage for ex vivo work in the VTA.
The idea that MOP receptor agonists activate VTA dopamine neurons by inhibiting local GABAergic interneurons was addressed by Johnson & North [74] who showed that most VTA neurons are inhibited by dopamine but not MOP receptor agonists (‘principal neurons’); out of the 8 principal neurons tested, 5 were cytochemically identified as dopaminergic. A smaller group (not cytochemically identified) was hyperpolarized by MOP agonists but not dopamine. Based on their similarity to putative GABA neurons in the SN, they proposed that these ‘secondary cells’ were GABAergic interneurons that inhibited neighboring dopamine neurons. Consistent with this idea, most principal cells showed spontaneous bicuculline-sensitive (i.e. GABAA receptor mediated) synaptic potentials that were prevented by the Na+ channel blocker tetrodotoxin, and therefore assumed to result from action potentials arising in local GABAergic interneurons (i.e. secondary cells) [54]. The frequency of these synaptic potentials, but not their amplitudes, was reduced by opioid agonists selective for MOP receptors. Johnson & North therefore proposed that MOP receptor agonists excite VTA dopamine neurons by inhibiting local GABAergic interneurons (Figure 3). Consistent with this model, we showed that half of cytochemically identified VTA GABAergic neurons in rat are hyperpolarized by the MOP receptor selective agonist DAMGO [57]. Similar findings were reported in all identified GAD67-GFP (i.e. GABAergic) VTA neurons in mouse [75]. At least some VTA GABA neurons synapse onto neighboring dopamine neurons [76] and a recent study in which ChR was selectively expressed in midbrain GABAergic neurons using GAD-67 Cre mice showed that activation of these neurons can inhibit dopaminergic neurons and reduce NAc dopamine release as measured by cyclic voltammetry [77]. Furthermore, selective inactivation of midbrain GABAergic neurons can excite VTA dopamine neurons [78]. Whether the VTA GABAergic neurons locally connected to dopamine neurons include those inhibited by MOP receptor agonists remains to be determined.
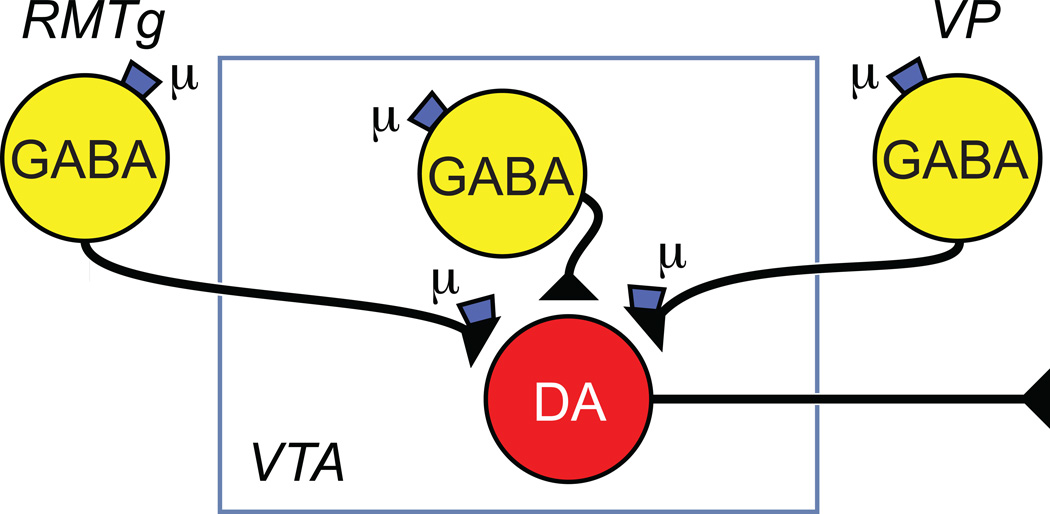
Figure 3.
Identified sites where MOP receptor action could disinhibit VTA neurons. MOP receptor agonists have been shown to directly hyperpolarize GABA neurons in the ventral pallidum (VP), rostromedial tegmental nucleus (RMTg) and within the VTA. In addition, MOR agonists inhibit release from the terminals of these three neuron groups.
Although the canonical two neuron model has the virtues of simplicity and completeness, i.e. a single VTA synaptic site of action for MOP receptor agonist reward, there are significant numbers of MOP sensitive GABAergic terminals that arise from neurons extrinsic to the VTA. One particularly interesting group of GABAergic neurons lies within the caudal VTA and continues caudally and dorsally well beyond the most caudal dopamine neurons in the VTA. These neurons, variously named the rostral medial tegmental nucleus (RMTg) or the tail of the VTA, densely project to the VTA and directly contact dopamine neurons [79]. Many RMTg neurons are hyperpolarized by the MOP receptor selective agonist DAMGO [80]. Selective optogenetic activation of RMTg afferents to VTA dopamine neurons produced large GABAergic inhibitory postsynaptic currents (IPSCs) that are inhibited by DAMGO [80, 81]. MOP receptor agonists also inhibit GABA release on to VTA dopamine neurons from the terminals of ventral pallidum neurons [82, 83] and from the terminals of intrinsic VTA GABAergic neurons [81](Figure 3). The degree to which MOP receptor agonists inhibit GABA release is much greater for RMTg inputs than those from intrinsic VTA or NAc neurons. In vivo, the degree of disinhibition of VTA neurons will depend upon the level of GABA terminal activity when MOP receptor agonists are introduced.
The generality of the disinhibition model is attractive; however, MOP receptor agonists have a variety of both inhibitory and excitatory synaptic actions in the VTA (Figure 4). In addition to the inhibition of GABAergic terminals synapsing on dopamine neurons, MOP receptor activation also inhibits GABA release onto non-dopamine neurons [84], and MOP receptor agonists can inhibit glutamate release from terminals synapsing onto VTA neurons [85, 86]. Despite the inhibitory effect of MOP on VTA glutamate transmission, Jalabert and colleagues [51] reported that an increase in putative VTA dopamine neuron firing following morphine requires glutamate neurotransmission in the VTA, and that morphine CPP requires glutamate signaling in the VTA [87].
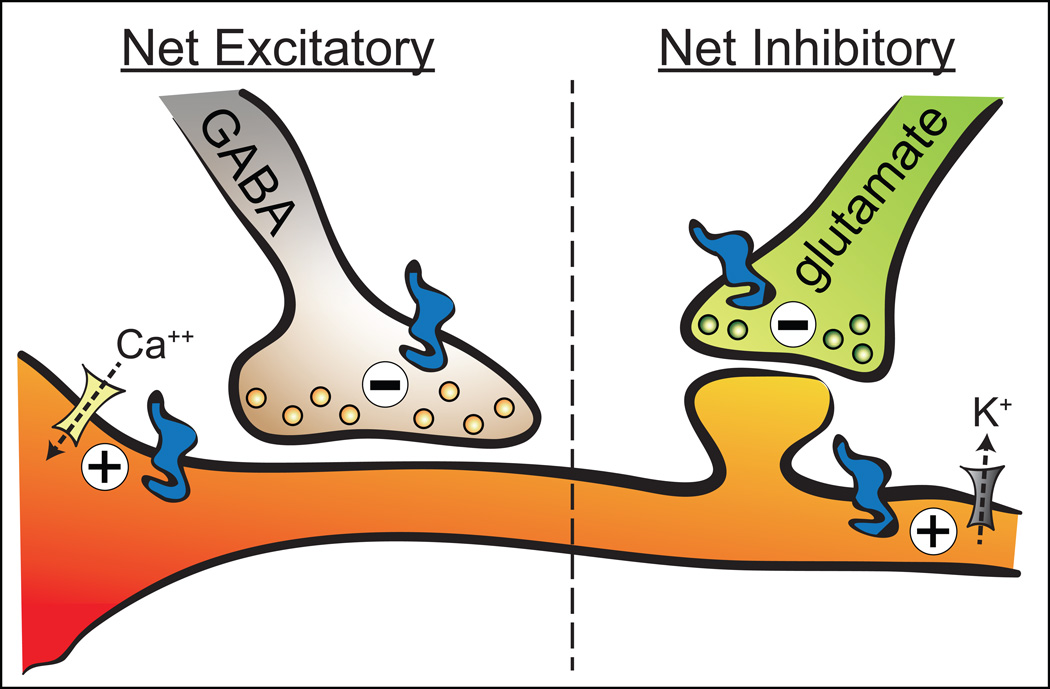
Figure 4.
Major pre- and postsynaptic mechanisms underlying MOP receptor (blue icon) control of VTA neurons. MOP receptor control of VTA neurons can have a net excitatory effect (directly by increasing Ca++ channel (yellow icon) conductance or indirectly by inhibiting GABA release) or a net inhibitory effect (directly by activating K+ channels (gray icon) or indirectly by inhibiting glutamate release).
Finally, we have recently discovered that MOP receptor activation by DAMGO can directly excite a significant subset of VTA neurons, including dopamine neurons [88]. With an EC-50 in the single nanomolar range, two orders of magnitude more sensitive than the inhibition of release from GABA terminals, this effect appears to require opening of a somatodendritic Cav2.1 channel. Unlike disinhibition, this mechanism does not require active GABA or glutamate inputs in order to excite VTA neurons. This direct excitatory effect predominates in about 20% of VTA neurons, raising the possibility that only certain circuits through the VTA can harness this direct excitatory mechanism.
Alternative circuits for MOP reward: dopamine and non-dopamine
The canonical model of opioid reward asserts that the critical dopaminergic terminal region is the ventral striatum. Indeed, dopamine D1 receptor antagonists microinjected into the NAc can reduce MOP receptor agonist reinforcement [89]. However, recent evidence suggests that dopamine can be released in the striatum independent of increases in VTA dopamine neuron activity: first, VTA GABA neurons that project to the NAc synapse onto cholinergic interneurons [39]; second, cholinergic interneuron activation in the NAc can stimulate dopamine release through nicotinic acetylcholine receptors on the striatal terminals of dopamine neurons [90, 91]. Therefore, MOP inhibition of VTA GABA neurons projecting to the NAc could increase NAc dopamine release, independent of somatic action potential activity in the VTA (Figure 2). There is also evidence implicating VTA projections to targets other than the NAc. For example, lesions of dopaminergic terminals in the anterior cingulate cortex prevents the acquisition of systemic or intra-VTA morphine CPP [92]. Dopamine D1 or D2 receptor antagonists microinjected into the amygdala can also block morphine CPP, depending on the state of the animal [93]. Future studies may reveal additional VTA projections that contribute to MOP reward.
While it is clear that there are distinct circuits involved in dopamine independent MOP reward in the VTA, our knowledge of them is very limited. The pedunculopontine tegmentum (PPTg) is required for VTA MOP CPP in opiate naïve animals [24]. However, the circuit connections and neurotransmitter(s) required for this effect are not known. It is possible that non-dopamine projections to such well-studied limbic targets as the NAc, prefrontal cortex, and amygdala are involved, but the role in VTA MOP reward of non-dopamine projections to other brain regions, such as the ventral pallidum, hippocampus, or periaqueductal gray needs to be investigated.
Can inhibition of dopamine neurons produce reinforcement?
Another robust MOP receptor effect on a subset of VTA dopamine neurons is direct postsynaptic inhibition [32, 88, 94, 95]. In fact, nearly half of all confirmed VTA dopamine neurons are inhibited by MOP activation ex vivo in the rat [88]. The heterogeneity of MOP receptor mediated actions on VTA dopamine neurons, in particular, the ubiquity of the direct inhibitory effect, undermines a critical simplifying assumption underpinning the two neuron model, i.e., that dopamine neurons in the VTA form a single functional group with uniform pharmacology. It is now abundantly clear that different groups of VTA dopamine neurons have distinct functional and pharmacological profiles that depend in part on their distinct projection targets.
One intriguing possibility is raised by the observation that a subset of VTA dopamine neurons is activated by noxious stimuli [3, 5, 96]. Consistent with this idea is a recent report that activation of lateral habenula inputs to the VTA produces an aversive effect through activation of a subset of dopamine neurons projecting to prefrontal cortex [97]. If these neurons are active and generating an aversive signal, their direct inhibition by MOP receptor activation should produce negative reinforcement (i.e. a rewarding effect due to a reduction of an ongoing aversive input).
In addition to the idea that MOP receptor agonists could have different synaptic actions on different subpopulations of VTA neurons depending upon their circuit connections, the variety of MOP receptor synaptic actions raises several alternative mechanisms by which MOP receptor agonists might increase dopamine release in downstream target regions. Local somadendritic release of dopamine provides a robust mechanism for inhibition of dopamine neurons by other nearby dopamine neurons via D2 dopamine receptor activation (e.g. [98]). Consequently, MOP receptor inhibition of some VTA dopamine neurons could lead to a decrease in local dopamine concentration and contribute to disinhibition of other dopamine neurons. Clearly, additional experiments are required to determine if any of these VTA synaptic mechanisms of MOP receptor agonists contribute(s) to reinforcement.
Concluding remarks
While it is clear that direct synaptic actions in the VTA are required for MOP receptor mediated reward, the goal of identifying the relevant mechanisms and sites of action is elusive for several reasons. On the one hand the process of reward itself consists of multiple elements dissociable in time and likely involving different circuits. This functional diversity may be reflected in the distinct connectivity and function of different subsets of VTA neurons. Despite this heterogeneity, a very large proportion of both axon terminals and somadendritic elements express functional MOP receptors. This ubiquitous distribution of MOP receptors in neurons with different neurotransmitter content and different projection targets makes a unitary mechanism of MOP receptor mediated reward unlikely. That more than one circuit running through the VTA can promote MOP reward is demonstrated by the observation that the reinforcing effect of MOP receptor actions in the VTA involves different circuits in opioid naïve and dependent rodents. In opioid naïve but not opioid exposed rats, VTA MOP reward is dopamine independent. In-depth studies of MOP receptor mediated control of VTA synaptic physiology have revealed a variety of possible mechanisms for activating both dopamine and non-dopamine projection neurons. Finally, the fact that MOP receptors directly inhibit a significant number of VTA dopamine neurons raises a variety of questions; does this happen in vivo? If it does, what is the normal contribution of these neurons to behavior? Are they the neurons that produce aversive effects when activated? Can inhibition of a subset of dopamine neurons produce reinforcement?
In addition to these unanswered questions about the functions of the different MOP sensitive circuits and their contribution to reinforcement there are still significant uncertainties about the synaptic mechanisms by which MOP receptors control these circuits. For example, in spite of broad acceptance of the canonical disinhibition model, it is unclear to what degree (if at all) postsynaptic inhibition of VTA GABAergic interneurons by MOP receptors contributes to DA neuron activation. Ex vivo experiments clearly demonstrate not only that MOP receptor activation robustly inhibits GABA terminals that synapse on to dopamine neurons, but that MOP receptors also signal through a direct excitatory effect on these neurons. As predicted by the canonical model, some VTA GABA neurons are hyperpolarized by MOP receptor agonists; however, we do not know whether these are local interneurons connected to dopamine neurons or are projection neurons contributing to dopamine independent reinforcement processes. As of this writing there have been no reported studies of MOP receptor control of VTA glutamate neurons, despite the fact that they project to limbic forebrain areas implicated in reinforcement. Clearly we are at a very early stage in our attempts to parse the contribution of each of these elements to reward and to define the conditions under which each is operative. Fortunately, the recent development of experimental tools (e.g. optogenetics) may provide the requisite level of temporal and anatomical precision necessary to address these questions in a rigorous way.
Glossary
- Drug self-administration
In this paradigm animals are required to perform an operant action (typically a lever press or nose poke) in order to receive an infusion of drug. If rats emit more operant actions for the drug than vehicle, it is evidence that the drug has a positively reinforcing action.
- Conditioned place preference (CPP)
In this paradigm, a three chamber apparatus is most commonly used, where each chamber possesses unique contextual cues. During training, drugs are administered and then the animal is confined for a period in one of the end chambers. In alternate training periods vehicle is administered prior to placing the rat in a different chamber. Animals are later tested in a drug free state by allowing them to roam freely with access to all chambers of the apparatus. If animals spend more time in the drug associated chamber we say the drug produces a CPP.
- Reinforcement
A process that leads to an increase in the probability of an action that was previously followed by a positive outcome. Negative reinforcement refers specifically to the benefit of removing an unpleasant stimulus or state (e.g. pain relief). Positive reinforcement occurs when the benefit does not require relief of an unpleasant state. Punishment refers to the process whereby a harmful outcome reduces the probability of the action preceding the harmful outcome.
- Channelrhodopsin (ChR)
A light activated channel natively expressed in green algae that is now commonly artificially expressed in neurons to enable acute, time-locked experimenter control of neural activity. When open, the channels non-selectively pass cations, including H+, Na+, K+, and Ca2+.
- Mu Opioid Peptide (MOP) receptor
The MOP receptor is a member of the opioid family of 7 transmembrane domain G protein coupled receptors (GPCRs) classified in part by their high amino acid sequence homology. Other members of the family include the delta, kappa, and orphanin receptors. MOP receptors are widely distributed throughout the peripheral and central nervous systems. MOP receptors can signal through a variety of downstream pathways but typically their actions are inhibitory; e.g. to inhibit glutamate or GABA release from terminals or to hyperpolarize neurons through G-protein coupled inwardly rectifying potassium channels (GIRK) [70].
- Ventral Tegmental Area (VTA)
A region in the midbrain that includes dopaminergic neurons of the A10 cell group [127]. It is immediately ventral to the red nucleus, caudal to the hypothalamus and medial to and contiguous with the substantia nigra [128]. The VTA has been divided into five subdivisions (see figure 3 in [129]). There are three midline nuclei: the interfascicular, rostral linear and caudal linear. The two lateral divisions are the parabrachial pigmented and paranigral nuclei which extend laterally from these midline nuclei to the medial lemniscus and the medial edge of the substantia nigra. The original description of the ventral tegmental area of Tsai did not include the midline nuclei (e.g. refs), however, there is general agreement that the catecholaminergic A10 group as originally defined by Dahlstroem & Fuxe [127] includes dopamine neurons in all five of these subnuclei. As of this writing there is no evidence that the cytoarchitectonically described subdivisions of the VTA differ functionally. VTA neurons in each of the subnuclei project widely to several limbic areas implicated in motivation and positive reinforcement [21, 26, 130, 131] (see figure 3 in [128]) and the weight of current evidence supports the idea that the critical organizational principal for grouping VTA neurons is their projection target and neurotransmitter content rather than location within the VTA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fields HL. The doctor's dilemma: opiate analgesics and chronic pain. Neuron. 2011;69:591–594. doi: 10.1016/j.neuron.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall AJ, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 3.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalley JW, et al. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg-Martin ES, et al. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunbridge EM, et al. The role of catechol-O-methyltransferase in reward processing and addiction. CNS Neurol Disord Drug Targets. 2012;11:306–323. doi: 10.2174/187152712800672409. [DOI] [PubMed] [Google Scholar]
- 7.Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting-A-Kee R, van der Kooy D. The neurobiology of opiate motivation. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Merrer J, et al. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology. 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- 11.Hong S. Dopamine system: manager of neural pathways. Frontiers in human neuroscience. 2013;7:854. doi: 10.3389/fnhum.2013.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara CG, et al. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieffer BL. Opioids: first lessons from knockout mice. Trends in pharmacological sciences. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 14.Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behavioral neuroscience. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Mu opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience. 2009;158:474–483. doi: 10.1016/j.neuroscience.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britt MD, Wise RA. Ventral tegmental site of opiate reward: antagonism by a hydrophilic opiate receptor blocker. Brain Res. 1983;258:105–108. doi: 10.1016/0006-8993(83)91232-5. [DOI] [PubMed] [Google Scholar]
- 17.Zangen A, et al. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- 19.David V, Cazala P. Differentiation of intracranial morphine self-administration behavior among five brain regions in mice. Pharmacol Biochem Behav. 1994;48:625–633. doi: 10.1016/0091-3057(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 20.David V, Cazala P. Anatomical and pharmacological specificity of the rewarding effect elicited by microinjections of morphine into the nucleus accumbens of mice. Psychopharmacology (Berl) 2000;150:24–34. doi: 10.1007/s002130000425. [DOI] [PubMed] [Google Scholar]
- 21.Phillips AG, LePiane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav. 1980;12:965–968. doi: 10.1016/0091-3057(80)90460-8. [DOI] [PubMed] [Google Scholar]
- 22.Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- 23.Bals-Kubik R, et al. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. The Journal of pharmacology and experimental therapeutics. 1993;264:489–495. [PubMed] [Google Scholar]
- 24.Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting". J Neurosci. 2014;34:4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields HL, et al. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annual review of neuroscience. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 27.Ornstein K, et al. Biochemical and radioautographic evidence for dopaminergic afferents of the locus coeruleus originating in the ventral tegmental area. J Neural Transm. 1987;70:183–191. doi: 10.1007/BF01253597. [DOI] [PubMed] [Google Scholar]
- 28.Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- 29.Beckstead RM, et al. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- 30.Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1981;1:1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain research bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 32.Ford CP, et al. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis EB, et al. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis EB, et al. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammel S, et al. Unique Properties of Mesoprefrontal Neurons within a Dual Mesocorticolimbic Dopamine System. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Lammel S, et al. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi T, et al. Mesocorticolimbic glutamatergic pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown MT, et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- 40.Greenwell TN, et al. Endomorphin-1 and-2 immunoreactive cells in the hypothalamus are labeled by fluoro-gold injections to the ventral tegmental area. J Comp Neurol. 2002;454:320–328. doi: 10.1002/cne.10464. [DOI] [PubMed] [Google Scholar]
- 41.Sesack SR, Pickel VM. Dual ultrastructural localization of enkephalin and tyrosine hydroxylase immunoreactivity in the rat ventral tegmental area: multiple substrates for opiate-dopamine interactions. J Neurosci. 1992;12:1335–1350. doi: 10.1523/JNEUROSCI.12-04-01335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bausch SB, et al. Colocalization of mu opioid receptors with GIRK1 potassium channels in the rat brain: an immunocytochemical study. Receptors Channels. 1995;3:221–241. [PubMed] [Google Scholar]
- 43.Garzon M, Pickel VM. Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41:311–328. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- 44.Spanagel R, et al. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devine DP, et al. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- 46.Yoshida M, et al. Facilitatory modulation of mesolimbic dopamine neuronal activity by a mu-opioid agonist and nicotine as examined with in vivo microdialysis. Brain research. 1993;624:277–280. doi: 10.1016/0006-8993(93)90087-4. [DOI] [PubMed] [Google Scholar]
- 47.Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann N Y Acad Sci. 1986;473:367–381. doi: 10.1111/j.1749-6632.1986.tb23629.x. [DOI] [PubMed] [Google Scholar]
- 48.Chefer VI, et al. Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice. Eur J Neurosci. 2003;18:1915–1922. doi: 10.1046/j.1460-9568.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- 49.Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- 50.Melis M, et al. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 51.Jalabert M, et al. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A. 2011;108:16446–16450. doi: 10.1073/pnas.1105418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- 53.Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- 54.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiyatkin EA, Rebec GV. Activity of presumed dopamine neurons in the ventral tegmental area during heroin self-administration. Neuroreport. 1997;8:2581–2585. doi: 10.1097/00001756-199707280-00032. [DOI] [PubMed] [Google Scholar]
- 56.Margolis EB, et al. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? The Journal of physiology. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margolis EB, et al. Identification of rat ventral tegmental area GABAergic neurons. PLoS One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen JY, et al. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012 doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hnasko TS, et al. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- 60.Bechara A, et al. Neurobiology of motivation: double dissociation of two motivational mechanisms mediating opiate reward in drug-naive versus drug-dependent animals. Behav Neurosci. 1992;106:798–807. doi: 10.1037//0735-7044.106.5.798. [DOI] [PubMed] [Google Scholar]
- 61.Laviolette SR, et al. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res. 2002;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- 62.Riad-Allen L, van der Kooy D. Social defeat stress switches the neural system mediating benzodiazepine conditioned motivation. Behav Neurosci. 2013;127:515–523. doi: 10.1037/a0032962. [DOI] [PubMed] [Google Scholar]
- 63.Vargas-Perez H, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bechara A, van der Kooy D. A single brain stem substrate mediates the motivational effects of both opiates and food in nondeprived rats but not in deprived rats. Behav Neurosci. 1992;106:351–363. doi: 10.1037//0735-7044.106.2.351. [DOI] [PubMed] [Google Scholar]
- 65.Witten IB, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim KM, et al. Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS One. 2012;7:e33612. doi: 10.1371/journal.pone.0033612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinberg EE, et al. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, et al. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain structure & function. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- 70.Williams JT, et al. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 71.Kelley AE, et al. Interactions between D-ala-met-enkephalin, A10 dopaminergic neurones, and spontaneous behaviour in the rat. Behav Brain Res. 1980;1:3–24. doi: 10.1016/0166-4328(80)90043-1. [DOI] [PubMed] [Google Scholar]
- 72.Nicoll RA, et al. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980;287:22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- 73.Lacey MG, et al. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. The Journal of physiology. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. The Journal of physiology. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chieng B, et al. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol. 2011;589:3775–3787. doi: 10.1113/jphysiol.2011.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Zessen R, et al. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bocklisch C, et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341:1521–1525. doi: 10.1126/science.1237059. [DOI] [PubMed] [Google Scholar]
- 79.Balcita-Pedicino JJ, et al. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsui A, et al. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron. 2014;82:1346–1356. doi: 10.1016/j.neuron.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hjelmstad GO, et al. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J Neurosci. 2013;33:6454–6459. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia Y, et al. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margolis EB, et al. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Margolis EB, et al. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol. 2005;93:3086–3093. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- 86.Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harris GC, et al. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Margolis EB, et al. Direct bidirectional mu-opioid control of midbrain dopamine neurons. J Neurosci. 2014;34:14707–14716. doi: 10.1523/JNEUROSCI.2144-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- 90.Cachope R, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Threlfell S, et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 92.Narita M, et al. Implication of dopaminergic projection from the ventral tegmental area to the anterior cingulate cortex in mu-opioid-induced place preference. Addiction biology. 2010;15:434–447. doi: 10.1111/j.1369-1600.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 93.Lintas A, et al. Identification of a dopamine receptor-mediated opiate reward memory switch in the basolateral amygdala-nucleus accumbens circuit. J Neurosci. 2011;31:11172–11183. doi: 10.1523/JNEUROSCI.1781-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cameron DL, et al. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- 95.Margolis EB, et al. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brischoux F, et al. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ford CP, et al. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Chiara G, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 100.Mitchell JM, et al. Intra-VTA Deltorphin, But Not DPDPE, Induces Place Preference in Ethanol-Drinking Rats: Distinct DOR-1 and DOR-2 Mechanisms Control Ethanol Consumption and Reward. Alcohol Clin Exp Res. 2014;38:195–203. doi: 10.1111/acer.12246. [DOI] [PubMed] [Google Scholar]
- 101.Barak S, et al. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J, et al. Nucleus accumbens-derived glial cell line-derived neurotrophic factor is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. J Neurosci. 2010;30:14502–14512. doi: 10.1523/JNEUROSCI.3909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamilton ME, Freeman AS. Effects of administration of cholecystokinin into the VTA on DA overflow in nucleus accumbens and amygdala of freely moving rats. Brain Res. 1995;688:134–142. doi: 10.1016/0006-8993(95)00518-u. [DOI] [PubMed] [Google Scholar]
- 104.Laitinen K, et al. Neurotensin and cholecystokinin microinjected into the ventral tegmental area modulate microdialysate concentrations of dopamine and metabolites in the posterior nucleus accumbens. Brain Res. 1990;523:342–346. doi: 10.1016/0006-8993(90)91511-e. [DOI] [PubMed] [Google Scholar]
- 105.Pettit HO, Mueller K. Infusions of cholecystokinin octapeptide into the ventral tegmental area potentiate amphetamine conditioned place preferences. Psychopharmacology (Berl) 1989;99:423–426. doi: 10.1007/BF00445571. [DOI] [PubMed] [Google Scholar]
- 106.Devine DP, et al. Mesolimbic dopamine neurotransmission is increased by administration of mu-opioid receptor antagonists. Eur J Pharmacol. 1993;243:55–64. doi: 10.1016/0014-2999(93)90167-g. [DOI] [PubMed] [Google Scholar]
- 107.Shippenberg TS, Bals-Kubik R. Involvement of the mesolimbic dopamine system in mediating the aversive effects of opioid antagonists in the rat. Behav Pharmacol. 1995;6:99–106. [PubMed] [Google Scholar]
- 108.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 109.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 110.Stuber GD, et al. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tecuapetla F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gorelova N, et al. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cereb Cortex. 2012;22:327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Root DH, et al. Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci. 2014 doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tritsch NX, et al. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stamatakis AM, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schalling M, et al. Analysis of expression of cholecystokinin in dopamine cells in the ventral mesencephalon of several species and in humans with schizophrenia. Proc Natl Acad Sci U S A. 1990;87:8427–8431. doi: 10.1073/pnas.87.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Savasta M, et al. The colocalization of cholecystokinin and tyrosine hydroxylase mRNAs in mesencephalic dopaminergic neurons in the rat brain examined by in situ hybridization. Neuroscience. 1989;29:363–369. doi: 10.1016/0306-4522(89)90063-8. [DOI] [PubMed] [Google Scholar]
- 118.Seroogy K, et al. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res. 1989;74:149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- 119.Seroogy K, et al. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Res. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 120.Seroogy KB, Gall CM. Expression of neurotrophins by midbrain dopaminergic neurons. Exp Neurol. 1993;124:119–128. doi: 10.1006/exnr.1993.1182. [DOI] [PubMed] [Google Scholar]
- 121.Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J Comp Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grieder TE, et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci. 2014;17:1751–1758. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamilton ME, et al. Overflow of dopamine and cholecystokinin in rat nucleus accumbens in response to acute drug administration. Synapse. 2000;38:238–242. doi: 10.1002/1098-2396(20001201)38:3<238::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 124.Yetnikoff L, et al. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014;282C:23–48. doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- 126.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol Scand Suppl. 1964;232(SUPPL):231–255. [PubMed] [Google Scholar]
- 128.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 129.Sanchez-Catalan MJ, et al. The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience. 2014;282C:198–216. doi: 10.1016/j.neuroscience.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 130.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol. 1978;180:533–544. doi: 10.1002/cne.901800309. [DOI] [PubMed] [Google Scholar]
- 131.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]