Abstract
Purpose/Objectives
Primary endpoint analysis of RTOG 0247 demonstrated preoperative RT with capecitabine plus oxaliplatin achieved a pCR pre-specified threshold (21%) to merit further study, whereas the RT with capecitabine plus irinotecan arm did not (10%). Secondary efficacy endpoints are reported here.
Methods and Materials
A randomized phase II trial evaluated preoperative RT (50.4 Gy in 1.8 Gy fractions) with two concurrent chemotherapy regimens: 1—capecitabine (1200 mg/m2/d M-F) plus irinotecan (50 mg/m2 /week × 4); and 2—capecitabine (1650 mg/m2/d M-F) plus oxaliplatin (50 mg/m2 /week × 5) for clinical T3 or T4 rectal cancer. Surgery was performed 4-8 weeks following chemoradiation, then 4-6 weeks later, adjuvant chemotherapy (oxaliplatin 85 mg/m2; leucovorin 400 mg/m2; 5FU 400 mg/m2; 5FU 2400 mg/m2) every 2 weeks × 9. Disease-free survival (DFS) and overall survival (OS) were estimated univariately by the Kaplan-Meier method. Local-regional failure (LRF), distant failure (DF), and second primary failure (SP) were estimated by the cumulative incidence method. No statistical comparisons were made between arms as each was evaluated individually.
Results
104 patients (median age 57) were treated; characteristics were similar for both arms. Median follow-up for RT with capecitabine/irinotecan arm was 3.77 years and for RT with capecitabine/oxaliplatin arm was 3.97 years. Four-year DFS, OS, LRF, DF, and SP estimates for capecitabine/irinotecan arm are 68%, 85%, 16%, 24%, and 2%, respectively. The 4-year DFS, OS, LRF, DF, and SP failure estimates for capecitabine/oxaliplatin arm are 62%, 75%, 18%, 30%, and 6%, respectively.
Conclusions
Efficacy results for both arms are similar to other reported studies but suggest that pCR is an unsuitable surrogate for traditional survival metrics of clinical outcome. While it remains uncertain if the addition of a second cytotoxic agent enhances the effectiveness of fluorouracil plus RT, these results suggest further study of irinotecan may be warranted.
Keywords: Rectal cancer, radiation, neoadjuvant, capecitabine, irinotecan, oxaliplatin, preoperative
Introduction
Adenocarcinoma of the rectum is a common disease affecting more than 40,000 new patients per year in the US. 1 The anatomy of the rectum differentiates rectal cancer from colon cancer and confers necessity for tri-modality therapy for locally advanced rectal cancer. Improved outcome of rectal cancer has been achieved over the past several decades by evolution of combined modality therapy and refinements of individual components of therapy--enhanced radiation therapy technology and improved surgical techniques. Whether integration of newer, more active chemotherapy or molecular targeted agents can significantly improve outcome has yet to be determined. Identification of the optimal chemotherapy regimen to combine with neoadjuvant pelvic irradiation has been this subject of extensive clinical investigation. Pathologic complete remission rate (pCR) has been the clinical benchmark by which many trials have sought to answer this question.
RTOG 0247 was a randomized phase 2 study that examined two concurrent neoadjuvant chemotherapy regimens for rectal cancer. 2 This study was designed to determine, on the basis of a predesignated level of pCR, a regimen worthy of further development. The primary endpoint of this study has been previously reported and showed that the capecitabine plus oxaliplatin regimen demonstrated significant clinical activity pCR rate of 21% (10/48 patients) and acceptable toxicity. The capecitabine plus irinotecan arm demonstrated a lower pCR rate of 10% (5/48). Because the value of pCR as a definitive clinical endpoint for neoadjuvant rectal cancer therapy is controversial, traditional survival endpoints and patterns of failure provide useful information as to the efficacy of neoadjuvant therapy regimens. The analyses of secondary efficacy endpoints of RTOG 0247 are reported here.
Methods and Materials
Patient Characteristics
All patients gave written informed consent in accordance with each center's institutional review board guidelines. Eligible patients were at least 18 years of age; had Zubrod performance of 0 to 2; adequate hematologic, renal, cardiac, and hepatic function; potentially resectable adenocarcinoma of the rectum originating at or below 12 cm from the anal verge without evidence of distant metastases; and clinical stage T3, based on endorectal ultrasound, or clinical stage T4, based on endorectal ultrasound or physical exam.
Exclusion criteria included pregnancy or lactation, distant metastasis, synchronous colon carcinomas, anal canal extension, prior chemotherapy or radiation for malignancies, serious uncontrolled concurrent medical or neurologic conditions, clinically significant cardiac disease, major surgery within 4 weeks of study entry, upper gastrointestinal disease that may interfere with drug absorption, or uncontrolled coagulopathy.
Pre-randomization evaluations included a medical history and physical examination, blood counts, serum chemistry and liver function panel, pregnancy testing, chest radiography, CT scan of abdomen and pelvis, and lower endoscopic examination.
Treatment
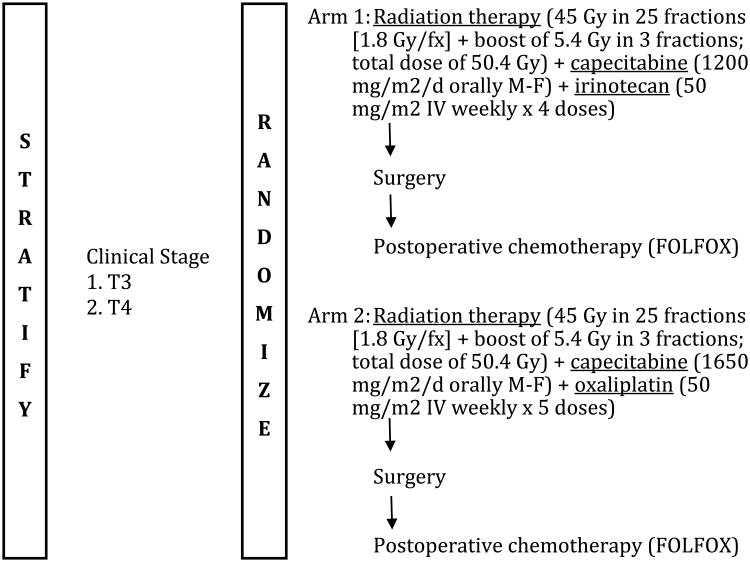
Planned treatment consisted of 1:1 randomization to one of two arms in which preoperative pelvic RT was administered with (1) concurrent capecitabine (1200 mg/m2/d orally Monday-Friday during RT) and irinotecan (50 mg/m2 IV weekly × 4 doses) (arm 1), or (2) concurrent capecitabine (1650 mg/m2/d orally Monday-Friday during RT) and oxaliplatin (50 mg/m2 IV weekly × 5 doses) (arm 2), see Figure 1. These chemotherapy doses represent a modification of the initial study design which demonstrated excessive toxicity in the initial 35 patients treated. Pelvic RT was delivered according to the conformational standards established by RTOG and consisted of 1.8 Gy/fraction, 5 fractions/week, with 45 Gy in 25 fractions plus a boost dose of 5.4 Gy in 3 fractions for a total dose of 50.4 Gy over 5 ½ weeks. Either two-dimensional or three-dimensional delivery was allowed. Surgery was planned for all patients at 4-8 weeks following completion of RT. For both arms, postoperative chemotherapy (FOLFOX) was administered 4-6 weeks after surgery as follows: oxaliplatin 85 mg/ m2 IV over 2 hours (day 1, every 14 days); leucovorin 400 mg/ m2 IV over 2 hours (day 1, every 14 days): 5-FU bolus 400 mg/ m2 IV push (day 1, every 14 days); 5-FU infusion 2400 mg/ m2 IV continuous infusion over 46 hours (beginning day 1, every 14 days).
Figure 1. Schema for Radiation Therapy Oncology Group 0247 phase II study.
Follow-up Evaluations
Patients were evaluated weekly during concurrent chemoRT, prior to surgery, and before each cycle of postoperative chemotherapy. Patients were then followed every 3 months for the first 2 years after completion of therapy; every six months for the next 3 years, and annually thereafter. Follow-up imaging studies were performed at the discretion of the treating physician.
Statistical Considerations
A permuted block randomization method was used to randomize patients to the treatment arms3. As stated earlier, secondary efficacy endpoints of each regimen being evaluated are the focus of this report. All analyses were performed using SAS/STAT® software. Overall survival (OS) failure is death due to any cause. Events for local-regional failure (LRF) are defined as any of the following: no clinical complete response (cCR) in the primary site and/or nodes at any time after treatment completion (persistence), recurrence and/or progression in the primary site and/or nodes after cCR, and non-protocol surgery to the primary site after cCR. Distant failure (DF) is the appearance of any distant metastases. Second/new primary failure (SP) is the appearance of any second primary tumor. Disease-free survival (DFS) events include death, LRF, DF, and SP. OS and DFS were estimated univariately with the Kaplan-Meier method 4 and LRF, DF, and SP rates were estimated by the cumulative incidence method. 5 Patterns of first failure were also evaluated. As each regimen is being evaluated individually, no statistical comparisons are made between the treatment arms.
Results
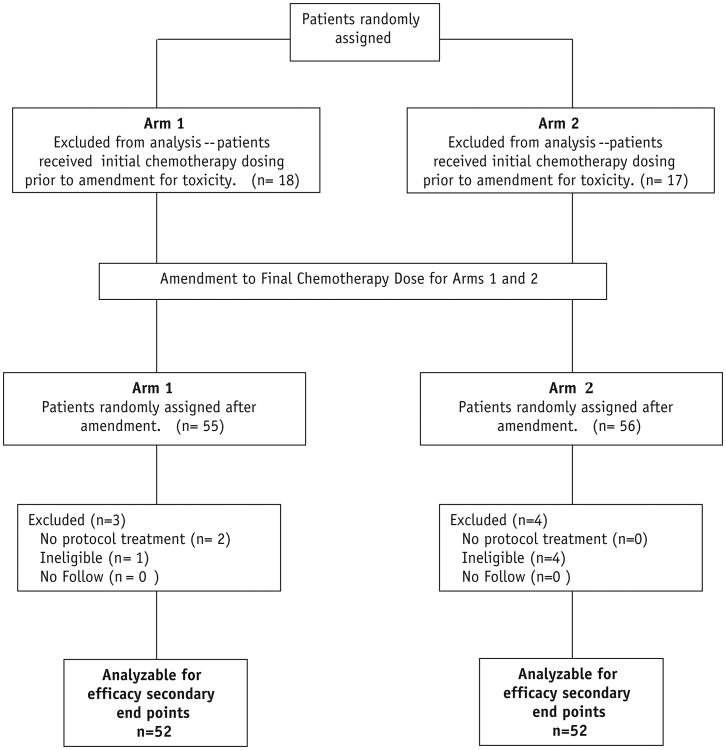
One hundred forty-six patients from 59 institutions were entered on the study from March 2004 to February 2007. In January of 2005, both arms were temporarily closed due to excessive GI adverse events (AE) associated with neoadjuvant therapy. The study reopened with the amended chemotherapy regimen (described above) in April 2005 with a new targeted sample size of 141 patients including the 35 patients previously enrolled. The 35 patients accrued prior to the amendment were not included in the primary endpoint analysis. The data and analysis reported below pertains to the 104 eligible and analyzable patients enrolled following this amendment. Five patients were retrospectively declared ineligible and 2 patients received no protocol therapy. The median follow-up for the irinotecan plus capecitabine arm is 3.77 years (min-max: 0.19-5.23 years) and for the oxaliplatin plus capecitabine arm is 3.97 years (min-max: 0.44-5.15 years). Patient disposition is presented in Figure 2. Patient characteristics were similar for both arms, as shown in Table 1. Compliance to radiation therapy and to chemotherapy was acceptable and similar in both arms: 2% unacceptable deviations in RT in each arm; significant deviations in neoadjuvant chemotherapy were ∼10% in both arms; protocol deviations to adjuvant chemotherapy was 30% in each arm. Surgical compliance was similar in each arm—including time from completion of chemoRT to surgery.
Figure 2. CONSORT patient flow diagram.
Table 1. Patient Characteristics.
| Capecitabine/Irinotecan/RT (n=52) | Capecitabine/Oxaliplatin/RT (n=52) | |
|---|---|---|
| Age (years) | ||
| Median | 57 | 56 |
| Min - Max | 27 - 78 | 30 - 76 |
|
| ||
| Gender | ||
| Male | 33 ( 63.5%) | 38 ( 73.1%) |
| Female | 19 ( 36.5%) | 14 ( 26.9%) |
|
| ||
| Race | ||
| American Indian or Alaskan native | 0 ( 0.0%) | 1 ( 1.9%) |
| Asian | 2 ( 3.8%) | 0 ( 0.0%) |
| Black or African-American | 4 ( 7.7%) | 6 ( 11.5%) |
| White | 46 ( 88.5%) | 45 ( 86.5%) |
|
| ||
| Ethnicity | ||
| Hispanic or Latino | 3 ( 5.8%) | 0 ( 0.0%) |
| Not Hispanic or Latino | 48 ( 92.3%) | 49 ( 94.2%) |
| Unknown | 1 ( 1.9%) | 3 ( 5.8%) |
|
| ||
| Zubrod performance status | ||
| 0 | 45 ( 86.5%) | 36 ( 69.2%) |
| 1 | 7 ( 13.5%) | 16 ( 30.8%) |
|
| ||
| Distance of tumor from anal verge (cm) | ||
| ≤ 6cm | 30 ( 57.7%) | 30 ( 57.7%) |
| 7-12 cm | 22 ( 42.3%) | 22 ( 42.3%) |
|
| ||
| T-stage | ||
| T3 | 46 ( 88.5%) | 45 ( 86.5%) |
| T4 | 6 ( 11.5%) | 7 ( 13.5%) |
|
| ||
| N-stage | ||
| N0 | 23 ( 44.2%) | 25 ( 48.1%) |
| N1 | 23 ( 44.2%) | 24 ( 46.2%) |
| N2 | 2 ( 3.8%) | 0 ( 0.0%) |
| NX | 4 ( 7.7%) | 3 ( 5.8%) |
|
| ||
| AJCC stage (6th edition) | ||
| Stage IIA | 20 ( 38.5%) | 21 ( 40.4%) |
| Stage IIB | 3 ( 5.8%) | 4 ( 7.7%) |
| Stage IIIB | 23 ( 44.2%) | 24 ( 46.2%) |
| Stage IIIC | 2 ( 3.8%) | 0 ( 0.0%) |
| Unknown | 4 ( 7.7%) | 3 ( 5.8%) |
Survival and Patterns of Failure
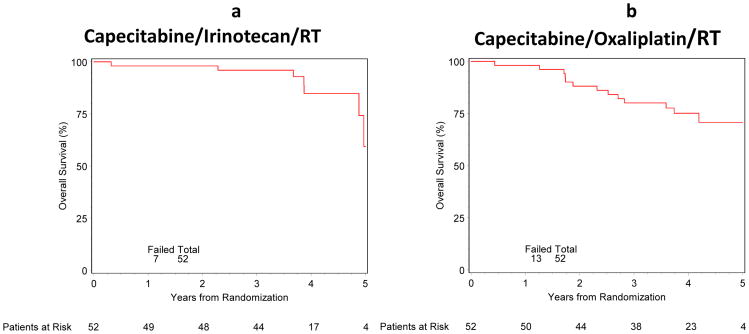
Survival and patterns of failure are shown in Table 2. Disease free survival and overall survival curves are shown in Figures 3a,b and 4a,b. At 4 years, for the irinotecan plus capecitabine arm, DFS, OS, LRF, DF, and SP were 68% (95% CI: 52%- 80%), 85% (95% CI: 66%- 94%), 16% (95% CI: 6%- 27%), 24% (95% CI: 11%- 38%), and 2% (95% CI: 0%- 6%), respectively. At 4 years, for the oxaliplatin plus capecitabine arm, DFS, OS, LRF, DF, and SP were 62% (95% CI: 47%- 74%), 75% (95% CI: 60%- 85%), 18% (95% CI: 7%- 28%), 30% (95% CI: 17%- 43%), and 6% (95% CI: 0%- 13%), respectively. Patterns of first failure were also examined and are shown in Table 3. Salvage therapy is described for patients on the irinotecan arm: of those with LRF, 4 (50%) got additional chemo and/or RT, 5 (62.5%) had metastasis; 4 (50%) have died on Arm 1. Salvage therapy is also described for the oxaliplatin arm: of those with LRF, 8 (88.9%) got additional chemo and/or RT on Arm 2, 8 (88.9 %) had metastasis/2nd primary on Arm 2; 6 (66.7%) have died on Arm 2.
Table 2. Survival and failure patterns.
| Endpoint | Capecitabine/Irinotecan/RT (n=52) | Capecitabine/Oxaliplatin/RT (n=52) | ||
|---|---|---|---|---|
| TF | 4y% (95% CI) | TF | 4y% (95% CI) | |
| Overall Survival | 7 | 85 (66, 94) | 13 | 75 (60, 85) |
|
| ||||
| Local-Regional Failure | 8 | 16 (6, 27) | 9 | 18 (7, 28) |
|
| ||||
| Distant Metastases Failure | 12 | 24 (11, 38) | 17 | 30 (17, 43) |
|
| ||||
| Second Primary Failure | 1 | 2 (0, 6) | 4 | 6 (0, 13) |
|
| ||||
| Disease-Free Survival | 15 | 68 (52, 80) | 22 | 62 (47, 74) |
Abbreviations: TF = total failures; 4y% = estimated 4-year survival/failure rate; 95% CI = 95% confidence interval
Figure 3. a and b. Overall Survival.
Figure 4. a and b. Disease-Free Survival.
Table 3. Patterns of first failure.
| Site | Capecitabine/Irinotecan/RT (n=52) | Capecitabine/Oxaliplatin/RT (n=52) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Local Only | 1 | 1.9 | 5 | 9.6 |
|
| ||||
| Regional Only | 2 | 3.8 | 0 | 0.0 |
|
| ||||
| Distant Only | 10 | 19.2 | 12 | 23.1 |
|
| ||||
| Second Primary Only | 0 | 0.0 | 2 | 3.8 |
|
| ||||
| Local and Regional | 2 | 3.8 | 1 | 1.9 |
|
| ||||
| Regional and Distant | 0 | 0.0 | 1 | 1.9 |
|
| ||||
| Death Only | 0 | 0.0 | 1 | 1.9 |
|
| ||||
| No Failure | 37 | 71.2 | 30 | 57.7 |
Summary of overall worst adverse event per patient is shown in Table 4. Gastrointestinal and metabolic toxicity were the most common causes of grade ≥ 3 adverse events before surgery and after surgery/prior to chemotherapy. Hematologic toxicity was the primary cause of ≥ 3 adverse events during post operative chemotherapy. No deaths occurred during treatment. During the 60 day period following completion of protocol treatment the mortality rate from any cause was 2% (one patient from each arm— both were deemed unrelated to treatment).
Table 4. Summary of Worst Overall Adverse Events per Patient Definitely, Probably, or Possibly Related to treatment.
| Capecitabine/Irinotecan/RT | Capecitabine/ Oxaliplatin/RT | ||||
|---|---|---|---|---|---|
| Timing | Grade | n | % | n | % |
| (n=52) | (n=52) | ||||
| Occurring Prior to Surgery | 1 | 12 | 23.1 | 9 | 17.3 |
| 2 | 25 | 48.1 | 29 | 55.8 | |
| 3 | 14 | 26.9 | 12 | 23.1 | |
| 4 | 0 | 0.0 | 2 | 3.8 | |
| 5 | 0 | 0.0 | 0 | 0.0 | |
|
| |||||
| (n=47*) | (n=51*) | ||||
| Occurring Post Surgery† | 1 | 4 | 8.5 | 9 | 17.6 |
| 2 | 16 | 34.0 | 14 | 27.5 | |
| 3 | 8 | 17.0 | 9 | 17.6 | |
| 4 | 1 | 2.1 | 1 | 2.0 | |
| 5 | 0 | 0.0 | 0 | 0.0 | |
|
| |||||
| (n=52) | (n=52) | ||||
| Occurring Anytime | 1 | 1 | 1.9 | 1 | 1.9 |
| 2 | 13 | 25.0 | 15 | 28.8 | |
| 3 | 31 | 59.6 | 31 | 59.6 | |
| 4 | 7 | 13.5 | 5 | 9.6 | |
| 5 | 0 | 0.0 | 0 | 0.0 | |
AEs occurring after surgery and prior to chemotherapy or within 60 days of surgery for patients who did not have post-operative chemotherapy
Patients who had surgery.
Discussion
The optimal selection of chemotherapy drugs for neoadjuvant chemoradiation for rectal cancer has yet to be determined. Recent clinical trials have focused on intensification of conventional chemotherapy in an attempt to improve the efficacy of therapy. This is exemplified by numerous phase 2 and 3 clinical trials that have examined the addition of a second cytotoxic drug to a fluorouracil backbone. 6-11 A logical biologic rationale for combining two cytotoxic agents can be postulated: addition of a second active systemic agent should enhance cytotoxicity and radiosensization that should translate into improved clearance of the primary tumor and, ultimately, improved resectability and locoregional control. Many neoadjuvant rectal cancer trials have therefore selected pCR as the primary clinical endpoint to gauge clinical activity. Whether this strategy is effective has not yet been proven. RTOG 0247 was predicated upon these assertions and was initiated to examine the efficacy of two separate combinations of neoadjuvant cytotoxic doublet regimens with agents active against metastatic disease.
The previously reported, primary endpoint results of RTOG 0247 demonstrated that capecitabine plus oxaliplatin achieved a predetermined pCR level (21%) sufficient to warrant further clinical study, whereas capecitabine plus irinotecan did not (10%). In the present analysis it is shown that both regimens demonstrate efficacy, as determined by survival and failure patterns, within a level that is acceptable and within an expected range based upon published data using neoadjuvant chemoradiation. This study was not designed to permit direct statistical comparisons between the two treatment arms. Thus the contradictory observation of overall survival that appears to favor the irinotecan arm cannot be substantiated and may be due to chance. The total cumulative dose of capecitabine was 37.5% higher in the oxaliplatin arm compared to the irinotecan arm, hence fluorouracil dose intensity is not likely to be a factor in favoring the efficacy of one regimen over another. We conducted exploratory analyses to identify clues for differences in survival such as ypT, ypN, and survival differences among pCR and non-pCR patients; these analyses failed to demonstrate useful signals of causality although this was limited by small number of events. A consensus from other groups, based upon pCR as a primary endpoint in early phase study results, have generally concluded that oxaliplatin is more active than irinotecan in this setting. If in fact the contrary is true, this begs the question as to whether pCR is a reasonable measure of clinical efficacy. It is also of interest to speculate why secondary efficacy endpoints from this trial appear similar between the two arms while pCR rates appeared to disparately favor the capecitabine plus oxaliplatin arm. The results of other recently reported studies shed some light as to a possible explanation for these observations.
The NSABP R04 study, initiated after RTOG 0247, was a phase 3, four arm study of 5-FU versus capecitabine plus or minus oxaliplatin and concurrent pelvic irradiation for stage 2 and 3 rectal cancer. 12 The study was designed to determine superiority of efficacy as determined by pCR. The results of the study showed no difference in pCR with or without the addition of oxaliplatin. In addition, neither sphincter preservation nor surgical downstaging was improved by oxaliplatin and no difference was observed between 5-FU versus capecitabine. The conclusion drawn from this trial is that oxaliplatin does not enhance the clinical effectiveness of fluorouracil as measured by pCR. The ACCORD 12/0405- Prodige 2 study (preoperative pelvic radiation plus concurrent capecitabine with or without oxaliplatin) also failed to demonstrate statistically significant improvement in pCR by the addition of oxaliplatin. 11
A number of studies have examined prognostic factors associated with neoadjuvant chemoradiation for rectal cancer—in particular, pCR. A recent meta-analysis of the FFDC and the EORTC studies examined correlation between pCR and survival endpoints and showed no correlation—strongly suggesting that pCR is an invalid study endpoint for this setting. 13 Multivariate analysis of the ACCORD 12 study showed that the Dworak tumor regression grade score significantly correlated with DFS suggesting that this may be explored as a useful study endpoint in future trials.11 These observations are in agreement with a recent analysis be Fokas et al demonstrating that, in multivariate analysis, residual lymph node status (ypN+) and tumor regression score were the only prognostic factors associated with disease free survival.14 Secondary efficacy endpoints of RTOG 0247 are also consistent with the findings of the above analyses suggesting that pCR rate does not adequately predict longer-term outcomes. Future reporting of long term results of NSABP R04 may permit definitive conclusions on this subject.
It is possible that the addition of oxaliplatin to concurrent fluorouracil with pelvic radiation for rectal cancer simply has no effect in enhancing efficacy of therapy. Revelation of this possible conclusion raises troubling questions as to how, other than dose intensification, to improve the overall effectiveness of combined modality therapy for rectal cancer. Integration biologic agents, such as cetuximab as in the EXPERT-C study, and/or enrichment favorable patient cohorts with molecular genetic testing may be a promising future approaches. 15 Retrospective analyses from the EXPERT-C study demonstrate potential utility of RAS mutations and TP53 as predictive biomarkers for beneficial effects of cetuximab.16,17 Analyses such as this are relevant to the design of future studies that test whether molecular profiling can be utilized to improve outcome of rectal cancer patients.
In summary, the efficacy results of RTOG 0247 support the possibility, particularly in the context of data from other large randomized studies, that pCR is an insensitive surrogate for traditional survival metrics of clinical outcome. This trial's efficacy results beyond pCR raise the question as to whether the efficacy of irinotecan has been underestimated, such that further studies to address this may be warranted. Additionally, the need for approaches, other than dose intensification, to improve the overall effectiveness of combined modality therapy for rectal cancer requires continual investigation. Integration of biologic agents, such as cetuximab in the EXPERT-C study, and/or enriched patient cohorts with molecular genetic testing may be promising future approaches.13
RTOG 0247 was a clinical trial of neoadjuvant chemoradiation for T3, T4 rectal cancer that demonstrated preoperative RT with capecitabine plus oxaliplatin yielded a pCR pre-specified threshold (21%) to merit further study, whereas the RT with capecitabine plus irinotecan did not (10%). We report long term outcome endpoints and demonstrate similar efficacy outcome for both arms suggesting that pathologic complete remission is an unsuitable surrogate for traditional survival metrics of clinical outcome.
Acknowledgments
“This project was supported by RTOG grant U10 CA21661, and CCOP grant U10 CA37422 from the National Cancer Institute (NCI).”
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wong SJ, Winter K, Meropol NJ, et al. Radiation therapy oncology group 0247: A randomized phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelen M. The randomization and stratification of patients to clinical trials. J Chron Dis. 1994;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. Journal of the American Statistical Association. 1958;53:457. [Google Scholar]
- 5.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 1980:167–169. [Google Scholar]
- 6.Rodel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol. 2003;21(16):3098–3104. doi: 10.1200/JCO.2003.02.505. [DOI] [PubMed] [Google Scholar]
- 7.Aschele C, Friso ML, Pucciarelli S, et al. A phase I-II study of weekly oxaliplatin, 5-fluorouracil continuous infusion and preoperative radiotherapy in locally advanced rectal cancer. Ann Oncol. 2005;16(7):1140–1146. doi: 10.1093/annonc/mdi212. [DOI] [PubMed] [Google Scholar]
- 8.Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation therapy oncology group trial 0012. J Clin Oncol. 2006;24(4):650–655. doi: 10.1200/JCO.2005.03.6095. [DOI] [PubMed] [Google Scholar]
- 9.Cancer and Leukemia Group B 89901. Ryan DP, Niedzwiecki D, et al. Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and leukemia group B 89901. J Clin Oncol. 2006;24(16):2557–2562. doi: 10.1200/JCO.2006.05.6754. [DOI] [PubMed] [Google Scholar]
- 10.Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU)-based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer: Pathologic response analysis of the studio terapia adiuvante retto (STAR)-01 randomized phase III trial. J Clin Oncol. 2009;27(170s) [Google Scholar]
- 11.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Clinical Outcome of the ACCORD 12/0405-PRODIGE 2 Randomized Trial in Rectal Cancer. J Clin Oncol. 2012;30(36):4558–4565. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 12.O'Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32:1927–1934. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnetain F, Bosset JF, Gerard JP, et al. What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: Surrogacy in question? Eur J Cancer. 2012;48(12):1781–1790. doi: 10.1016/j.ejca.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Siegel R, Ghadimi M, Mrak K, Merkel S, Raab HR, Sauer R, Wittekind C, Rödel C. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014 May 20;32(15):1534–6. doi: 10.1200/JCO.2013.54.3769. [DOI] [PubMed] [Google Scholar]
- 15.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30(14):1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 16.Sclafani F, Gonzalez D, Cunningham D, et al. RAS mutations and cetuximab in locally advanced rectal cancer: Results of the EXPERT-C trial. Eur J Cancer. 2014;50:1430–1436. doi: 10.1016/j.ejca.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Sclafani F, Gonzalez D, Cunningham D, et al. TP53 mutational status and cetuximab benefit in rectal cancer: 5-year results of the EXPERT-C trial. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju121. Print 2014 Jul. [DOI] [PubMed] [Google Scholar]