Highlights
-
•
fMRI research with infants and toddlers has increased rapidly over the past decade.
-
•
Infant fMRI has provided unique insight into early functional brain development.
-
•
Complex methodological challenges associated with infant fMRI warrant careful consideration and ongoing research.
-
•
Infant fMRI has potential to contribute to multiple fields of study.
-
•
The study of early life stress is a prime example of a field that is ripe to benefit from this technique.
Keywords: Infancy, Natural sleep fMRI, Resting state functional connectivity MRI, Developmental neuroimaging, Early life stress, Developmental neuroscience
Abstract
Functional magnetic resonance imaging (fMRI) research with infants and toddlers has increased rapidly over the past decade, and provided a unique window into early brain development. In the current report, we review the state of the literature, which has established the feasibility and utility of task-based fMRI and resting state functional connectivity MRI (rs-fcMRI) during early periods of brain maturation. These methodologies have been successfully applied beginning in the neonatal period to increase understanding of how the brain both responds to environmental stimuli, and becomes organized into large-scale functional systems that support complex behaviors. We discuss the methodological challenges posed by this promising area of research. We also highlight that despite these challenges, early work indicates a strong potential for these methods to influence multiple research domains. As an example, we focus on the study of early life stress and its influence on brain development and mental health outcomes. We illustrate the promise of these methodologies for building on, and making important contributions to, the existing literature in this field.
1. The emerging field of fMRI with infants and toddlers
Functional magnetic resonance imaging (fMRI) has become a widely utilized tool to characterize brain function across multiple fields of inquiry, including cognitive neuroscience, developmental and clinical psychology, and medical science (Biswal et al., 2010, Lee et al., 2013, Luna et al., 2010, Mather et al., 2013, Matthews et al., 2006, Uddin et al., 2010). This methodology has provided a means of non-invasively examining how brain systems efficiently process and organize information. The use of this tool during infancy and toddlerhood to characterize brain function is a more recent, yet quickly expanding application of fMRI. In the current report, we provide a critical review of the current state of the literature employing fMRI with infants and toddlers during natural sleep. We include the pros and cons of utilizing task-based fMRI, as well as the benefits and considerations of using resting state functional connectivity MRI (rs-fcMRI) to study early brain development. We argue that the research to date demonstrates the feasibility of conducting both forms of fMRI with infants and toddlers. The utility of these methods for increasing our understanding of early periods of brain development will be discussed.
We conclude our report by providing an example of an area of research in which task-based and rs-fcMRI in early development has potential to build on the existing literature and make a unique contribution. We focus on the study of early life stress (ELS) and its influence on mental health outcomes across the lifespan. We argue that the research to date employing task-based fMRI and rs-fcMRI with infants and toddlers indicates the potential for these methods to build on the existing, rich body of human and animal literature in this area by providing insight into the influence of ELS on early developing functional brain systems. We highlight several specific ways in which infant fMRI can build on existing work in this area and make unique contributions.
1.1. A brief history of and introduction to infant fMRI
The use of fMRI methods with infants and toddlers in research settings is a relatively recent phenomenon. While the earliest studies in the field were conducted over a decade ago (Anderson et al., 2001, Dehaene-Lambertz et al., 2002), only recently has infant functional imaging begun to show marked growth across institutions. The reasons for this delay relative to the use of fMRI in other special populations are several. Collecting high quality MR images typically requires a participant to remain still throughout one or more scans, lasting 4–8 min on average. In childhood (including children as young as 4 years-of-age [Gabard-Durnam et al., 2014, Tottenham et al., 2012]) and beyond, this is accomplished by providing instructions, practice and incentives. For infants, this is not an option. Early on this barrier was overcome with the use of sedation (Souweidane et al., 1999, Yamada et al., 1997), which relegated scanning to clinical settings, and likely produced blunted functional responses (Qiu et al., 2008). Several researchers then pioneered the technique of conducting fMRI scans with infants during natural sleep (Anderson et al., 2001, Dehaene-Lambertz et al., 2002, Redcay et al., 2007). The success of studies utilizing the natural sleep method has led to increasing utilization of fMRI with infants in research contexts, resulting in over twenty currently published studies (Table 1, Table 2). Despite this success, many important methodological issues require careful consideration, including the effects of sleep on brain signaling, and are discussed in Section 1.5.
Table 1.
Review of functional activation studies with infants and toddlers during natural sleep.
| Article | Population | No of analyses | No of excluded | Stimuli | Motion | Atlas | Statistical threshold | Main findings |
|---|---|---|---|---|---|---|---|---|
| Anderson et al. (2001) | Healthy term and preterm infants (M = 21 days), adult males (M = 34 yrs) | 9 Term, 5 Preterm, 4 Adults | 6 infants for motion or not enough images | Tone 60–80 dB (gradually increased and decreased to prevent startle) | Frame removal: images with >2 mm or 3 degrees; Scans excluded: <50% of images retained or SD >1 for translation or rotation | NA | % Signal change | (1) BOLD signal decrease to auditory for 9 and increase for 5 infants; (2) Signal change in B superior temporal regions |
| Blasi et al. (2011) | Healthy infants (M = 159 days) | 21 | 24 sleep difficulties | Nonvocal (environmental); Nonspeech vocalizations (Neutral, Happy, Sad) | Rigid body transform based on spin-history correction | Infant DL and transformed to Tal | p < 0.005 uncorrected, cluster size ≥3 voxels | (1) Age + associated with L STG activity to Neutral > Nonvoice; (2) L Insula and gyrus rectus activity for Sad > Neutral |
| Dehaene-Lambertz et al. (2002) | Healthy infants (M = 79 days) | 20 (6 awake, 5 asleep, 9 both) | 6 fussiness; 5 artifact or problems with experiment | Forward speech (children's stories); Backward speech (reversed forward) | Frame removal: visual examination | Infant DL and transformed to MNI | Voxel p < 0.01, cluster p < 0.05 corrected for multiple comparisons | (1) All sounds > rest: L STG; (2) Forward > Backward: L angular gyrus and mesial parietal lobe; (3) Forward > Backward for Awake > Asleep: R PFC |
| Dehaene-Lambertz et al. (2010) | Healthy infants (M = 72 days) | 7 (1 awake, 2 asleep, 4 both) | 6 fussiness; 11 no activation to sound > rest | Classical music; Mother's speech; Stranger's speech; For all-Repeated and Varied | Frame removal: visual examination; Adjusted analysis to limit influence of large deviation in signal (≥2.5 SD) | Infant DL | Random-effects: voxel p < 0.01, cluster p < 0.05 corrected; Fixed-effects: voxel p < 0.001, cluster p < 0.05 corrected | (1) Repetition Suppression Effect: L STG; (2) Laterality effect speech > music in L planum temporale for music > speech in R planum temporale; (3) Mother > stranger in B anterior PFC, L posterior temporal and rest > mother in R amygdala, R insula, R STS, R occipital sulcus |
| Eyler et al. (2012) | Typically developing (TD) children (12–48 mo, M = 32.0 mo); Children with current or subsequently confirmed ASD diagnosis (12–48 mo, M = 25.6 mo) | 80 (40 TD, 40 ASD) | 10 TD and 12 ASD waking before 2/3 scan complete; 5 ASD not confirmed; 4 various reasons | Simple forward speech; complex forward speech; backward speech (Redcay et al., 2008) | AFNI software for motion correction; Subject removal: visually apparent residual motion on >1/3 of images (none excluded); Covary for residual motion in analyses | Tal | p < .05 Monte Carlo correction for multiple comparisons (t > 2.86 for within group; t > 2.42 for a one-tailed between-group, at least 32 voxels) | (1) Each type of speech stimuli > rest, TD > ASD: L STG; (2) In ASD group, L STG activation to simple forward > rest is negatively correlated with age; (3) In TD group, left laterality to forward speech in STG; (4) In ASD group, right laterality to forward speech in anterior STG |
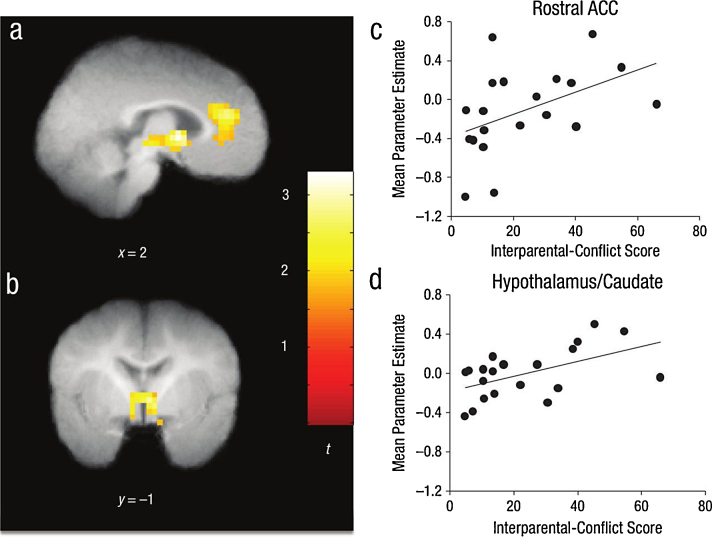
| Graham et al. (2013) | Healthy infants from families reporting a range of interparental conflict (M = 8.33 mo) | 20 | 15 for motion or sleep difficulties; 4 no activation to sound > rest | Nonsense speech with varying prosody (Very Angry, Angry, Happy and Neutral) | Frame removal: visual examination; Maximum motion remaining = 1.07 mm | Infant 8–11 MRI NBD | Voxel p < 0.05, cluster p < 0.05 corrected for multiple comparisons | (1) Higher conflict associated with very angry > neutral in rostral anterior cingulate cortex, hypothalamus, thalamus and caudate; (2) Emotion controlling for conflict: Very Angry > Neutral: L temporal Pole; Angry > Neutral: None; Happy > Neutral: lingual gyrus, fusiform, parahippocampal gyrus, putamen, midcingulate, SMA, SFG, MFG |
| Redcay and Courchesne (2008) | Children with provisional ASD (M = 34.9 mo), age- (CA; M = 35.7 mo), and mental age (MA; M = 19.6 mo) controls | 13 ASD, 12 CA, 11 MA | 8 sleep difficulties; 1 motion; 2 did not meet criteria for ASD | Simple forward speech; complex forward speech; backward speech | Frame removal: Images with sum of root mean square of parameters >0.4 | Tal | p < .01, corrected at 960 mm3; Trend level p < .05, corrected at 384 mm3 | (1) Forward > rest, both MA > ASD and CA > ASD: frontal, temporal, parietal, occipital regions and cerebellum; (2) Forward > rest for ASD > CA: right hemisphere activation; (3) Receptive language + correlated with R frontal and temporal region activation in ASD group |
| Redcay et al. (2007) | Healthy children (M = 45.8 mo) | 19 (12 with visual, 13 with auditory) | Auditory: 2 for motion and 6 for waking; Visual: 9 for waking | Vocal (nonspeech); Nonvocal (environmental); Tones; Flashing lights | Frame removal: Images with sum of root mean square of parameters >0.4; Scans excluded: >10% of images lost | Tal | p < .005, cluster corrected at 740 mm3 | (1) Nonvocal > vocal: frontal, temporal (R STG), occipital (B lingual gyrus) and cerebellum; (2) Tones > Vocal: frontal, temporal (R STG), and parietal and cerebellum; (3) Rest < visual: B cuneus, B lingual gyrus, L superior occipital gyrus |
| Redcay et al. (2008) | Healthy toddlers (M = 21 mo) and 3-yo's (M = 39 mo) | 10 toddlers; 10 3-yo's | 5 sleep difficulties; 1 experimenter error; 2 did not attend | Simple forward speech; complex forward speech; backward speech | Summed distance of translational and rotational parameters >0.3 | Tal | Voxel p < .01, cluster p < .05 (cluster volume = 960 mm3); Relaxed for certain contrasts to voxel p < .05 | (1) Forward > rest for 3-yo's > toddler: B STG, frontal, parietal and occipital regions; (2) Forward > rest for toddler > 3-yo's: Frontal, parietal, occipital and subcortical regions (no temporal regions) |
Note. Studies with sedation not included. All experiments conducted during natural sleep with the exception of Dehaene-Lambertz et al. (2002), Dehaene-Lambertz et al. (2010), in which infants were scanned awake and during natural sleep. Atlas abbreviations: Infant DL = Template created by Dehaene-Lambertz et al. (2002); Infant 8–11 MRI NBD = 8–11 month version of atlas from MRI Study of Normal Brain Development (Fonov et al., 2009, Fonov et al., 2011); Tal = Talairach & Tournoux. Other abbreviations: wks = weeks; mo = months; yrs = years; yo's = year olds; B = Bilateral; L = left; R = right; STG = superior temporal gyrus; SFG = superior frontal gyrus; MFG = middle frontal gyrus; SMA = supplementary motor areas; ASD = Autism Spectrum Disorder.
Table 2.
Review of resting state functional connectivity studies with infants and toddlers during natural sleep.
| Article | Population | No of analyses | No of excluded | Analysis type | Motion | Atlas | Statistical threshold | Findings |
|---|---|---|---|---|---|---|---|---|
| Alcauter et al. (2013) | Healthy singleton and twin (only one twin per pair) neonates (M = 33 days), 1-yo's (M = 397 days), 2-yo's (M = 762 days); Longitudinal ≥2 time points | 112 neonates, 129 1-yo's, 92 2-yo's | No information provided, data identified from previous studies | Insula segmentation based on adult atlas; K means clustering with correlation matrix of insula voxels; Whole brain seed-based correlation with insula clusters; ROIs based on 2 yo's whole brain connectivity map; Graph theory metrics | Frame removal: DVARs <.5% signal change and FD <.5 mm | Individual Infant and then MNI | For cluster confirmation: validity indicator and cluster consensus measures, bootstrapping method (% of consistent cluster membership for each voxel based on 1000 samplings); p < .05, FDR corrected for tests with connectivity maps | (1) Two clusters for all ages with high consistency of voxel assignment to anterior or posterior based on bootstrapping (range of consistency = 74–95% for all insula voxels and each age group); (2) Dissociable whole brain connectivity for clusters across ages: AI connectivity with ACC, medial temporal lobe, thalamus, orbitofrontal cortex, dlPFC; PI connectivity with superior temporal sulcus, middle cingulate, motor and somatosensory cortices; (3) Change with age: AI = decreased local and increased long range; PI = increases and decreases for local and long range; greatest change 0–1 yo for mean connectivity strength and graph theory metrics |
| Alcauter et al. (2014) | See Alcauter et al. (2013) | See Alcauter et al. (2013) | See Alcauter et al. (2013) | Adult ROIs (Smith et al., 2009) to identify 9 cortical networks; Non-overlapping network masks based on “winner-takes-all”; Thalamus ROI from Harvard-Oxford atlas; Thalamus subdivided by partial correlations of voxels with network masks and “winner-takes-all”; Longitudinal analysis with mixed-effect regression models | Frame removal: DVARs <.5% signal change and FD <.5 mm; Remaining FD as covariate; Subject removal: ≤90 frames remaining | Individual Infant and then MNI | p < .05 FDR for whole brain seed-based connectivity maps; r ≥ .1 to create masks of cortical networks | (1) In neonates, sensorimotor network connectivity to large central thalamic cluster and salience networks to anterior thalamus; (2) In 1-yo's, medial-visual network connectivity to posterior lateral thalamus and default to central posterior thalamus; (3) 2-yo's show similar thalamic parcellations to 1-yo's; (4) 1-yo's thalamus-salience connectivity predicted visual-spatial working memory and Mullen Early Learning Composite Score at 2 yrs |
| Dinstein et al. (2011) | Toddlers with autism (M = 29 mo), language delay (LD; M = 19 mo), and typically developing (M = 28 mo) | 29 with autism, 13 with LD, 30 typical | No information provided, data from previous studies | Regressed out stimuli; Anatomically defined ROIs; Whole brain seed-based correlations | Frame removal: criteria not specified | Tal | r > .3 for whole brain seed correlations; Two-tailed t-test p < .05 for group differences | (1) Weaker interhemispheric connectivity for IFG and STG in autism; (2) Autism classification based on connectivity: 21/29 correctly and 7/43 incorrectly identified; (3) IFG connectivity +associated with expressive language and − associated with autism severity |
| Fransson et al. (2009) | Healthy infants delivered by cesarean (M = 40 wks GA) | 19 | 2 for motion | Probabilistic approach to ICA (PICA) | Frame removal: criteria not specified; Scans excluded: criteria not specified | Infant DL | p < .05 (activation versus null across whole brain and time series) | (1) 6 networks identified (% variance explained): medial occipital (1.63%), B sensorimotor (3.18%), B temporal (0.70%), parietal (4.78%), anterior PFC (1.60%), B basal ganglia (0.1%); (2) PCC/precuneus to bilateral parietal connectivity observed |
| Fransson et al. (2011) | Healthy infants from Fransson et al. (2009); Healthy adults (M = 29 yrs) | 18 Infants, 18 Adults | See Fransson et al. (2009) | Voxel-based graph theoretical analysis; Whole brain seed-based correlations of hub regions | For infants see Fransson et al. (2009) | Neonatal (Kazemi et al., 2007) | Peak Z-values >15 mm apart for hubs; p < .0005 for seed-based connectivity; Networks at 0.20 < r < 0.40, iteratively | (1) Infants: hubs and networks in sensory and motor cortices except for DLPFC, insula and parietal lobule; (2) Adults: hubs and networks in heteromodal cortex especially in default and frontoparietal attention networks; (3) Small-world network organization in infants |
| Fransson et al. (2013)* | Healthy infants from Fransson et al. (2009); Healthy adults (M = 29 yrs) | 18 Infants, 17 adults | See Fransson et al. (2009) | Spherical ROIs based on adult and infant atlas coordinates; Power analysis | For infants see Fransson et al. (2009) | Neonatal (Kazemi et al., 2007) | NA | (1) Infants > adults for average power-law exponent; (2) For adults power-law exponents higher in associative networks and for infants higher in primary sensory networks |
| Gao et al. (2009) | Healthy neonates (M = 24 days), 1-yo's (M = 13 mo), 2-yo's (M = 25 mo) and adults (M = 30 yrs) | 20 neonates, 24 1-yo's, 27 2-yo's, 15 adults | 22 for motion or medical problem (e.g. preterm birth) | ICA; graph theory | Frame removal: Criteria not specified, but based on screening unpreprocessed images for abrupt BOLD signal changes | Individual Infant and then MNI | For default network definition: z > 1 to determine voxel-wise connectivity; For correlation matrices p < .05 FDR corrected | (1) # default regions identified: Neonates 6; 1 yo's 10, 2 yo's 13; (2) MPFC and PCC identified in all groups with volume of cluster – associated with age; (3) Nonlinear development of default network; (4) PCC as default network hub in neonates |
| Gao et al. (2011) | Healthy neonates (M = 23 days), 1-yo's (M = 13 mo), and 2-yo's (M = 24 mo) | 51 neonates, 50 1-yo's, 46 2-yo's | 51 for motion or medical problem (e.g. preterm birth) | ROIs from adult atlas based on sulcal patterns; graphy theory | Frame removal: Screening unpreprocessed images for abrupt BOLD signal changes (criteria not specified) | Individual Infant and then MNI | Whole brain analysis: p < .05 FDR; Regional analysis: p < .05 uncorrected | (1) Connection density increases from neonate to 1 yo, but stable from 1yo's to 2 yo's; (2) Strength of connectivity for anatomically distant nodes increases with age; (3) Increase in small-worldness with age; (4) B insula consistent hub across age groups |
| Gao et al. (2013) | See Gao et al. (2011) | See Gao et al. (2011) | See Gao et al. (2011) | Adult ROIs based on Fox et al. (2005); Whole brain seed-based correlations; Additional ROIs based on 1yo connectivity map; nonparametric rank-sum test for age differences | Frame removal: Criteria not specified, but based on screening unpreprocessed images for abrupt BOLD signal changes | Individual Infant and then MNI | p < .05 FDR, cluster size >10 voxels | (1) Default (PCC seed) and dorsal attention networks (IPS seed) show adult-like topology in 1yo's (with exception of frontal eye fields in dorsal attention); (2) Greater change from 0 to 1yo and less from 1yo to 2yo for both networks; (3) Increasing connection strength within each network, and increasing segregation between networks over time (less overlap and negative correlations seen in 1 yo's and 2 yo's) |
| Gao et al. (2014b) | See Alcauter et al. (2013) | See Alcauter et al. (2013) | See Alcauter et al. (2013) | ICA; Spatial correlation to match components to 9 adult networks; voxel-wise “winner-takes-all” to define functional regions based on ICA; Growth models of mean inter-regional connectivity, network connectivity and inter-regional connectivity between 2 networks | Frame removal: DVARs <.5% signal change and FD <.5 mm, and examined results with thresholds of <.3% and <.2 mm; Remaining FD as covariate | Individual Infant and then UNC Infant | p < .05 FDR for connectivity | (1) Adult-like topology in neonates for medial visual and sensorimotor networks; (2) Adult-like topology for all networks in 1 yos (medial visual, sensorimotor, occipital pole, lateral visual, default, auditory/language, salience, frontoparietal) with ≥.40 spatial correlation between 1 yo component and adult network; (3) Network structure in 2 yo's consistent with 1 yo's; (4) Significant non-linear growth of inter-regional connectivity for all networks with greater change from 0 to 1 yo's (except medial visual, sensorimotor, occipital pole); (5) Inter-network connectivity decreased over time; (6) Greater growth in frontoparietal connectivity from 0 to 2 yo's for boys vs girls |
| Gao et al. (2014a) | Healthy infants scanned longitudinally (<1-mo, 3-mo, 6-mo, 9-mo, 12-mo); Healthy adults (27–40 yrs) | 65 infants (45 <1-mo, 34 3-mo, 33 6-mo, 29 9-mo, 35 12-mo); 19 adults | Retrospectively identified from larger longitudinal sample | Adult ROIs (Smith et al., 2009); Whole brain seed-based correlations; t-tests to compare networks; network matching score = mean connectivity within adult network mask – mean conn outside mask; longitudinal modeling of network matching score | Frame removal: DVARs <.5% signal change and FD <.5 mm; Subject removal for >1/3 volumes removed; Remaining FD and volumes removed as covariates | Individual Infant and then MNI | p < .05 FDR | (1) Network specific growth periods: most pronounced changes in V1, V2, default, salience and frontoparietal in 1 st 3 months; (2) Over 12 months, significant log-linear growth in network matching score (except sensorimotor and auditory networks) with fastest growth in following order: visual, default, salience, frontoparietal; (3) Sensorimotor and auditory show significant decreases in outside network connectivity; (4) Higher SES (income and maternal education) associated with greater sensorimotor (higher matching score and within network conn) and default (lower outside network conn) development at 6 months. SES results did not survive correction for multiple comparisons |
| Gao et al. (2014c) | Healthy singleton neonates (M = 22 days), 1-yo's (M = 378 days), 2-yo's (M = 741 days); Healthy monozygotic (MZ) twin neonates (M = 46 days), 1-yo's (M = 410 days), 2-yo's (M = 766 days); Healthy dyzgotic (DZ) twin neonates (M = 36 days), 1-yo's (M = 402 days), 2-yo's (M = 779 days) | Singletons: 36 neonates, 46 1-yo's, 26 2-yo's; MZ pairs: 31 neonates, 18 1-yo's, 18 2-yo's; DZ pairs: 40 neonates, 25 1-yo's, 19 2-yo's | Identified from larger longitudinal sample (Alcauter et al., 2013) | Whole brain voxel-wise connectivity maps; Variability map = one minus correlation between corresponding columns of correlation matrix for twin pairs and age matched singleton pairs; Network variability maps based on masks from Smith et al. (2009); Percentage of shared genes (0%, 50%, 100%) and environment (0, 1, 1) as predictors of similarity between twin and singleton pairs (voxel-wise regression) | Frame removal: DVARs <.3% signal change and FD <.2 mm; Remaining FD as covariate; Subject removal: <75 frames remaining | Individual Infant and then MNI | p < .005, 13 voxels, based on 3dClustSim in AFNI | (1) At all ages, greater intersubject variability in association areas vs primary functional areas (similar to adults); (2) Increasing similarity to adult spatial variability pattern with age; (3) U shaped growth in intersubject variability from 0 to 2 yrs; (4) Greater intersubject variability associated with more long range connectivity (similar to adults); (5) Higher percentage shared genes predicts lower intersubject variability; (6) Genetic effects on connectivity vary by brain region and by age; (7) Genetic effects grow stronger from 0 to 1 yr and weaker from 1 to 2 yrs; (8) Weaker effects of shared environment in contrast to genetic effects |
| Lin et al. (2008) | Healthy neonates (Range = 2–4 wks), 1-yo's, and 2-yo's | 16 neonates, 12 1-yo's, 7 2-yo's | 18 for motion; 14 premature birth, medical problems, or parent disorder | Manually drawn ROIS; whole brain seed-based correlations | Frame removal: Criteria not specified, but based on screening unpreprocessed images for abrupt BOLD signal changes | Individual infant | z < 1; p < .05 corrected for t-test comparison of the 2 ROIs and for ANOVA of the 3 groups | (1) Difference between maximum and minimum signal intensity: 2-yo's > neonates; (2) Average strength of connectivity and brain volume evidencing connections to visual and sensorimotor regions increases with age |
| Liu et al. (2008) | Healthy infants (M = 12.8 mo) | 11 | From larger structural MRI study with 63% scan success | PICA; Bold time series and power spectra computed for each component | Frame removal: Images with >1 mm or >1 degree of motion | Infant DL | NA | (1) 16–36 spatially independent components for each subject with 3 in sensorimotor area; (2) More intra- versus interhemispheric connectivity |
| Smith et al. (2011) | Preterm infants <30 wks (Range = 36–44 wks PMA for scan); Healthy term infants | Preterm: 10 low and 10 high stress; 10 term | Significant cerebral injury (N not reported) | Whole brain seed-based correlations (ROIs not specified); Group maps compared qualitatively | Frame removal: criteria not specified | Not reported | Qualitative comparisons for rs-fcMRI maps | (1) Interhemispheric correlations with R temporal lobe in low stress and term infants, but not high stress infants; (2) Total brain injury + associated with stress; (3) R temporal lobe anisotropy – correlated with stress |
| Smyser et al. (2010) | Preterm infants scanned longitudinally; Term infants (Range = 2–3 days) | 28 preterm with longitudinal data, 10 term | 8 for prominent neuropathology; 10 for motion | Spherical ROIs based on adult atlas coordinates; Whole brain seed-based correlations | Frame removal: Software used to identify frames with motion based on signal change; Scans excluded: <4 min | Individual Infant and then Tal | For t-tests of correlation maps z > 1.65, p < .05; Correlations between connectivity and age p < .05 | (1) Increasing interhemispheric connectivity with age; (2) Term infants > term age preterm infants: local, long range and interhemispheric connectivity; (3) Connectivity between MPFC and PCC in half of term control infants, but not in preterm infants |
| Smyser et al. (2013) | Preterm infants <30 wks with moderate to severe white matter injury (WMI; Range = 36–39 wks PMA for scan); Preterm infants <30 wks with mild white matter injury (PT; Range = 36–40 wks PMA for scan); Healthy term infants (HT; Range = 37–41 wks PMA for scan) | 14 WMI; 25 PT; 25 HT | 10 from WMI group for not meeting data quality standards | ROIs based on Brier et al. (2012) and manually adjusted for anatomic variation due to injury; Correlations and covariances between ROI pairs (homotopic counterparts, PCC to MPFC); t-tests to compare correlations | Frame removal: DVARs <.3% signal change and FD <.25 mm; Subject removal: <100 frames remaining | Individual Infant and then Tal | Bonferroni multiple comparison correction for t-tests and rank-sum tests | (1) WMI group < both control groups for connectivity between homotopic regions (motor cortex, visual cortex, medial cerebellum) and between motor cortex and thalamus; (2) WMI group < HT only for connectivity between other homotopic regions (thalamus, auditory cortex) and PCC to MPFC (but PCC-MPFC did not survive Bonferroni); (3) For WMI group, reduced connectivity more pronounced in injured hemisphere; (4) In motor cortex and thalamus (regions usually close to injury), greater injury severity associated with reduced connectivity |
Note: Studies with sedation not included. All experiments conducted during natural sleep with the exception of Smyser et al. (2010), in which infants were scanned awake and during natural sleep. Atlas abbreviations: Infant DL = Template created by Dehaene-Lambertz et al. (2002); Tal = Talairach & Tournoux; Individual Infant = template based on single infant with longitudinal data; UNC Infant = Template created by Shi et al. (2011). Other abbreviations: wks = weeks; mo = months; yrs = years; yo's = year olds; ICA = Independent component analysis; PMA = postmenstrual age; GA = gestational age; B = Bilateral; L = left; R = right; AI = anterior insulal; ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; MPFC = medial prefrontal cortex; IFG = inferior frontal gyrus; PCC = posterior cingulate cortex; PI = posterior insula; STG = superior temporal gyrus.
The two forms of functional MRI employed in infant studies are task-based fMRI and rs-fcMRI. While both methods are based on the Blood Oxygen Level Dependent (BOLD) signal (Fox and Raichle, 2007, Logothetis and Wandell, 2004, Raichle and Mintun, 2006), they allow for examining two critical aspects of healthy brain functioning. Task-based fMRI facilitates examination of the brain's response to specific aspects of the environment, including cues known to be important for infant development. These include tactile stimulation, and smells or sounds associated with caregivers. Rs-fcMRI provides information about how the brain is intrinsically functionally organized. It is this organization, which likely allows for proper stimulus response, as well as complex mental processes necessary for cognitive, emotional and social functioning (Raichle, 2010). Both of these methods rely on the capacity of fMRI to index brain functioning in specific cortical and subcortical regions throughout the brain. In Section 1.4, we will highlight the unique advantages of fMRI in the context of other functional neuroimaging techniques employed in developmental research.
1.2. Task-based fMRI with infants and toddlers
Implementing task-based fMRI with infants is unquestionably challenging. It requires infants to sleep through the excessive noise of the MRI during data acquisition, as well as the sensory stimulation of the paradigm. Despite these challenges, nine studies successfully using this methodology with participants ranging from seven days postnatal (Anderson et al., 2001) to four years-of-age (Redcay et al., 2007) have now been reported (Table 1). The sample sizes and findings reported in these studies (Table 1) indicate the feasibility of using this methodology with infants and toddlers to collect adequate amounts of data to address study aims.
Beyond the feasibility of data collection, another important issue is whether sleeping infants process stimuli presented during task-based paradigms, and whether they do so in a manner captured by BOLD fMRI. Auditory stimuli are most commonly used in infant fMRI research. Positive BOLD response in sleeping infants and toddlers has been observed in auditory processing brain regions (auditory cortex and auditory processing regions of the middle temporal gyri) in response to tones, nonvocal naturalistic sounds, vocal sounds and speech (Blasi et al., 2011, Dehaene-Lambertz et al., 2002, Graham et al., 2013, Redcay et al., 2007). However, it appears that this response may be decreased from baseline in very young infants during presentation of non-naturalistic stimuli (a tone; Anderson et al., 2001). (See Section 1.5.6 for further discussion about employing the BOLD signal as an index of neural activity beginning in infancy.) In addition to basic sensory processing of auditory stimuli, research to date provides evidence for differentiation between distinct types of auditory stimuli during natural sleep. Redcay and colleagues reported distinct patterns of brain activation in sleeping toddlers (two to four years-old) during presentations of tones versus nonvocal naturalistic sounds or vocal sounds (Redcay et al., 2007). By establishing the capacity to characterize neural processing of stimuli at a basic sensory level, this work provides a foundation for using natural sleep fMRI to examine additional aspects of stimulus processing.
Several studies have demonstrated the utility of natural sleep fMRI for examining early language and emotion processing. Patterns of brain activation in sleeping infants indicate registration of speech and vocal properties. For example, Dehaene-Lambertz and colleagues (2002) observed greater activation in the left angular gyrus and precuneus in response to forward versus backward speech in sleeping two to three month-old infants. In adults, these regions have been associated with differentiating between words and non-words (Binder et al., 2000) and with memory retrieval of verbal information (Krause et al., 1999, Nyberg et al., 2002) respectively. These results were replicated in a study of two year-old children during natural sleep, which further identified developmental changes in speech processing from two to three years-of-age (Redcay et al., 2008). In comparison to two year-olds, three year-olds demonstrate greater engagement of brain regions in line with speech processing in adults, such as the superior temporal gyrus, in response to forward versus backward speech (Redcay et al., 2008). Recent findings also indicate differential brain processing of vocal non-speech sounds (e.g. crying or laughing) based on emotion category for three to seven month-old infants during natural sleep. Specifically, during sad versus neutral vocalizations, infants demonstrate greater activation in the insula and part of the orbitofrontal cortex (Blasi et al., 2011). These studies indicate the capacity for natural sleep fMRI to lead to increased understanding of how the brain processes language and emotion beginning in infancy.
An important consideration for fMRI with sleeping infants was raised by Dehaene-Lambertz and colleagues (2010) in their examination of BOLD responses to sensory stimuli on an individual subject level. The authors reported that less than half of sleeping two-month-old infants in their sample demonstrated activation in auditory brain regions when contrasting stimulus presentation to no sound (at a statistical threshold of alpha less than .05 uncorrected for multiple comparisons). They suggest that developmental characteristics of the BOLD signal at this early age (discussed in Section 1.5.6) may account for the lack of observed activation in auditory regions for some infants. However, there are multiple factors that may influence whether or not a BOLD response is captured during acquisition. These factors might include sleep state, developmental stage, scanner noise and statistical power (these issues will be discussed in more detail in Section 1.5). Thus, it seems appropriate to consider this larger context when interpreting the presence or absence of activation within an individual subject. Including or excluding a subject based on the presence or absence of activity can only be considered in this context, and should be explained and justified.
1.3. Resting state functional connectivity MRI with infants and toddlers
In contrast to task-based fMRI, rs-fcMRI allows for examination of intrinsic correlated brain activity in the absence of a specific task or external stimulus. Rs-fcMRI typically involves examining changes in brain signal over the course of a scan lasting between 5 and 10 min, or more. During the scan, older children and adult participants rest with eyes open or closed, while infants or toddler participants are asleep. Rs-fcMRI focuses on correlating fluctuations in the brain signal between a region and all other voxels in the brain, or between specific brain regions (Biswal et al., 1995). Low frequency changes in brain signal observed in the absence of stimuli presentation likely reflect endogenous neural activity (Murayama et al., 2010, Schölvinck et al., 2010, Shmuel and Leopold, 2008). Correlations between spontaneous brain activity over time are posited to represent connectivity between brain regions. Recent findings provide support for this conceptualization, with patterns of functional connectivity frequently in line with direct or indirect structural connectivity between brain regions (Damoiseaux and Greicius, 2009, Greicius et al., 2009, Honey et al., 2009, Miranda-Dominguez et al., 2014, Shmuel and Leopold, 2008).
Rs-fcMRI data can be characterized at three levels of analysis (Churchland and Sejnowski, 1990). At the first level of analysis (circuit level), connections between brain regions are defined by the strength of the correlation between their signals. The second level of analysis (network level) characterizes functional brain networks as a whole instead of as individual connections within the system. Examples of functional brain networks that have been characterized with rs-fcMRI include the default network, a group of brain regions that demonstrate higher levels of activity in the absence of specific external tasks (Greicius et al., 2003, Raichle et al., 2001), and networks underlying specific aspects of cognitive control, such as the fronto-parietal and cingulo-opercular networks (Dosenbach et al., 2007, Dosenbach et al., 2008). The third level of analysis (global topology) focuses on interactions within and between functional networks (Rubinov and Sporns, 2010). All three of these levels of analysis provide information about the functional organization of the brain that have been shown to be relevant to mental health and specific aspects of cognitive and emotional functioning (Fair et al., 2010, Fair et al., 2012, Power et al., 2010, Stevens et al., 2012).
For developmental research, rs-fcMRI has the distinct advantage of documenting coordinated brain functioning across the whole brain in the absence of tasks, which are challenging to adjust appropriately for a wide age range (Casey et al., 2005, Uddin et al., 2010). Moreover, research with adults has indicated that while a particular paradigm employed in task-based fMRI may only activate a subset of regions involved in a broad domain of functioning, such as memory, rs-fcMRI can yield a more complete representation of the network of brain regions involved (Fox and Raichle, 2007, Vincent et al., 2006). This is particularly relevant for infant fMRI. Because scans are conducted during natural sleep, there are even more limitations on the types of stimuli and tasks that can be used in the scanner, decreasing the chances of activating the full set of regions involved in a behavioral domain of interest.
Investigations using rs-fcMRI have indicated the utility of this methodology for studying the functional development of brain networks across a wide age range (Fair et al., 2007, Fair et al., 2008, Fair et al., 2009, Grayson et al., 2014, Supekar et al., 2009, Supekar et al., 2010). For example, several reports have documented a pattern of developmental changes in the functional connectivity of brain networks from childhood to adulthood that is characterized by increasing long-range connectivity (between anatomically distant regions) and decreasing short-range (or localized) connectivity (Fair et al., 2007, Fair et al., 2008, Fair et al., 2009, Kelly et al., 2009, Supekar et al., 2009). A similar pattern has been observed across the first two years-of-life (Gao et al., 2009, Gao et al., 2011, Gao et al., 2013, Gao et al., 2014b). Changes across this earlier age range occur at an extremely rapid rate, such that from two-weeks to one year-of-age, the number of regions overlapping with the adult default network that evidence significant functional connectivity during natural sleep increases from six to thirteen (Gao et al., 2009). Development does not appear to be linear, with many measures (including the number of connections showing statistically significant changes in strength) indicating less change across the second year-of-life in comparison to the first (Gao et al., 2011, Gao et al., 2013, Gao et al., 2014a, Gao et al., 2014b, Gao et al., 2014c; Fig. 1).
Fig. 1.
Functional connectivity maps for the default and dorsal attention networks in (a) neonates, (b) 1 year-olds, (c) and 2 year-olds. Right column shows composite maps of default and dorsal attention networks (green = default, yellow = dorsal attention, overlap = red). Color bar indicates correlation values (Gao et al., 2013).
Both environmental input and normative changes within the developing brain, such as myelination, are posited to account for the observed changes in connectivity during development (Fair et al., 2009) with different processes likely at work depending on the age range of interest (Gao et al., 2011). These changes are described as reflecting increasing connectivity among regions within a network (integration) and increasing segregation between regions in distinct networks (segregation; Fair et al., 2007, Fair et al., 2009, Gao et al., 2013). This demonstration of the development of large scale, dissociable functional networks has led to increased understanding of how information is efficiently processed and integrated to support a range of complex behaviors (Power et al., 2011).
Recent work has drawn attention to the effects of subject “micro-movements” on developmental rs-fcMRI results (Fair et al., 2012, Power et al., 2012, Satterthwaite et al., 2012, Satterthwaite et al., 2013, Van Dijk et al., 2012). This issue and the proposed solutions will be discussed in more detail in the next section. However, it should be noted that this work focuses on the biasing effects of motion in research spanning childhood to adulthood, during which time motion decreases systematically with age. The potential for confounding motion effects and developmental changes in studies of sleeping infants and toddlers will likely be different, as movement during scanning is not expected to track with age in the same manner during this time frame. Specifically, while an awake middle-aged adult is expected to have greater self-control and therefore move less than a child during scanning, there is no reason to expect that a sleeping two-year-old will move less than a sleeping one-year-old during scanning (indeed the opposite may be true; Gao et al., 2014c).
In addition to capturing developmental changes in the functional organization of the brain, rs-fcMRI with infants allows for examination of functional brain networks that support higher order cognitive and emotional processes, which cannot be reliably assessed at a behavioral level during infancy. For example, although the functional significance of the default network is not definitively known, variability in default network connectivity has repeatedly been associated with higher order cognitive skills (including executive functioning [EF]) and related mental health disorders in children and adults (e.g. Attention Deficit Hyperactivity Disorder; Castellanos et al., 2008, Fair et al., 2010, Fair et al., 2012, Kelly et al., 2008). In addition to the default network, rs-fcMRI with infants has been used to characterize the early development of the dorsal attention network (Gao et al., 2013; Fig. 1), which underlies goal driven selective and sustained attention in adults (Corbetta et al., 2008), as well as the salience and frontoparietal networks (Gao et al., 2014b). Advances in behavioral assessment have allowed for reliable measurement of higher order cognitive skills beginning at two years-of-age (Beck et al., 2011). However, rs-fcMRI makes it possible to examine the development of relevant functional brain networks beginning at birth, prior to the emergence of complex behaviors, potentially allowing for earlier identification of individual differences that may confer risk or resiliency.
1.4. fMRI in the context of other developmental neuroimaging methods
The primary methods used to date to study brain functioning in infants are functional near infrared spectroscopy (fNIRS) and electroencephalography (EEG). These methods have been widely utilized such that even the more recent of these techniques, fNIRS, has now been used to examine infant brain functioning in nearly 100 studies (Lloyd-Fox et al., 2014). The specific advantages and disadvantages of fMRI are complementary to these other methods. For example, EEG and fNIRS have superior temporal resolution in comparison to fMRI and can be conducted in settings of reduced noise (in contrast to noisy fMRI sequences). EEG and fNIRS can also be conducted with awake infants engaged in a variety of tasks, which allows for results that may generalize more readily to real world contexts (Lloyd-Fox et al., 2009, Lloyd-Fox et al., 2010). In addition, fNIRS facilitates examining aspects of brain metabolism that are not possible to differentiate with fMRI (Boas et al., 2003, Dunn et al., 2003). Due to this capacity it has contributed to our understanding of the BOLD signal during infancy (see Section 1.5.6; Liao et al., 2010). fMRI complements these other methods by providing higher spatial resolution throughout the brain. The specific location of brain activity at the level of millimeters across the cortex is only possible with fMRI. It is also unique among these techniques in that it provides the capacity to index functioning of specific subcortical brain regions, which are not accessible by these other methods. In the concluding section of this review we provide examples of how infant fMRI can build on the existing body of EEG and fNIRS research in the context of ELS research.
Structural MRI (sMRI) is another important developmental neuroimaging tool that provides information about brain morphology not captured by functional neuroimaging methods. fMRI and sMRI methods can be seen as complementary in several ways. First, they provide unique sources of information regarding typical and atypical courses of brain development. For example, preterm infants without prominent pathology in sMRI scans show differences in how various brain regions are functionally connected (Smyser et al., 2010). It has been suggested that functional signal examined with BOLD fMRI may be more sensitive to individual differences, or atypical brain organization, in comparison to the structural phenomenon we are currently able to characterize with sMRI. For this reason, it may be possible to use fMRI to identify differences in brain functioning at early stages of development that subsequently manifest as morphological changes observable in older age groups (Tottenham and Sheridan, 2010). sMRI also represents a complementary methodology because high resolution sMRI scans are necessary for aligning fMRI scans with a common brain space to conduct statistical analysis and localize activity. Thus, the two measures are well suited to be used in tandem to examine brain development from multiple perspectives. As with other neuroimaging techniques, the level of complexity involved in fMRI methods is extremely high, allowing for multiple sources of error in measurements. Thus, convergent evidence across modalities (e.g. Fransson et al., 2013) will be crucial for advancing the field.
1.5. Methodological and ethical considerations
1.5.1. Successful scan completion
Despite this promising foundational work with task-based fMRI and rs-fcMRI during natural sleep, significant methodological challenges and considerations require attention. As noted earlier, these methods require infants to sleep in a novel environment with the loud noises of the MRI machine. Potential sleep disturbances arising from stimuli in task-based fMRI are a concern as well. Several procedures have been implemented to improve scan success, and warrant consideration by research teams. To prepare for a scan, families can be provided with a CD of scanner noises to acclimate an infant to sleeping with scanner noises in the background (Redcay et al., 2007). Additionally, research staff can put caregivers and infants at ease through good communication about what to expect prior to the scan, and demonstrations of patience and confidence throughout the scan appointment. The scanning environment can be set up to facilitate sleep by keeping the room relatively dark, and providing an MRI compatible rocking chair, crib and weighted blankets.
In order to reduce disturbance of sleep due to the sounds of the MRI machine, researchers often use MRI compatible head phones small enough to fit an infant or toddler (Blasi et al., 2011, Dehaene-Lambertz et al., 2010, Redcay et al., 2007). Neonatal ear shields (“Minimuffs,” Natus Medical Inc., San Carlos, CA; Anderson et al., 2001, Blasi et al., 2011) or foam ear plugs (Redcay et al., 2007) can be used as additional sound protection under headphones (Blasi et al., 2011, Redcay et al., 2007), or in conjunction with a vacuum pillow that molds to an infant's head and provides additional noise protection (Lin et al., 2008). Several groups have also added sound shielding foam to the inside of the scanner bore to reduce the noise of scan sequences (Blasi et al., 2011, Dehaene-Lambertz et al., 2002). Additionally, a scan sequence with a lower volume or less jarring quality of sound can be presented at the beginning of an experiment to help infants gradually adjust to the noise of the scanner (e.g., a proton density weighted scan). Finally, to avoid waking infants due to sudden changes in volume when a scan sequence ends, researchers can play consistent background noise (Blasi et al., 2011), or minimize time between scan sequences. Due to the challenging nature of this methodology, systematic study of malleable factors likely to impact scan success is warranted.
Individual differences, such as age, temperament and health, may impact scan success, and bias results due to exclusion of highly sensitive infants. In studying factors that impact scan success, it will be important to take into account such individual differences as different approaches may be more or less effective depending on the population being studied. For example, approaches that increase scan success rate in neonates may be less effective, or even counter-productive, for obtaining good data with one-year-old infants. However, this issue is not unique to fMRI with infants, and also applies to behavioral and physiological measures (e.g. Azak et al., 2012). Moreover, successful use of task-based fMRI with toddlers with ASD (reviewed by Pierce, 2011), which is often associated with sensory sensitivity (Lane et al., 2010), provides support for the potential utility of this method even with highly sensitive infants and toddlers. See Table 1, Table 2 for a review of successful scans included in analyses (‘N Analyses’) and scans lost due to sleep difficulties, motion or other reasons (‘N Excluded’) in published studies employing fMRI or rs-fcMRI with infants during natural sleep.
1.5.2. Effects of sleep on brain signaling
Another important issue involves understanding effects of sleep and variations in sleep stage on brain signal. Methodological challenges have thus far prevented simultaneous use of EEG and fMRI in infants and toddlers to allow for tracking sleep state during scans with this age group (although this work is likely soon to appear as such research has been conducted in adults; Boly et al., 2012, Czisch et al., 2002, Horovitz et al., 2008, Kaufmann et al., 2006, Larson-Prior et al., 2009, Portas et al., 2000, Sämann et al., 2011, Tagliazucchi et al., 2012). While this confound will need to be addressed in future work, there is evidence that comparable, albeit not identical, neural processing of auditory stimuli (i.e. infant EEG and fMRI) and rs-fcMRI occurs during sleep and wake states (Czisch et al., 2002, Horovitz et al., 2008, Larson-Prior et al., 2009, Portas et al., 2000). For example, one ERP study revealed similar processing of auditory stimuli during sleep and wake states with preservation of response amplitude and latency across different sleep stages (Martynova et al., 2003). Moreover, it appears that learning involving auditory and basic somatosensory stimuli occurs during sleep for infants (Cheour et al., 2002, Fifer et al., 2010, Reeb-Sutherland et al., 2011), suggesting that these stimuli are processed beyond a basic, sensory level during sleep.
Patterns of brain activation involved in distinguishing between different types of auditory stimuli (in particular, a subject's name versus a tone) also demonstrate similarities across sleep and wake states (Portas et al., 2000). These results are in line with findings of sleeping infants and toddlers showing distinct patterns of brain activation in response to different types of auditory stimuli (Redcay et al., 2007) and different emotional tones (Blasi et al., 2011, Graham et al., 2013). Finally, simultaneous EEG and fMRI studies with adults indicate a potentially reduced spatial extent of activation in the auditory cortex during sleep versus wakefulness, but consistent amplitude of activation across sleep stages (Czisch et al., 2002, Portas et al., 2000).
Combined rs-fcMRI and EEG research with adults indicate functional connectivity within higher order brain networks, including the default network (Horovitz et al., 2009), an attention network, and a cognitive control network (Larson-Prior et al., 2009), remains consistent across the transition from an awake state to light sleep (non-slow wave and non-rapid eye movement [non-REM] sleep, respectively). Rs-fcMRI with sleeping infants has demonstrated functional connectivity between default network regions (Gao et al., 2009, Gao et al., 2011, Smyser et al., 2010) and patterns of negative correlations (also referred to as anticorrelations) between default and dorsal attention network regions (Gao et al., 2013). These patterns are in line with those observed in adults in an awake state (Fox et al., 2005, Fransson, 2005). Taken together this work indicates the potential for capturing the functional architecture of multiple higher order brain networks with rs-fcMRI during sleep.
Despite these similarities, differences in task-based fMRI and rs-fcMRI results during sleep versus wake, or across different sleep stages have also been observed. For example, in an fMRI study with adults, deactivation in the visual cortex during presentation of an auditory stimulus was observed during sleep stages 1 and 2, and interpreted as a potential sleep protective mechanism (or a process that prevents waking despite sensory stimulation; Czisch et al., 2002). Lower levels of activation during sleep in higher level processing regions have also been observed, including reduced parietal, prefrontal and cingulate activation to auditory stimuli during sleep versus wake (Portas et al., 2000). With regard to rs-fcMRI, simultaneous use of EEG and rs-fcMRI has revealed preserved functional connectivity between cortical regions, during light sleep, but decreased connectivity during slow wave sleep (Spoormaker et al., 2010). Although connectivity among default network regions is generally preserved across sleep stages, there is also evidence for region specific differences, such as decreased posterior cingulate cortex (PCC) connectivity with other default regions during light sleep (stages 1 and 2; Sämann et al., 2011), and decreased medial prefrontal cortex (MPFC) connectivity with default network regions during slow wave sleep (Horovitz et al., 2009, Sämann et al., 2011). These findings indicate the importance of continued efforts to characterize variation in fMRI and rs-fcMRI results due to sleep stage. However, these issues are not unique to rs-fcMRI and fMRI during sleep as variation in attention (Sander et al., 2005) and arousal (Logothetis, 2008, Tagliazucchi et al., 2012) impacts results in studies conducted during awake states.
1.5.3. Motion
As noted previously, subject motion constitutes an important methodological consideration in developmental fMRI and rs-fcMRI studies. Two key issues include violation of assumptions underlying traditional motion correction strategies, and the potential for small amounts of motion (i.e. “micro-movements”) to have systematic biasing effects on results (Fair et al., 2012, Power et al., 2012, Satterthwaite et al., 2012, Van Dijk et al., 2012, Yan et al., 2013). Although these issues are pertinent to all fMRI and rs-fcMRI work, they are particularly important in developmental samples typically characterized by larger amounts of motion. Recent work indicates that parameters calculated with traditional motion correction strategies are not tightly linked to changes in signal due to motion (Fair et al., 2012, Power et al., 2012, Satterthwaite et al., 2012, Van Dijk et al., 2012), and that the association between motion and signal change is not necessarily linear (Fair et al., 2012, Van Dijk et al., 2012). Traditional motion correction methods thus leave substantial amounts of motion related signal unaccounted for. Motion related signal has been found to have a specific effect on rs-fcMRI data that is particularly problematic for developmental studies. Motion appears to bias results toward fewer/weaker long range connections and more/stronger local connections (Fair et al., 2012, Power et al., 2012, Satterthwaite et al., 2012, Van Dijk et al., 2012, Yan et al., 2013). Although averaging across multiple trials may diminish effects of motion to some degree in task-based fMRI compared to rs-fcMRI, similar efforts to understand confounds of motion are critical for all types of fMRI research (Power et al., 2012, Siegel et al., 2013, Van Dijk et al., 2012, Yuan et al., 2009).
Recent work has outlined improved strategies to account for effects of motion on brain signal. First, examination of motion from frame to frame (known as framewise displacement; FD), as opposed to a single reference frame, provides an index more closely related to motion induced changes in brain signal (Power et al., 2012). In addition, examination of frame to frame changes in BOLD signal allows for differentiation of motion versus biologically based signal due to magnitude (Power et al., 2012, Smyser et al., 2010). These two measures can be used for removal of individual frames (“scrubbing”; Power et al., 2012), and as regressors to account for motion (Fair et al., 2012, Van Dijk et al., 2012). Other aspects of data processing relevant for motion effects on rs-fcMRI include the number of confound regressors, regression of global whole brain signal and selection of frequencies to include (band pass filtering) (Satterthwaite et al., 2013). Studies utilizing these new procedures indicate attenuated strength of previously reported associations between age and increasing long range and decreasing short range functional connectivity, although the pattern of results remains (Fair et al., 2012, Satterthwaite et al., 2012).
Patterns of motion may be markedly different for sleeping infants and toddlers compared to awake children and adolescents. Sleeping infants have been noted to startle at the beginning of a new scan sequence and settle down as the scan progresses, although efforts have been made to reduce startle by playing consistent background scanner noise (Blasi et al., 2011, Dehaene-Lambertz et al., 2002). A sleeping infant may also have one or two large, circumscribed movements while adjusting sleeping position during a scan. Proactive approaches to reducing such motion during infant scans include swaddling infants prior to scanning (Anderson et al., 2001, Blasi et al., 2011, Dehaene-Lambertz et al., 2002), and use of a vacuum pillow fixation device around the body and head (Blasi et al., 2011), or the head only (Lin et al., 2008). Further investigation of the optimal approach for handling motion in fMRI and rs-fcMRI research with sleeping infants and toddlers will be necessary to understand potential advantages and disadvantages of different methods. However, the methods outlined for older developmental samples provide a solid framework and powerful tools for this work.
1.5.4. Atlas space
Aligning each participant into a common coordinate system or common stereotaxic space is critical for group studies, but is a particular challenge for infant studies. Most algorithms to align a brain to an atlas rely on gray and white matter contrast. Because that contrast is weak in the newborn (due to reduced myelination), using traditional means to register the infant brain is problematic. The other difficulty is brain size. By the time children reach school age the brain is approximately 90% the size of the adult brain (Lenroot and Giedd, 2006). Thus, registering children and adult brains to similar atlas space (e.g. Talairach – see below) is relatively straightforward. However, the neonatal brain is approximately one-half the volume of the adult brain (Knickmeyer et al., 2008), making accurate registration to an adult atlas more challenging. Several approaches have been taken thus far. The adult Talaraich atlas (Talairach and Tournoux, 1988) has been used in studies of toddlers (Dinstein et al., 2011, Eyler et al., 2012, Redcay and Courchesne, 2008, Redcay et al., 2008). The authors provide evidence for similar within group variability for toddlers and adults with regard to central sulcus alignment of individual structural images with the atlas (Redcay et al., 2008). However, coordinates of the Talaraich atlas do not accurately indicate specific brain regions in toddlers, necessitating the use of anatomical landmarks to identify location of fMRI signal (Redcay et al., 2008). This registration procedure also involves stretching infant brain images to fit an adult atlas. As an infant brain image is composed of fewer voxels, this can lead to less independence among voxels. The use of infant specific atlases, discussed below, represents a potential solution to this issue. However, direct comparison across age groups requires transformation to a common stereotaxic space, and therefore reintroduces this problem. Ongoing efforts are needed to establish an optimal solution for direct comparison of fMRI results across a wide age span.
The use of an adult or even a pediatric atlas with infant brain images (including those of neonates, one and two year-olds) can also lead to misclassification of brain tissue (Altaye et al., 2008, Kazemi et al., 2007, Shi et al., 2011), which is improved by using an age-appropriate infant atlas (Altaye et al., 2008, Kazemi et al., 2007, Shi et al., 2011). Some investigators have created an atlas based on one subject in the age range of interest for the particular study (Alcauter et al., 2013, Gao et al., 2009, Gao et al., 2011, Gao et al., 2013). This approach facilitates focus on the age range of interest and does not require stretching to an adult size brain. However, creating an atlas based on one or two individuals can lead to biases based on the particular morphology of an individual brain. Moreover, continued use of different atlases across studies may lend to difficulties in synthesizing the literature as the field grows.
High quality atlases for infants within various age ranges have recently become publicly available (Fonov et al., 2009, Sanchez et al., 2012, Shi et al., 2011). One of these is particularly noteworthy for age-appropriate atlases (for the neonatal period through adulthood), and conversion algorithms for coordinates corresponding to frequently used adult atlases (Fonov et al., 2011, Fonov et al., 2009). Even with these advancements, the best solution for making comparisons across age groups remains unclear. Due to rapid early brain development (Knickmeyer et al., 2008), researchers have suggested using fine grained atlases for each three month period or greater during the first year-of-life (Almli et al., 2007, Sanchez et al., 2012). It may therefore be most appropriate to register to a specific atlas for each age group in a study and then transform to a common child or adult atlas for making comparisons across ages. However, research into the effects of such transformations will be important. Finally, it will be necessary to take into account variation in the number of voxels for specific atlases and how this impacts correction for multiple comparisons.
1.5.5. Types of stimuli for task-based fMRI
Auditory stimuli have been most frequently used in task-based fMRI with infants. This work has focused on basic differentiation among different types of sounds (Redcay et al., 2007), as well as language (Dehaene-Lambertz et al., 2002, Redcay and Courchesne, 2008, Redcay et al., 2008), and emotion processing (including vocal non-speech emotional stimuli [Blasi et al., 2011] and emotional prosody of speech [Graham et al., 2013]). Auditory stimuli specific to the infants’ caregiving environment have also been employed in a study in which Dehaene-Lambertz and colleagues examined processing of maternal voice in 2 month-old infants (Dehaene-Lambertz et al., 2010). Visual stimuli have been employed in infant fMRI in one study, which examined response to flashing lights during natural sleep (Redcay et al., 2007).
Conducting scans during natural sleep creates some limitations for task-based fMRI as only passive stimuli presentation can be employed, and stimuli of greater intensity may wake infants. However, within these limitations there are a great range of possible stimuli, which are relevant to infants’ social, emotional and cognitive development. For example, adult fMRI work has employed hand holding to examine response to social somatosensory cues (Coan et al., 2006). Response to touch by caregivers and other forms of somatosensory stimulation can be similarly examined with infants’ during natural sleep scans. Additional variations on auditory stimuli involving personally relevant social and emotional cues, such as parents’ calling an infant's name or engaging in conflict with one another, have also yet to be explored in the infant fMRI literature. Research with infants documenting basic learning with auditory and somatosensory stimuli during natural sleep (Cheour et al., 2002, Fifer et al., 2010, Reeb-Sutherland et al., 2011), also suggests the intriguing possibility of studying the neural basis of early learning with fMRI. At present, the range of potential stimuli for task-based fMRI studies with sleeping infants and toddlers remains largely unexplored.
1.5.6. Other methodological considerations
With the limited gradient capability, the spatial resolution of task-based fMRI is normally limited to 2 × 2 × 2 millimeters. While this spatial resolution is generally adequate for adult studies, with the small size of the infant brain, it imposes limitations for detailed separation of specific brain regions. Furthermore, preprocessing of task-based fMRI and rs-fcMRI data typically involves spatially smoothing images prior to group level analyses, further worsening spatial resolution. It may be beneficial to skip this spatial smoothing step in analysis of fMRI data with infants, particularly when trying to localize signal to smaller brain regions, such as the nucleus accumbens and amygdala. Although improved signal to noise ratio associated with spatially smoothing fMRI data has been demonstrated (Strother et al., 2004, Van Dijk et al., 2010), recent work indicates that the effects on both task-based results and rs-fcMRI correlations are minimal (Molloy et al., 2014). In the case of examining small brain structures, it can even be detrimental. If researchers choose to employ spatial smoothing it is important to consider that due to dramatic brain volume growth across infancy and early childhood, effective voxel size (relative to the whole brain volume) changes over time (given the same absolute voxel size). One potential remedy is to use adaptive smoothing strategies (e.g., using brain size normalized smoothing kernels) to reduce the impact of different “relative voxel sizes” inherent in developmental studies (Gao et al., 2014b).
Standard analysis methods for both task-based fMRI and rs-fcMRI can be directly applied to infant studies. However, developmental considerations and the relative paucity of existing literature should be taken into account when selecting an analytic method. This is especially true for rs-fcMRI since there are a multitude of different analysis methods. One example is the choice between seed-based analysis and more data-driven independent component analysis (ICA). While seed-based analysis requires identifying a priori brain regions of interest for analysis, ICA can be used to examine signal from voxels across the whole brain and to categorize voxels into networks based on statistically identified shared variance. ICA has been utilized in initial infant studies to examine putative precursors of adult functional brain networks without biasing results by anchoring networks in regions identified in adult research as part of specific brain networks (Fransson et al., 2009, Gao et al., 2009, Liu et al., 2008). However, ICA results can be biased due to decisions about how many components to incorporate into the model, and how to interpret which components represent functional brain networks (Fox et al., 2007). Both types of analyses have strengths and weaknesses (Fox and Raichle, 2007, Hagmann et al., 2012), and there is some evidence for convergent results across these methodologies. Such evidence has been provided through direct comparison of results in healthy adult subjects (Rosazza et al., 2012), and through consistent findings regarding the emergence of functional networks in infant studies that utilize seed-based analytic techniques (Gao et al., 2011, Gao et al., 2013) and ICA (Gao et al., 2009, Gao et al., 2014b).
Finally, both task-based fMRI and rs-fcMRI rely on the BOLD signal as an index of brain functioning. It is therefore very important to take into account potential developmental changes in neurovascular coupling, which refers to the relationship between neural activity and the ratio of oxygenated to deoxygenated hemoglobin (oxyhemoglobin and deoxyhomoglobin) – the source of the BOLD signal. For example, some studies suggest differences in the BOLD signal between older and younger adults may be attributable to aging-related changes in non-neuronal factors (D’Esposito et al., 2003), such as resting cerebral blood flow (Ances et al., 2009). In contrast, developmental fMRI research provides evidence for consistency in the association between neural activity and the BOLD signal among children and adults (Kang et al., 2003, Wenger et al., 2004), and among children with and without neurodevelopmental disabilities (Feczko et al., 2012). This work provides support for interpreting differences in the BOLD signal between these groups as reflective of differences in neural activity.
However, this work has been conducted in children, and unique aspects of brain vasculature during early infancy warrant attention, as divergent patterns of neurovascular coupling may exist (see Hagmann et al., 2012). Findings with animals and humans have indicated potential differences in the latency, amplitude (Arichi et al., 2012, Colonnese et al., 2008), and direction (Kozberg et al., 2013) of the BOLD response to stimulus in early infancy compared to later developmental stages. More direct evidence from fNIRS studies with infants reveals a pattern of response to stimuli (increasing oxy- and decreasing deoxyhomoglobin) that is qualitatively similar to the adult pattern of neurovascular coupling (Karen et al., 2008, Liao et al., 2010, Taga et al., 2003, Watanabe et al., 2008). However, additional research in this area is needed as fNIRS studies have also yielded some mixed evidence (Bortfeld et al., 2007, Fava et al., 2013, Kusaka et al., 2004, Meek et al., 1998). Perhaps most importantly, functional neuroimaging modalities that do not depend on the BOLD signal, including EEG and diffuse optical tomography, have demonstrated consistency with fMRI findings in neonates (Fransson et al., 2013, White et al., 2013). Thus, although additional research into developmental differences in the link between neural activity and the BOLD signal will be of great importance, the findings to date provide support for the capacity of infant fMRI to meaningfully characterize early brain functioning.
1.5.7. Ethical considerations
In addition to methodological considerations, important ethical issues in conducting fMRI research with infants and toddlers warrant attention. Many of these issues, while highly relevant to research with infants, are not unique to fMRI, and are beyond the scope of the present review (see Axelin and Salanterä, 2008, Diekema, 2009). Ethical considerations specific to infant fMRI research include the need to adequately explain the fMRI procedure to caregivers with varying levels of education, and from different cultural backgrounds, to allow them to give informed consent. As part of this process, caregivers need to have a good understanding of safety protocols related to fMRI, including fMRI contraindications (Downie and Marshall, 2007, Hinton, 2002). As an additional safety measure for infants, pre-screening for fMRI contraindications should include caregivers and the primary care physician to ensure that there is no reason an fMRI protocol would pose a risk to a particular infant.
Beyond ensuring safety, the extent to which an fMRI protocol may be distressing to an infant is an important ethical consideration. First, it should be noted that because infants are sleeping during the protocol it has potential to be less distressing than common behavioral paradigms used in research with infants to illicit stress responses (for a review see Gunnar et al., 2009). However, infants often wake up during scans and may experience distress due to the novel surroundings and fMRI scan noises. These issues can be addressed by the use of high quality sound protection throughout scanning (as discussed in Section 1.5.1), and careful monitoring throughout the scan. Careful monitoring involves having a researcher in close proximity to the infant throughout the scan, and use of various techniques to monitor infant wakefulness (including infrared cameras in darker settings or strategically placed mirrors in lighter settings that allow for viewing the infant's face in the scanner). Through careful monitoring, researchers can observe and respond quickly to any signs of wakefulness or distress. Contingent response to distress by caregivers, or researchers experienced in working with infants, is an important component of ensuring that infant fMRI protocols don’t serve as a significant source of stress to infants. As with the methodological factors discussed previously, systematic study of these issues will add to researchers’ ability to make informed decisions about the best protocols to employ. For example, peripheral measures of the autonomic nervous system or neuroendocrine stress response system could be employed to assess physiological arousal before and after different scanning protocols.
2. Application of infant fMRI to build on current approaches for studying early life stress (ELS)
The foundational work employing fMRI with infants during natural sleep indicates the potential utility of this method for advancing research in multiple areas. However, at present, few domains of study have harnessed this potential. As a notable exception, investigators examining the etiology of autism have successfully employed infant fMRI to identify early biological markers of autism, and provide a model for the early neurodevelopmental trajectory of this disorder (Eyler et al., 2012, Pierce, 2011). In the remainder of this article we provide an example of one area of research that has yet to take full advantage of the potential contributions of infant fMRI, but seems particularly well poised to benefit from this methodology. We focus on the study of ELS, and specifically research into the effects of ELS on functional brain development with implications for subsequent mental health. The guiding theoretical principles and extensive empirical work in this area involving fMRI with older children and adults, and other imaging approaches with infants, set the stage for infant fMRI research to make a significant contribution. While it is beyond the scope of this article to provide a thorough review of the research examining effects of ELS on brain development and mental health (for reviews see Loman and Gunnar, 2010, Lupien et al., 2009, Shonkoff and Garner, 2012, Tottenham and Sheridan, 2010), we highlight aspects of this literature that illustrate the potential role of infant fMRI in advancing the state of this field.
2.1. Typical development and developmental programming
The concept of developmental programming represents a guiding theoretical principle in the study of ELS on brain development. This concept suggests that during times of rapid growth, systems are more vulnerable to disorganizing influences (Buss et al., 2012b, Gluckman and Hanson, 2004, Seckl, 2008). During the prenatal period, brain development proceeds from the basic foundational level of neural tube formation, to the migration of neurons, the initial myelination of axons and the formation of synapses (reviewed by Fair and Schlaggar, 2008, Lenroot and Giedd, 2006). Rapid brain development continues during infancy with frontal and primary sensory cortex showing 70–80% increases in synaptic density over the first year and half of life (Huttenlocher and Dabholkar, 1997), and brain volume increasing four-fold from birth to four years of age, accompanied by a rapid decline in the ratio of gray to white matter volume (indicative of increasing myelination; Courchesne et al., 2000). During these periods of rapid change, the brain is sensitive to environmental input, which is both necessary for healthy development, and potentially harmful depending on multiple factors, including the nature and timing of the input (Fox et al., 2010, Knudsen, 2004). In order to understand healthy or maladaptive patterns of brain development, it has been suggested that examination of brain systems begin as early as possible to tease apart environmental influences from development of compensatory strategies or psychopathology (Lenroot and Giedd, 2006).
2.2. Building on the dominant functional neuroimaging techniques used with infants to study ELS
To date, fNIRs and EEG have been the most common functional neuroimaging methodologies used to study effects of ELS on brain functioning in infancy. fNIRs and EEG studies have shed light on infants’ neural processing of a range of stimuli highly relevant to experiences of ELS and the caregiving environment including touch (Kida and Shinohara, 2013), human vocalization (Grossmann et al., 2010), emotional tone of voice (Grossmann et al., 2005, Santesso et al., 2007), maternal voice (Saito et al., 2009) and breast milk odor (Aoyama et al., 2010). This work has been conducted in normative samples, as well as in infants exposed to ELS and at risk for poor outcomes. Infant fMRI has potential to contribute to this literature by increasing capacity to localize neural processing of stressor relevant stimuli, and to identify emerging differences in specific brain systems relevant to mental health outcomes.
2.2.1. Application of infant fMRI to preterm birth and experiences of pain in the NICU
Infants born prematurely are a population at increased risk of exposure to ELS, as well as poor mental health and neurodevelopmental outcomes (Grunau, 2013, Johnson and Marlow, 2014). This phenomenon has been well studied with fNIRS and EEG. For example, fNIRS has been used to shed light led on preterm infants’ neural processing of routine, painful medical procedures (e.g. heel stick) in the neonatal intensive care unit (NICU). This work has identified cortical involvement in pain processing beginning at 25 weeks postmenstrual age (Bartocci et al., 2006, Slater et al., 2006). Additional fNIRS and EEG research with preterm infants has indicated heightened sensitivity of the neonatal brain (compared to term infants) to painful stimuli (Slater et al., 2010), and limited capacity to distinguish painful stimuli from other forms of somatosensory stimulation prior to 35 weeks gestational age (Fabrizi et al., 2011). This cortical involvement in pain processing and heightened sensitivity is proposed to impact developing brain systems, and contribute to long term differences in pain perception, and other neurodevelopmental outcomes (Fabrizi et al., 2011, Grunau, 2013, Slater et al., 2010). However, the specific brain regions and systems involved, and how these systems are implicated in mental health outcomes known to be impacted by preterm birth, remain unknown.
The examination of preterm infants with fMRI during natural sleep has the potential to fill this void. Rs-fcMRI studies with preterm infants have demonstrated the feasibility and sensitivity of this method for examining brain functioning in this population. Specifically, preterm birth has been associated with decreased connectivity among two key default network regions (Smyser et al., 2010). Other work has shown that a high number of cumulative medical procedures from birth until term age equivalent predicts decreased inter-hemispheric connectivity in the temporal lobe (Smith et al., 2011). The association between preterm birth and emerging default network connectivity may be relevant for subsequent mental health, as connectivity of this network has repeatedly been associated with mental health outcomes in children and adults (Berman et al., 2011, Fair et al., 2010, Fair et al., 2012, Fox and Greicius, 2010, Uddin et al., 2010, Whitfield-Gabrieli and Ford, 2012). Future studies can build on this initial work by examining functional brain systems implicated in pain processing or in aspects of cognition and emotion often impacted by preterm birth.
In addition to lending insight into cumulative effects of preterm birth and medical procedures on brain functioning, infant fMRI can likely contribute to understanding the immediate neural sequelae of processing pain and somatosensory stimuli. Although it would not be possible to conduct fMRI with infants simultaneous to painful medical procedures (as in the EEG and fNIRS research), scans conducted soon afterwards may identify residual effects of stress on functional brain systems. This possibility is suggested by rs-fcMRI work in adults demonstrating alterations in functional connectivity among brain regions important for processing stress following the conclusion of a stress induction paradigm (van Marle et al., 2010). Task-based fMRI can also contribute information about specific cortical and subcortical brain regions involved in processing of somatosensory stimuli, which will likely be relevant for understanding preterm infants’ processing of pain (Fabrizi et al., 2011). This work will be important for building on the extant EEG and fNIRS research to increase understanding of preterm infants’ experiences in the NICU that may contribute to differences in neurodevelopmental and mental health outcomes.
2.2.2. Application of infant fMRI to understanding effects of moderate, common forms of familial stress
Preterm birth and medical procedures represent relatively extreme forms of ELS. fMRI with infants can also build on the extant literature focused on functional brain development in the context of more normative variation in the early environment, or moderate forms of ELS. EEG work with infants has indicated that normative variation in maternal caregiving behaviors (e.g. maternal sensitivity and intrusiveness during feeding and changing) during the first year of life is associated with differences in brain activity. For example, lower quality maternal caregiving at 9 months has been associated with infants showing greater right frontal EEG asymmetry at a concurrent assessment (Hane and Fox, 2006) and at 3 years of age (Hane et al., 2010). Frontal EEG asymmetry is of interest for research into the effects of ELS on development due to associations of this measure with temperament (Fox et al., 1995, Fox et al., 2001, McManis et al., 2002) and internalizing and externalizing behaviors in young children (Smith and Bell, 2010), and symptoms of depression and anxiety in adults (Stewart et al., 2010, Thibodeau et al., 2006). However, frontal EEG asymmetry is a broad measure of brain functioning that does not distinguish among the contributions of specific frontal or subcortical brain regions.
Recent work in adults has demonstrated the utility of fMRI to localize asymmetry to the functioning of specific frontal brain regions in order to increase understanding of the associations between asymmetry and behavioral outcomes and psychopathology (Miller et al., 2013; Herrington et al., 2010). Miller reviews evidence suggesting that inconsistencies in the associations between frontal EEG asymmetry and behavioral outcomes can be addressed to some extent by taking advantage of information about distinct roles for different parts of the frontal cortex in cognitive and emotional functioning. Along these lines, fMRI research in infants has potential to build on the rich foundation of frontal EEG asymmetry research to examine effects of ELS on functional asymmetry within specific frontal and subcortical brain regions. While frontal asymmetry has not yet been examined with infant fMRI, existing work has demonstrated the capacity for infant fMRI to reveal an association between a moderate form of ELS and functioning of specific regions of the frontal cortex (Graham et al., 2013). Specifically, higher levels of nonphysical conflict between parents appear to be associated with 6–12 month-old infants showing greater reactivity of the rostral anterior cingulate cortex (rACC; along with subcortical regions including the thalamus and hypothalamus) to very angry tone of voice (Graham et al., 2013; Fig. 2). Although this study did not identify a lateralized prefrontal region, it demonstrates the sensitivity of infant fMRI to identify an association between a moderate source of ELS and the functioning of a specific prefrontal brain region implicated in mental health outcomes at later developmental stages.
Fig. 2.
Association between interparental-conflict scores and brain reactivity to very angry relative to neutral speech (p < .05, family-wise-error corrected for multiple comparisons) displayed on the group mean structural image. The image in (a) shows activation in the rostral anterior cingulate cortex (rACC; infant-atlas: x = 3, y = 29, z = 13; MNI: x = 4, y = 36, z = 17). The images in (a) and (b) show activation in a subcortical cluster including hypothalamus, caudate, and thalamus (infant-atlas: x = 3, y = −1, z = 1; MNI: x = 4, y = −1, z = 1), in which higher conflict scores predicted greater neural response to very angry than to neutral speech. The scatter plots (c and d; with best-fitting regression lines) reillustrate the association between conflict score and parameter estimates for these two regions (Graham et al., 2013).
2.3. Building on the fMRI literature in children to study ELS
Research with children old enough to comply with instructions for scanning while awake has taken advantage of the greater spatial resolution of fMRI to study effects of ELS on the functioning of specific brain systems. Differences in brain functioning in school-aged children and adolescents have been associated with various forms of ELS ranging from more severe (e.g. maltreatment [Bruce et al., 2013, McCrory et al., 2013, Mueller et al., 2010] and institutionalized rearing [Mehta et al., 2010, Tottenham et al., 2011]) to more moderate (e.g. differences in socioeconomic status [Muscatell et al., 2012, Sheridan et al., 2012]). These differences in brain functioning are evident in the context of tasks targeting multiple domains relevant to mental health, and cognitive and emotional functioning more generally, such as inhibitory control (Bruce et al., 2013, Mueller et al., 2010), reward processing (Mehta et al., 2010), encoding of social information (Muscatell et al., 2012) and emotion processing (Gee et al., 2013, McCrory et al., 2011, McCrory et al., 2013, Suzuki et al., 2014, Tottenham et al., 2011). Multiple cortical and subcortical brain regions appear to be involved in mediating effects of ELS on functioning in these domains, including among others, the amygdala (McCrory et al., 2011, McCrory et al., 2013, Muscatell et al., 2012, Tottenham et al., 2011), rACC (Gee et al., 2013, Suzuki et al., 2014), dorsal ACC (Bruce et al., 2013, Mueller et al., 2010), and striatum (Mehta et al., 2010). Rs-fcMRI research in adolescents also indicates associations between ELS and coordinated functioning of the amygdala and MPFC in the absence of a specific task (Burghy et al., 2012, Herringa et al., 2013).
This work suggests widespread effects of ELS involving multiple brain systems both at rest and during engagement in a range of cognitive, social and emotional tasks. In addition, this research has provided an important bridge to the extensive animal literature documenting the particular sensitivity of certain brain regions, such as the amygdala, to ELS (Malter Cohen et al., 2013, Sánchez et al., 2001, Tottenham and Sheridan, 2010, Tottenham, 2014). Extending this work down to infancy with natural sleep fMRI can facilitate investigation of several topics that are challenging to examine at older ages. First, infant fMRI has potential to address unanswered questions about the effects of timing of ELS on functional brain systems. Second, infant fMRI may be used to hone in on initial, core effects of ELS that precede a more widespread impact on multiple brain systems and behavioral domains. In a related line of inquiry, this tool will likely be helpful for distinguishing between aspects of brain functioning that precede ELS exposure and confer risk for poor outcomes, versus aspects of brain functioning that are particularly malleable in the context of ELS. Investigation of such topics with infant fMRI has potential to increase understanding of the heterogeneous outcomes associated with ELS, and the methods to best support optimal outcomes.
2.3.1. Potential for infant fMRI to examine timing effects of ELS
Due to the rapid pace of brain development during the pre- and postnatal periods, the timing of exposure to ELS has important implications for how brain systems and mental health will be impacted. This has been demonstrated repeatedly in the animal literature (Sánchez et al., 2001, Tottenham and Sheridan, 2010, Uchida et al., 2010, Vázquez et al., 1996), and to some extent in the human literature (Buss et al., 2012a, Rutter, 1998, Tottenham et al., 2010). However, basic questions regarding effects of timing remain unanswered in the human literature. For example, potentially distinct effects of pre- versus postnatal stress on functional brain development are poorly understood. As stressful environments are not usually confined to the postnatal period, this is an important area of inquiry. Because infant fMRI can characterize neural response to environmental stimuli and intrinsic functional brain networks shortly after birth, it increases the capacity to isolate potential effects of the prenatal environment. Moreover, neonatal brain functioning can be used as a baseline to examine effects of postnatal stress on functional brain development over time. Importantly, the animal literature has shown that pre- and postnatal stress can have opposing effects on biological systems (Lehmann et al., 2000, Love and Williams, 2008, Vallée et al., 1999). Thus, examining brain functioning with fMRI beginning in childhood, or even late infancy, may obscure opposing effects of stress at different time points.
Although only experimental manipulations with animal models can distinguish causal effects of pre- versus postnatal stress, and specific effects of timing within the pre- and postnatal periods, infant fMRI can move human research closer to this foundational work. Infant fMRI studies beginning in the neonatal period cannot feasibly be conducted in certain settings, such as in the context of institutionalized rearing. However, such work is possible in other samples at risk for exposure to ELS. For example, previous study designs have involved recruitment of mothers with psychopathology during pregnancy, and scanning of infants beginning in the neonatal period (e.g. Gilmore et al., 2010, Shi et al., 2012).
2.3.2. Infant fMRI to increase understanding of the initial impact of ELS on core brain regions and networks
Related to the issue of timing of ELS, is the timing of emerging differences in brain functioning following exposure to ELS. Infant fMRI has potential to increase understanding of the core, initial effects of ELS on functional brain systems. Different brain regions and networks will be more vulnerable to the impact of ELS at different stages of development (Knudsen, 2004, Le Grand et al., 2003, Tottenham and Sheridan, 2010). However, environmentally induced changes affecting one aspect of brain functioning are likely to have a ripple effect as brain regions and networks function in concert to support higher level emotional and cognitive processes (Fox et al., 2010, Le Grand et al., 2001). The fMRI work in children suggests a widespread impact of ELS on multiple brain systems. However this work has not addressed whether altered functioning in certain brain regions or networks represents a core feature of the initial impact of ELS, which precedes broader effects. fMRI research with infants beginning within a proximal time frame of ELS provides an opportunity to examine the initial impact of ELS on brain functioning, and subsequent potential ripple effects. For example, a recent rs-fcMRI study with infants demonstrated effects of low socioeconomic status within the first year of life on the connectivity of the default mode network, but not on later developing functional networks involved in cognitive control (Gao et al., 2014b). Continued longitudinal work with such a sample could examine whether these initial differences in the strength of default network connectivity predict subsequent differences in other functional networks, and in cognitive and mental health outcomes of interest.
2.3.3. Preexisting aspects of brain functioning that confer risk
Similarly, there is a lack of clarity regarding preexisting aspects of brain functioning that predispose infants to maladjustment following stress exposure (i.e. risk factors), versus aspects of brain functioning impacted by ELS that confer risk for poor outcomes. Research with adults demonstrates how longitudinal fMRI studies can begin to shed light on this issue. For example, in adult combat paramedics, preexisting high levels of amygdala reactivity (prior to deployment) predict emergence of stress related symptoms following deployment, while changes in reactivity and functional connectivity of the hippocampus over the course of deployment are associated with increases in stress related symptoms (Admon et al., 2009). Additional work suggests that greater preexisting amygdala reactivity (prior to deployment) in combination with decreased nucleus accumbens activity over the course of deployment is a particularly strong predictor of increases in stress symptomatology (Admon et al., 2012). It remains unknown whether mental health outcomes associated with ELS are similarly influenced by preexisting activity in certain brain regions and plasticity in the functioning of others. Infants with certain neural risk factors may require additional support to avoid poor outcomes even if only exposed to very mild levels of ELS, while infants without such risk factors may thrive even when exposed to higher levels of ELS. Infant fMRI is the only methodology that allows for examining the early functioning of specific subcortical brain regions that are likely to be important for understanding maladaptive and adaptive responses to ELS.
2.4. Relevance of infant fMRI for intervention related to ELS
Application of infant fMRI to the study of ELS has potential to inform prevention and intervention efforts during early developmental periods when such services are likely to be most impactful for establishing healthy developmental trajectories. First, increased understanding of individual differences which contribute to vulnerability or resilience to ELS can contribute to targeted prevention work. Although fMRI scans do not represent a feasible means of identifying at-risk infants and toddlers, such work can lead to an increasingly in depth understanding of risk and resilience, and more readily identifiable behavioral or physiological markers. Second, prevention work can be informed by associations between sources of ELS during specific time frames, and changes in brain functioning associated with later developing behavioral symptoms. Identification of early changes in brain functioning may provide a link between specific sources of ELS and behavioral symptoms that emerge later when the complexity of behavioral repertoires and environmental demands increase. These methods additionally provide a means of examining neural mechanisms underlying effective intervention for children exposed to ELS. Targeted prevention and intervention strategies based on this work can facilitate efficient use of resources.
3. Conclusion
The use of fMRI during natural sleep represents a promising methodology for advancing understanding of functional brain development. Research employing task-based and rs-fcMRI methods with infants during natural sleep has become increasingly popular to the extent that a foundational literature has been established. This foundational work demonstrates the feasibility and utility of these methods for examining how the infant brain responds to environmental stimuli, and how it becomes organized into functional networks that facilitate efficient communication among brain regions. Considered in the context of more widely used functional neuroimaging methods for infants (including EEG and fNIRs), fMRI has great potential to complement and advance the existing literature due to its unique advantage with regard to spatial resolution. Indeed, convergent evidence across modalities will be essential for deepening the current understanding of early brain development.
The methodological challenges associated with task-based fMRI and rs-fcMRI with infants and toddlers during natural sleep necessitate careful consideration and further study. However, progress has already been made in understanding and addressing these challenges. Moreover, the literature to date documenting the approaches of various research groups provides a framework to inform careful decision making about methodological issues. We hope that the increasing use of sleep fMRI, and systematic study of methodological questions will lead to further insight into the best means of addressing these challenges. Some of the challenges may also highlight aspects of brain development that receive little attention. For example, increasing study of the issues regarding sleep state may draw attention to the sensitivity of the infant brain to environmental conditions even during sleep.
Despite the methodological challenges, task-based fMRI and rs-fcMRI during natural sleep have potential to advance research across multiple domains. The literature examining effects of ELS on brain functioning and associated mental health difficulties is a prime example of an area that stands to benefit from the application of infant fMRI. The research to date in this area indicates an important role for specific brain systems in mediating effects of ELS on mental health outcomes. The use of infant fMRI allows for examining these systems within a proximal time frame of ELS, and within the window of rapid brain development and heightened vulnerability to disorganizing influences. The wide ranging effects of ELS on multiple domains of functioning may be understood through a new lens by examining early emerging effects on developing brain systems and their potential to subsequently influence additional aspects of brain functioning. Comprehensive developmental models stemming from such work are needed to increase understanding of how brain development proceeds under a variety of environmental conditions.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
Support for this work was provided by NRSA F31-10667639 (AG); R00 MH091238 (DF), and R01 MH096773 (DF).
Footnotes
Available online 16 October 2014
References
- Admon R., Lubin G., Rosenblatt J.D., Stern O., Kahn I., Assaf M., Hendler T. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- Admon R., Lubin G., Stern O., Rosenberg K., Sela L., Ben-Ami H., Hendler T. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S., Lin W., Smith J.K., Gilmore J.H., Gao W. Consistent anterior–posterior segregation of the insula during the first 2 years of life. Cereb. Cortex. 2013:1–12. doi: 10.1093/cercor/bht312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S., Lin W., Smith J.K., Short S.J., Goldman B.D., Reznick J.S., Gilmore J.H., Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J. Neurosci. 2014;34(27):9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli C.R., Rivkin M.J., McKinstry R.C. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage. 2007;35(1):308–325. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Altaye M., Holland S., Wilke M., Gaser C. Infant brain probability templates for MRI segmentation and normalization. Neuroimage. 2008;43(4):721–730. doi: 10.1016/j.neuroimage.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances B.M., Liang C.L., Leontiev O., Perthen J.E., Fleisher A.S., Lansing A.E., Buxton R.B. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum. Brain Mapp. 2009;30(4):1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.W.D.P., Marois R., Colson E.R.D.M., Peterson B.S. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn. Reson. Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Aoyama S., Toshima T., Saito Y., Konishi N., Motoshige K., Ishikawa N. Maternal breast milk odour induces frontal lobe activation in neonates: a NIRS study. Early Hum. Dev. 2010;86(9):541–545. doi: 10.1016/j.earlhumdev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Arichi T., Fagiolo G., Varela M., Melendez-Calderon A., Allievi A., Merchant N. Development of BOLD signal hemodynamic responses in the human brain. Neuroimage. 2012;63(2):663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelin A., Salanterä S. Ethics in neonatal pain research. Nurs. Ethics. 2008;15(4):492–499. doi: 10.1177/0969733007086017. [DOI] [PubMed] [Google Scholar]
- Azak S., Murison R., Wentzel-Larsen T., Smith L., Gunnar M.R. Maternal depression and infant daytime cortisol. Dev. Psychobiol. 2012;55(4):334–351. doi: 10.1002/dev.21033. [DOI] [PubMed] [Google Scholar]
- Bartocci M., Bergqvist L.L., Lagercrantz H., Anand K.J.S. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(1–2):109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Beck D.M., Schaefer C., Pang K., Carlson S.M. Executive function in preschool children: test–retest reliability. J. Cognit. Dev. 2011;12(2):169–193. doi: 10.1080/15248372.2011.563485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. Depression, rumination and the default network. Soc. Cognit. Affect. Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Bellgowan P.S.F., Springer J.A., Kaufman J.N., Possing E.T. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S.M. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. http://www.ncbi.nlm.nih.gov/pubmed/8524021 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Blasi A., Mercure E., Lloyd-Fox S., Thomson A., Brammer M., Sauter D. Early specialization for voice and emotion processing in the infant brain. Curr. Biol. 2011;21(14):1–5. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Boas D.A., Strangman G., Culver J.P., Hoge R.D., Jasdzewski G., Poldrack R.A. Can the cerebral metabolic rate of oxygen be estimated with near-infrared spectroscopy? Phys. Med. Biol. 2003;48(15):2405–2418. doi: 10.1088/0031-9155/48/15/311. http://www.ncbi.nlm.nih.gov/pubmed/12953906 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Boly M., Perlbarg V., Marrelec G., Schabus M., Laureys S., Doyon J. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc. Natl. Acad. Sci. U. S. A. 2012;109(15):5856–5861. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H., Wruck E., Boas D. Assessing infants’ cortical response to speech using near-infrared spectroscopy. Neuroimage. 2007;34(1):407–415. doi: 10.1016/j.neuroimage.2006.08.010. http://www.sciencedirect.com/science/article/pii/S105381190600869X Retrieved from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier M.R., Thomas J.B., Snyder A.Z., Benzinger T.L., Zhang D., Raichle M.E. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 2012;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Fisher P.A., Graham A.M., Moore W.E., Peake S.J., Mannering A.M. Patterns of brain activation in foster children and nonmaltreated children during an inhibitory control task. Dev. Psychopathol. 2013;25(4 Pt 1):931–941. doi: 10.1017/S095457941300028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L., Molloy E.K., Armstrong J.M., Oler J.A. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 2012;(November):1–8. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U. S. A. 2012;109(20) doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Entringer S., Wadhwa P.D. Fetal programming of brain development – role of intrauterine stress and stress biology in susceptibility for psychopathology. Sci. Signal. 2012;5(245) doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Galvan A., Hare T.A. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005;15(2):239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly A.M.C., Uddin L.Q., Ghaffari M., Kirsch A. Cingulate–precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M., Martynova O., Näätänen R., Erkkolall R., Sillanpää M., Kero P. Speech sounds learned by sleeping newborns. Nature. 2002;415(6872):599–600. doi: 10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- Churchland P.S., Sejnowski T. Neural representation and neural computation. In: Tomberlin J.E., editor. Philosophical Perspectives, Vol. 4, Action Theory and Philosophy of Mind. Ridgeview; Atascadero, CA: 1990. pp. 343–382. [Google Scholar]
- Coan J.A., Schaefer H.S., Davidson R.J. Lending a hand: social regulation of the neural response to threat. Psychol. Sci. 2006;17(12):1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Colonnese M.T., Phillips M.A., Constantine-Paton M., Kaila K., Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat. Neurosci. 2008;11(1):72–79. doi: 10.1038/nn2017. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Chisum H.J., Townsend J., Cowles A., Covington J., Egaas B. Normal brain development and aging: quantitative analysis at in vivo MR. Neuroradiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Czisch M., Wetter T.C., Kaufmann C., Pollmächer T., Holsboer F., Auer D.P. Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage. 2002;16(1):251–258. doi: 10.1006/nimg.2002.1071. [DOI] [PubMed] [Google Scholar]
- D’Esposito M., Deouell L.Y., Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Greicius M.D. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 2009;213(6):525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Montavont A., Jobert A., Allirol L., Dubois J., Hertz-Pannier L., Dehaene S. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang. 2010;114(2):53–65. doi: 10.1016/j.bandl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Diekema D.S. Ethical issues in research involving infants. Semin. Perinatol. 2009;33(6):364–371. doi: 10.1053/j.semperi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Dinstein I., Pierce K., Eyler L.T., Solso S., Malach R., Behrmann M., Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J., Marshall J. Pediatric neuroimaging ethics. Camb. Q. Healthc. Ethics. 2007;16(02):147–160. doi: 10.1017/s096318010707017x. [DOI] [PubMed] [Google Scholar]
- Dunn A.K., Devor A., Bolay H., Andermann M.L., Moskowitz M.A., Dale A.M., Boas D.A. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt. Lett. 2003;28(1):28–30. doi: 10.1364/ol.28.000028. http://www.ncbi.nlm.nih.gov/pubmed/12656525 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Eyler L.T., Pierce K., Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135:949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi L., Slater R., Worley A., Meek J., Boyd S., Olhede S., Fitzgerald M. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr. Biol. 2011;21(18):1552–1558. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., Church J.A., Miezin F.M., Barch D.M. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. http://www.pnas.org/content/105/10/4028.short Retrieved from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M. Functional brain networks develop from a local to distributed organization. PLoS Comput. Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U. S. A. 2007;104(33):13507–13512. [Google Scholar]
- Fair D.A., Nigg J.T., Iyer S., Bathula D., Mills K.L., Dosenbach N.U.F. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2012;6(February):80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Posner J., Nagel B.J., Bathula D., Dias T.G.C., Mills K.L. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Schlaggar B.L. Encyclopedia of Infant and Early Childhood Development. Academic Press; 2008. Brain development. [Google Scholar]
- Fava E., Hull R., Baumbauer K., Bortfeld H. Hemodynamic responses to speech and music in preverbal infants. Child Neuropsychol. 2013;20(4):430–448. doi: 10.1080/09297049.2013.803524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E., Miezin F.M., Constantino J.N., Schlaggar B.L., Petersen S.E., Pruett J.R. The hemodynamic response in children with Simplex Autism. Dev. Cognit. Neurosci. 2012;2(4):396–408. doi: 10.1016/j.dcn.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifer W.P., Byrd D.L., Kaku M., Eigsti I.-M., Isler J.R., Grose-Fifer J. Newborn infants learn during sleep. Proc. Natl. Acad. Sci. U. S. A. 2010;107(22):10320–10323. doi: 10.1073/pnas.1005061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V.S., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V.S., Evans A.C., Mckinstry R.C., Almli C.R., Collins D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47(Suppl. 1):S102. [Google Scholar]
- Fox M.D., Greicius M.D. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 2010;4:1–13. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56(1):171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Rubin K.H., Calkins S.D., Schmidt L.A. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Rubin K.H., Calkins S.D., Marshall T.J., Coplan R.J., Porges S.W. Frontal activation asymmetry and social competence at four years of age. Child Dev. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Fox S.E., Levitt P., Nelson C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cerebral Cortex. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fransson P., Metsäranta M., Blennow M., Aden U., Lagercrantz H., Vanhatalo S. Early development of spatial patterns of power-law frequency scaling in fMRI resting-state and EEG data in the newborn brain. Cereb. Cortex. 2013;23(3):638–646. doi: 10.1093/cercor/bhs047. [DOI] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Engström M., Hallberg B., Mosskin M., Aden U. Spontaneous brain activity in the newborn brain during natural sleep – an fMRI study in infants born at full term. Pediatr. Res. 2009;66(3):301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Elton A., Hernandez-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb. Cortex. 2014:1–10. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Smith J.K. Development of human brain cortical network architecture during infancy. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Elton A., Zhu H., Alcauter S., Smith J.K., Gilmore J.H., Lin W. Intersubject variability of and genetic effects on the brain's functional connectivity during infancy. J. Neurosci. 2014;34(34):11288–11296. doi: 10.1523/JNEUROSCI.5072-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Giovanello K.S., Smith J.K., Shen D., Zhu H., Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS ONE. 2011;6(9):e25278. doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Shen D., Smith J.K., Zhu H., Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb. Cortex. 2013;23(3):594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U. S. A. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. http://www.pnas.org/content/106/16/6790.short Retrieved from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Kang C., Evans D.D., Wolfe H.M., Smith J.K., Lieberman J.A. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am. J. Psychiatry. 2010;167(9):1083–1091. doi: 10.1176/appi.ajp.2010.09101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Fisher P.A., Pfeifer J.H. What sleeping babies hear: a functional MRI study of interparental conflict and infants’ emotion processing. Psychol. Sci. 2013;24(5):782–789. doi: 10.1177/0956797612458803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Ray S., Carpenter S., Iyer S., Dias T.G.C., Stevens C. Structural and functional rich club organization of the brain in children and adults. PLOS ONE. 2014;9(2):e88297. doi: 10.1371/journal.pone.0088297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Oberecker R., Koch S.P., Friederici A.D. The developmental origins of voice processing in the human brain. Neuron. 2010;65(6):852–858. doi: 10.1016/j.neuron.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Striano T., Friederici A.D. Infants’ electric brain responses to emotional prosody. Neuroreport. 2005;16(16):1825–1828. doi: 10.1097/01.wnr.0000185964.34336.b1. http://www.ncbi.nlm.nih.gov/pubmed/16237335 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Grunau R.E. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med. J. 2013;4(4):e0025. doi: 10.5041/RMMJ.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Talge N.M., Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Grant P.E., Fair D.A. MR connectomics: a conceptual framework for studying the developing brain. Front. Syst. Neurosci. 2012;6(June):43. doi: 10.3389/fnsys.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane A.A., Fox N.A. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychol. Sci. 2006;17(6):550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hane A.A., Henderson H.A., Reeb-Sutherland B.C., Fox N.A. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: a follow-up study. Dev. Psychobiol. 2010;52(6):558–567. doi: 10.1002/dev.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J.D., Heller W., Mohanty A., Engels A.S., Banich M.T., Webb A.G., Miller G.A. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47(3):442–454. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton V.J. Ethics of neuroimaging in pediatric development. Brain Cogn. 2002;50(3):455–468. doi: 10.1016/s0278-2626(02)00521-3. http://www.ncbi.nlm.nih.gov/pubmed/12480490 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Honey C., Sporns O., Cammoun L., Gigandet X., Thiran J.P., Meuli R., Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):2035. doi: 10.1073/pnas.0811168106. http://www.pnas.org/content/106/6/2035.short Retrieved from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz S.G., Braun A.R., Carr W.S., Picchioni D., Balkin T.J., Fukunaga M., Duyn J.H. Decoupling of the brain's default mode network during deep sleep. Proc. Natl. Acad. Sci. U. S. A. 2009;106(27):11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz S.G., Fukunaga M., de Zwart J.A., van Gelderen P., Fulton S.C., Balkin T.J., Duyn J.H. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum. Brain Mapp. 2008;29(6):671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comparat. Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. http://www.ncbi.nlm.nih.gov/pubmed/9336221 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Johnson S., Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin. Fetal Neonatal. Med. 2014;19(2):97–104. doi: 10.1016/j.siny.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Karen T., Morren G., Haensse D., Bauschatz A.S., Bucher H.U., Wolf M. Hemodynamic response to visual stimulation in newborn infants using functional near-infrared spectroscopy. Hum. Brain Mapp. 2008;29(4):453–460. doi: 10.1002/hbm.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann C., Wehrle R., Wetter T.C., Holsboer F., Auer D.P., Pollmächer T., Czisch M. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2006;129(Pt 3):655–667. doi: 10.1093/brain/awh686. [DOI] [PubMed] [Google Scholar]
- Kazemi K., Moghaddam H.A., Grebe R., Gondry-Jouet C., Wallois F. A neonatal atlas template for spatial normalization of whole-brain magnetic resonance images of newborns: preliminary results. Neuroimage. 2007;37(2):463–473. doi: 10.1016/j.neuroimage.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kelly A.M.C., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly A.M.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kida T., Shinohara K. Gentle touch activates the prefrontal cortex in infancy: an NIRS study. Neurosci. Lett. 2013;541:63–66. doi: 10.1016/j.neulet.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kozberg M.G., Chen B.R., Deleo S.E., Bouchard M.B., Hillman E.M.C. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc. Natl. Acad. Sci. U. S. A. 2013;110(11):4380–4385. doi: 10.1073/pnas.1212785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B.J., Schmidt D., Mottaghy F.M., Taylor J., Halsband U., Herzog H. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates. A PET study. Brain. 1999;122:255–263. doi: 10.1093/brain/122.2.255. http://www.ncbi.nlm.nih.gov/pubmed/10071054 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Kusaka T., Kawada K., Okubo K., Nagano K., Namba M., Okada H. Noninvasive optical imaging in the visual cortex in young infants. Hum. Brain Mapp. 2004;22(2):122–132. doi: 10.1002/hbm.20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A.E., Young R.L., Baker A.E.Z., Angley M.T. Sensory processing subtypes in autism: association with adaptive behavior. J. Autism Dev. Disord. 2010;40(1):112–122. doi: 10.1007/s10803-009-0840-2. [DOI] [PubMed] [Google Scholar]
- Larson-Prior L.J., Zempel J.M., Nolan T.S., Prior F.W., Snyder A.Z., Raichle M.E. Cortical network functional connectivity in the descent to sleep. Proc. Natl. Acad. Sci. U. S. A. 2009;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand R., Mondloch C.J., Maurer D., Brent H.P. Early visual experience essential for facial recognition. Nature. 2001;410(6831):890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- Le Grand R., Mondloch C.J., Maurer D., Brent H.P. Expert face processing requires visual input to the right hemisphere during infancy. Nat. Neurosci. 2003;6(10):1108–1112. doi: 10.1038/nn1121. [DOI] [PubMed] [Google Scholar]
- Lee M., Smyser C.D., Shimony J.S. Resting-state fMRI: a review of methods and clinical applications. Am. J. Neuroradiol. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Stöhr T., Feldon J. Long-term effects of prenatal stress experience and postnatal maternal separation on emotionality and attentional processes. Behav. Brain Res. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. http://www.sciencedirect.com/science/article/pii/S0166432899001229 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Liao S.M., Gregg N.M., White B.R., Zeff B.W., Bjerkaas K.A., Inder T.E., Culver J.P. Neonatal hemodynamic response to visual cortex activity: high-density near-infrared spectroscopy study. J. Biomed. Opt. 2010;15(2):026010. doi: 10.1117/1.3369809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Zhu Q., Gao W., Chen Y. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. Am. J. Neuroradiol. 2008;29(10):1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.-C., Flax J.F., Guise K.G., Sukul V., Benasich A.A. Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Res. 2008;1223:42–49. doi: 10.1016/j.brainres.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Volein A., Everdell N., Elwell C.E., Johnson M.H. Social perception in infancy: a near infrared spectroscopy study. Child Dev. 2009;80(4):986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Papademetriou M., Darboe M.K., Everdell N.L., Wegmuller R., Prentice A.M. Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Sci. Rep. 2014;4:4740. doi: 10.1038/srep04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Wandell B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Loman M.M., Gunnar M.R. Early experience and the development of stress reactivity and regulation in children. Neurosci. Biobehav. Rev. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love O.P., Williams T.D. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm. Behav. 2008;54(4):496–505. doi: 10.1016/j.yhbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynova O., Kirjavainen J., Cheour M. Mismatch negativity and late discriminative negativity in sleeping human newborns. Neurosci. Lett. 2003;340(2):75–78. doi: 10.1016/s0304-3940(02)01401-5. http://www.ncbi.nlm.nih.gov/pubmed/12668240 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Mather M., Cacioppo J.T., Kanwisher N. Introduction to the special section: 20 years of fMRI – what has it done for understanding cognition? Perspect. Psychol. Sci. 2013;8(1):41–43. doi: 10.1177/1745691612469036. [DOI] [PubMed] [Google Scholar]
- Matthews P.M., Honey G.D., Bullmore E.T. Applications of fMRI in translational medicine and clinical practice. Nat. Rev. Neurosci. 2006;7(9):732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Kelly P.A., Bird G., Sebastian C.L., Mechelli A. Amygdala activation in maltreated children during pre-attentive emotional processing. Br. J. Psychiatry. 2013;202(4):269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Sebastian C.L., Mechelli A., Bird G., Kelly P.A., Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McManis M.H., Kagan J., Snidman N.C., Woodward S.A. EEG asymmetry, power, and temperament in children. Dev. Psychobiol. 2002;41(2):169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- Meek J.H., Firbank M., Elwell C.E., Atkinson J., Braddick O., Wyatt J.S. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr. Res. 1998;43(6):840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Mehta M.A., Gore-Langton E., Golembo N., Colvert E., Williams S.C.R., Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cogn. Neurosci. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Crocker L.D., Spielberg J.M., Infantolino Z.P., Heller W. Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front. Integr. Neurosci. 2013;7:1–9. doi: 10.3389/fnint.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O., Mills B.D., Grayson D., Woodall A., Grant K.A., Kroenke C.D., Fair D.A. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. J. Neurosci. 2014;34(16):5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy E.K., Meyerand M.E., Birn R.M. The influence of spatial resolution and smoothing on the detectability of resting-state and task fMRI. Neuroimage. 2014;86:221–230. doi: 10.1016/j.neuroimage.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C., Maheu F.S., Dozier M., Peloso E., Mandell D., Leibenluft E. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y., Biessmann F., Meinecke F.C., Müller K.-R., Augath M., Oeltermann A., Logothetis N.K. Relationship between neural and hemodynamic signals during spontaneous activity studied with temporal kernel CCA. Magn. Reson. Imaging. 2010;28(8):1095–1103. doi: 10.1016/j.mri.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Muscatell K., Morelli S., Falk E., Way B., Pfeifer J.H., Galinksy A.D. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60(3):1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Forkstam C., Petersson K.M., Cabeza R., Ingvar M. Brain imaging of human memory systems: between-systems similarities and within-system differences. Cognit. Brain Res. 2002;13(2):281–292. doi: 10.1016/s0926-6410(02)00052-6. http://www.ncbi.nlm.nih.gov/pubmed/11958972 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Pierce K. Early functional brain development in autism and the promise of sleep fMRI. Brain Res. 2011;1380:162–174. doi: 10.1016/j.brainres.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas C.M., Krakow K., Allen P., Josephs O., Armony J.L., Frith C.D. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28(3):991–999. doi: 10.1016/s0896-6273(00)00169-0. http://www.ncbi.nlm.nih.gov/pubmed/11163282 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M., Ramani R., Swetye M., Rajeevan N., Constable R.T. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: an fMRI study in normal human subjects. Magn. Reson. Med. 2008;60(4):987–996. doi: 10.1002/mrm.21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. Two views of brain function. Trends Cogn. Sci. 2010;14(4):180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Macleod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Mintun M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol. Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Haist F., Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev. Sci. 2008;11(2):237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Redcay E., Kennedy D.P., Courchesne E. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland B.C., Fifer W.P., Byrd D.L., Hammock E.A.D., Levitt P., Fox N.A. One-month-old human infants learn about the social world while they sleep. Dev. Sci. 2011;14(5):1134–1141. doi: 10.1111/j.1467-7687.2011.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C., Minati L., Ghielmetti F., Mandelli M.L., Bruzzone M.G. Functional connectivity during resting-state functional MR imaging: study of the correspondence between independent component analysis and region-of-interest-based methods. Am. J. Neuroradiol. 2012;33(1):180–187. doi: 10.3174/ajnr.A2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. J. Child Psychol. Psychiatry. 1998;39(4):465–476. http://journals.cambridge.org/abstract_S0021963098002236 Retrieved from: [PubMed] [Google Scholar]
- Saito Y., Fukuhara R., Aoyama S., Toshima T. Frontal brain activation in premature infants’ response to auditory stimuli in neonatal intensive care unit. Early Hum. Dev. 2009;85(7):471–474. doi: 10.1016/j.earlhumdev.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Sämann P.G., Wehrle R., Hoehn D., Spoormaker V.I., Peters H., Tully C. Development of the brain's default mode network from wakefulness to slow wave sleep. Cereb. Cortex. 2011;21(9):2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- Sanchez C.E., Richards J.E., Almli C.R. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev. Psychobiol. 2012;54(1):77–91. doi: 10.1002/dev.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M.M., Ladd C.O.C.O., Plotsky P.M.P.M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sander D., Grandjean D., Pourtois G., Schwartz S., Seghier M.L., Scherer K.R., Vuilleumier P. Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage. 2005;28(4):848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Schmidt L.A., Trainor L.J. Frontal brain electrical activity (EEG) and heart rate in response to affective infant-directed (ID) speech in 9-month-old infants. Brain Cogn. 2007;65(1):14–21. doi: 10.1016/j.bandc.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck M.L., Maier A., Ye F.Q., Duyn J.H., Leopold D.A. Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. U. S. A. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl J.R. Glucocorticoids, developmental programming and the risk of affective dysfunction. Prog. Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Sheridan M.A., Sarsour K., Jutte D., D’Esposito M., Boyce W.T. The impact of social disparity on prefrontal function in childhood. PLoS ONE. 2012;7(4):e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Yap P.-T., Gao W., Lin W., Gilmore J.H., Shen D. Altered structural connectivity in neonates at genetic risk for schizophrenia: a combined study using morphological and white matter networks. Neuroimage. 2012;62(3):1622–1633. doi: 10.1016/j.neuroimage.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Yap P.-T., Wu G., Jia H., Gilmore J.H., Lin W., Shen D. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS ONE. 2011;6(4):e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A., Leopold D.A. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum. Brain Mapp. 2008;29(7):751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J.P., Garner A.S. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Church J.A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2013 doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater R., Cantarella A., Gallella S., Worley A., Boyd S., Meek J., Fitzgerald M. Cortical pain responses in human infants. J. Neurosci. 2006;26(14):3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater R., Fabrizi L., Worley A., Meek J., Boyd S., Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage. 2010;52(2):583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- Smith C.L., Bell M.A. Stability in infant frontal asymmetry as a predictor of toddlerhood internalizing and externalizing behaviors. Dev. Psychobiol. 2010;52(2):158–167. doi: 10.1002/dev.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.C., Gutovich J., Smyser C.D., Pineda R., Newnham C., Tjoeng T.H. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 2011;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z., Neil J.J. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Snyder A.Z., Shimony J.S., Blazey T.M., Inder T.E., Neil J.J. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PloS One. 2013;8(7):e68098. doi: 10.1371/journal.pone.0068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souweidane M.M., Kim K.H.S., McDowall R., Ruge M.I., Lis E., Krol G., Hirsch J. Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatr. Neurosurg. 1999;30(2):86–92. doi: 10.1159/000028768. [DOI] [PubMed] [Google Scholar]
- Spoormaker V.I., Schröter M.S., Gleiser P.M., Andrade K.C., Dresler M., Wehrle R. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J. Neurosci. 2010;30(34):11379–11387. doi: 10.1523/JNEUROSCI.2015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A.A., Tappon S.C., Garg A., Fair D.A. Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLoS ONE. 2012;7(1):e30468. doi: 10.1371/journal.pone.0030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.L., Bismark A.W., Towers D.N., Coan J.A., Allen J.B. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 2010;119(3):502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother S., La Conte S., Kai Hansen L., Anderson J., Zhang J., Pulapura S., Rottenberg D. Optimizing the fMRI data-processing pipeline using prediction and reproducibility performance metrics: I. A preliminary group analysis. Neuroimage. 2004;23(Suppl. 1):S196–S207. doi: 10.1016/j.neuroimage.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Luby J.L., Botteron K.N., Dietrich R., McAvoy M.P., Barch D.M. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J. Am. Acad. Child Adolesc. Psychiatry. 2014 doi: 10.1016/j.jaac.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G., Asakawa K., Maki A., Konishi Y., Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proc. Natl. Acad. Sci. U. S. A. 2003;100(19):10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E., von Wegner F., Morzelewski A., Borisov S., Jahnke K., Laufs H. Automatic sleep staging using fMRI functional connectivity data. Neuroimage. 2012;63(1):63–72. doi: 10.1016/j.neuroimage.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Talairach P., Tournoux J. Georg Thieme Verlag; Stuttgart, Germany: 1988. Co-planar Stereotaxic Altas of the Human Brain. [Google Scholar]
- Thibodeau R., Jorgensen R.S., Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J. Abnorm. Psychol. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. In: Pine D.S., Andersen S.L., editors. Current Topics in Behavioral Neuroscience, Volume 16: The Neurobiology of Childhood. Springer-Verlag; Berlin: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Shapiro M., Telzer E.H., Humphreys K.L. Amygdala response to mother. Dev. Sci. 2012;15(3):307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3(68):1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Hara K., Kobayashi A., Funato H., Hobara T., Otsuki K. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J. Neurosci. 2010;30(45):15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Front. Syst. Neurosci. 2010;4:1–12. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M., MacCari S., Dellu F., Simon H., Le Moal M., Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur. J. Neurosci. 1999;11(8):2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. http://www.ncbi.nlm.nih.gov/pubmed/10457187 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle H.J.F., Hermans E.J., Qin S., Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53(1):348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Vázquez D.M., Van Oers H., Levine S., Akil H. Regulation of glucocorticoid and mineralocorticoid receptor mRNAs in the hippocampus of the maternally deprived infant rat. Brain Res. 1996;731(1–2):79–90. doi: 10.1016/0006-8993(96)00465-9. http://www.ncbi.nlm.nih.gov/pubmed/8883857 Retrieved from: [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Snyder A.Z., Fox M.D., Shannon B.J., Andrews J.R., Raichle M.E., Buckner R.L. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Homae F., Nakano T., Taga G. Functional activation in diverse regions of the developing brain of human infants. Neuroimage. 2008;43:346–357. doi: 10.1016/j.neuroimage.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Wenger K.K., Visscher K.M., Miezin F.M., Petersen S.E., Schlaggar B.L. Comparison of sustained and transient activity in children and adults using a mixed blocked/event-related fMRI design. Neuroimage. 2004;22(2):975–985. doi: 10.1016/j.neuroimage.2004.02.028. [DOI] [PubMed] [Google Scholar]
- White B.R., Liao S.M., Ferradal S.L., Inder T.E., Culver J.P. Bedside optical imaging of occipital resting-state functional connectivity in neonates. Neuroimage. 2013;59(3):2529–2538. doi: 10.1016/j.neuroimage.2011.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Ann. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Yamada H., Sadato N., Konishi Y., Kimura K., Tanaka M., Yonekura Y., Ishii Y. A rapid brain metabolic change in infants detected by fMRI. Neuroreport. 1997;8(17):3775–3778. doi: 10.1097/00001756-199712010-00024. http://journals.lww.com/neuroreport/Abstract/1997/12010/A_rapid_brain_metabolic_change_in_infants_detected.24.aspx Retrieved from: [DOI] [PubMed] [Google Scholar]
- Yan C.-G., Craddock R.C., He Y., Milham M.P. Addressing head motion dependencies for small-world topologies in functional connectomics. Front. Hum. Neurosci. 2013;7:910. doi: 10.3389/fnhum.2013.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Altaye M., Ret J., Schmithorst V., Byars A.W., Plante E., Holland S.K. Quantification of head motion in children during various fMRI language tasks. Hum. Brain Mapp. 2009;30(5):1481–1489. doi: 10.1002/hbm.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]