Abstract
Imatinib will become generic in 2016; assuming that its price will decrease precipitously, we expect that the economic forces will change our current practice habits. We reviewed the literature on the current recommendations to treat chronic myeloid leukemia and highlight how we plan to deal with these changes. Specifically, we propose to better characterize patients according to prognostic scores, to allow more attention to those at high risk for disease progression, e.g., three-month guidelines and BCR/ABL1 message half-time, emphasize compliance by using contemporary technologies, and increase the importance of early monitoring. We hope that our message will open communication between providers, insurance companies and healthcare authorities to offer the best care for our patients.
Keywords: Chronic myeloid leukemia, tyrosine kinase inhibitors, price of drugs, generic
Introduction
Imatinib was approved for chronic myeloid leukemia (CML) in the United States of America (USA) by the Food and Drug Administration (FDA) in 2001.1 Since its approval, two other tyrosine kinase inhibitors (TKIs), nilotinib and dasatinib, were approved by the FDA as frontline treatment for CML based on two separate randomized trials comparing these second generation TKIs to imatinib.2, 3 Both trials showed “faster” cytogenetic and molecular responses at 12 months with the second generation TKIs which persisted up to three years (Figures 1, Panels A and B). Dasatinib’s improved cytogenetic outcome at 12 months was also confirmed by an independent trial.4 Interestingly, high-dose imatinib improved cytogenetic and molecular responses in one randomized study5 but not in another.6 Those effects were mainly observed in patients with a higher risk for disease progression based on the Sokal,7 the Hasford8 or the European Treatment and Outcome Study (EUTOS)9 prognostic systems (Tables 1 and 2). Progression to accelerated/blastic phases was statistically less frequent with the use of nilotinib compared to imatinib (ENESTnd, Figure 1, Panel C). No such data are available for dasatinib or high-dose imatinib. Yet, none of these differences translated into longer disease-free or overall survival. In spite of these facts, the second generation TKIs have been adopted in the first line setting for all patients by many practitioners in the USA. Their long-term safety data are summarized in Table 3.
Figure 1.
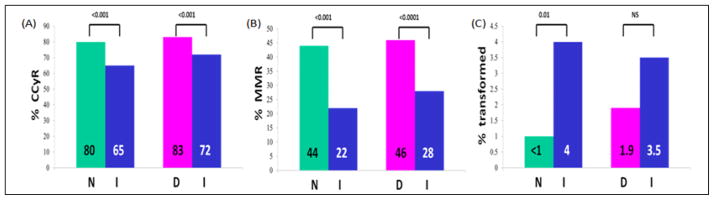
Response to nilotinib and dasatinib compared to imatinib. Panel A and B demonstrate complete cytogenetic response (CCyR) and major molecular response (MMR) achieved at 12 months in ENESTnd [nilotinib (N) vs. imatinib (I)] and DASISION [dasatinib (D) vs. imatinib (I)] trials. Panel C shows progression to accelerated/blastic phase with nilotinib (N) and dasatinib (D) in comparison to imatinib (I), at the conclusion of the first 12 months of follow-up. Numbers at the top represent P values.
Table 1.
Current prognostication systems for chronic myeloid leukemia.
| Parameters | Sokal Risk Score | Hasford Risk Score | EUTOS score |
|---|---|---|---|
| Age (years) | 0.116 (Age – 43.4) | 0.666 when Age ≥50 | |
| Spleen (cm) | 0.0345 (Spleen – 7.51) | 0.042 x Spleen | 0.0402 x Spleen |
| Platelet Count (x109/L) | 0.188 [(Plt/700)2−0.563] | 1.0956 when Platlet ≥1500 | |
| Blood Myeloblasts (%) | 0.0887 (Myeloblasts -2.10) | 0.0584 x Myeloblasts | |
| Blood Basophils (%) | - | 0.20399 when Basophils >3% | 0.07 x Basophils |
| Blood Eosinophils (%) | - | 0.0413 x Eosinophils | |
| Relative Risk | Exponential of the Total | Total x1000 | |
| Low Risk | <0.8 | ≤780 | ≤87 |
| Intermediate Risk | 0.8 – 1.2 | 781–1480 | |
| High Risk | >1.2 | >1480 | <87 |
Abbreviations: EUTOS, EUropean Treatment and Outcome Study.
Table 2.
Patients stratified by Sokal risk score and response to imatinib and nilotinib.
| Sokal Risk Imatinib (IRIS) |
Low Risk (%) |
Intermediate Risk (%) |
High Risk (%) |
|||
|---|---|---|---|---|---|---|
| CCyR (p<0.001) | 89 | 82 | 69 | |||
| Disease progression at 60 months | 3 | 8 | 17 | |||
| No disease progression in patients with CCyR | 99 | 95 | 95 | |||
| Nilotinib vs. imatinib (ENESTnd) | I | N | I | N | I | N |
| Best cumulative MMR by 3 years* | 62.5 | 76.7 | 54.5 | 75.2 | 38.5 | 66.7 |
After the 2-year visit, cytogenetic assessments were not required for all patients, hence no CCyR rates are available.
Abbreviations: CCyR, complete cytogeneric remission; I, imatinib; IRIS, International Randomized study of Interferon and STI571; N, nilotinib.
Table 3.
Long-term safety data for Imatinib, nilotinib and Dasatinib. Data are presented as percentages (unless otherwise described); data were extracted from IRIS, ENESTnd and DASISION trials.
| Imatinib | Nilotinib* | Dasatinib | |
|---|---|---|---|
| Anemia | 5.7 | 3.9–4.7 | 6–11 |
| Neutropenia | 21.4 | 10.8–11.8 | 15–24 |
| Thrombocytopenia | 8.9 | 10.4–12.3 | 10–19 |
| Nausea/Vomiting | 20–10 | 11–19/5–9 | 10/5 |
| Diarrhea | 21 | 6–8 | 21 |
| Fluid Retention | 56.4 | 18.6–23.5 | 13 |
| Effusions | 1.8 | 0.7–1.8 | 19 |
| Myalgia | 12 | -- | 23 |
| Muscle Inflammation | 17 | -- | 4 |
| Musculoskeletal pain | 14 | -- | 11 |
| Muscle Spasm | 24 | 6–7 | -- |
| Fatigue | 10 | -- | 9 |
| Rash | 22.1 | 41.2–46.9 | 13 |
| Alopecia | 4 | 8–13 | -- |
| Pruritus | 5 | 13–15 | -- |
| Headache | 10 | 14–21 | 13 |
| Pulmonary Arterial Hypertension | 0 | -- | 8 patients |
| Peripheral Arterial Occlussive Disease | 0 | 1.1–1.4 | -- |
| Ischemic Heart Disease | 1.1 | 3.2–4 | -- |
| An increase of >60 miliseconds in QTcF from baseline | 0.4 | 0.4–1.1 | -- |
| Hypophosphatemia | 28 | 5 | 7 |
| Pancreatitis | 0.7 | 1.8–2.2 | -- |
| Hepatotoxicity | 2.5 | 1.4–4 | -- |
| Glucose increased | 0 | 5.4–6.1 | -- |
Data include toxicities from both nilotinib 300 mg twice daily and 400 mg twice daily.
How will we treat this patient in 2016?
Imatinib’s patent expires on February 1st, 2016. Currently, imatinib sells in the USA for $92,000 per year.10 The second generation TKIs are even more expensive; nilotinib costs $115,500 per year and dasatinib $123,500 per year.10 The prices for these drugs vary in other countries. For example, in the United Kingdom, imatinib and nilotinib cost the same ($33,500) while dasatinib is more expensive ($48,500). In South Africa, nilotinib is less expensive than imatinib ($28,000 vs. $43,000) and dasatinib is more expensive than imatinib ($54,500). It is logical to assume that given a broad cost differential after patent expiration, insurance companies and healthcare authorities in the USA will favor generic imatinib as of 2016. The question for clinicians in the face of this anticipated change in drug coverage is how to optimize or codify the upfront use of second generation TKIs for patients at higher risk of progression on imatinib.
Clinical Parameters for Evaluation of Treatment Response
Clinical response to TKIs is measured by three main parameters which are acknowledged by the European Leukemia Net (ELN)11 and the National Comprehensive Cancer Network (NCCN).12 Complete hematologic response (CHR) is defined as reduction in white blood cell count to less than 10×109/L, reduction in platelet count to less than 450×109/L, disappearance of immature cells in the peripheral blood, no signs or symptoms of disease, and disappearance of splenomegaly. Cytogenetic response is divided into complete, partial and minor responses. Complete cytogenetic response (CCyR) is defined as 0% Philadelphia-positive (Ph+) metaphase cells upon evaluation of a minimum of 20 cells; partial cytogenetic response is ≤35% Ph+ metaphase cells, and minor cytogenetic response is >35% Ph+ metaphase cells. Major cytogenetic response applies only to large studies and combines complete and partial cytogenetic responses (0% to 35% metaphase cells with the Ph+ chromosome).13
Molecular response is the most sensitive measure currently used to monitor the disease. It is determined by quantifying the BCR/ABL1 transcript level through quantitative real-time reverse-transcriptase polymerase chain reaction of a sample from either the peripheral blood or the bone marrow. Major molecular response (MMR), a term that arose from the International Randomized study of Interferon and STI571 (IRIS),14 is defined as greater than 3 log reduction (<0.1%) in BCR/ABL1 transcript level based on the International Standard (IS).13 The sensitivity of this assay allows for a new treatment goal of a “deep molecular response” described as 4.5-log fold reduction (MR4.5) of BCR/ABL1 transcript with prognostic value for overall survival.5 Fluctuation of BCR/ABL1 transcript levels at the very low end of the detection level has a poor accuracy in defining a relapse risk.15, 16 Complete molecular response denotes inability to detect the transcript.
Imatinib data indicate that timing and degree of CCyR and MMR achieved have prognostic significance. For instance, attainment of CCyR or MMR within the first 12 months of imatinib treatment predicts a low risk for disease progression (Figure 2).13, 17 Furthermore, achievement of MMR in the first year indicates long-lasting CCyR.13 However, waiting for 12 months is not appropriate and therefore several groups have looked at earlier time points. The three-month time point was chosen by the ELN11 and the NCCN12 as a decision point based on imatinib data showing better outcome if patients achieved 10% or less BCR/ABL1 transcript by IS at the three-month time point (Figure 3, Panel A).18, 19 Others have challenged this time point and proposed the six-month time point, especially when using second generation TKIs because of their more robust response.20 When one compares nilotinib to imatinib data from ENESTnd, (Figure 3, Panel B) one can clearly notice that 33% of patients on imatinib did not achieve the 10% BCR/ABL1 message level by IS at the three-month time point and those patients are at risk for disease progression,21 especially if they had intermediate or high Sokal or Hasford Scores at diagnosis.3 However, no data showing that a change in treatment will modify the prognosis of these patients are available. A study offering nilotinib (400 mg orally twice daily) for patients with suboptimal response by ELN11 showed improved responses in some patients but many did not achieve CCyR.22 It is possible that patients with suboptimal responses inherently have worse disease and therefore are likely to progress regardless of change in treatment.19 We propose the three-month time point as a decision point because we predict that generic imatinib will become the drug of choice based on insurance coverage after 2016, and we therefore should be monitoring these patients more closely for disease progression. Alternatively, though with minimal data on longer disease-free or overall survival, insurance companies and healthcare authorities should be encouraged to pay for the use of second generation TKIs for all patients with intermediate and high Sokal, Hasford or EUTOS scores at the time of diagnosis given their higher risk of disease progression and imatinib failure.
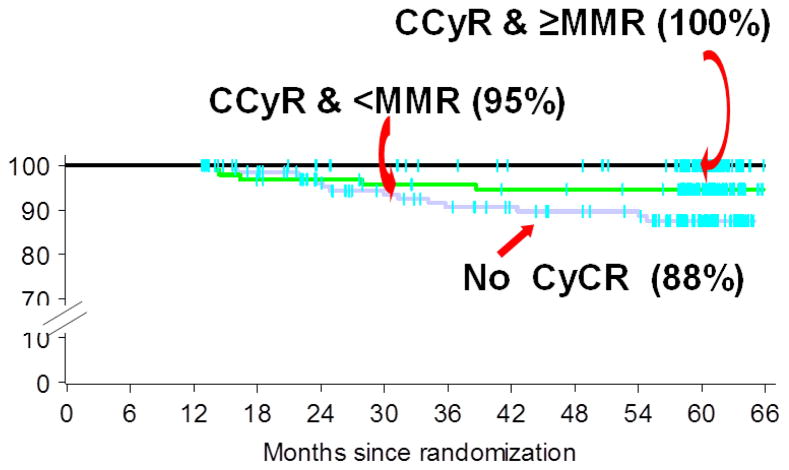
Figure 2.
Survival of newly diagnosed CML patients. Data from IRIS showed that for patients who did not achieve CCyR within the first 12 months of imatinib treatment fared worse. Adapted from Druker et al., New Engl J Med 355:2408–17, 2006 with Permission. Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response.
Figure 3.
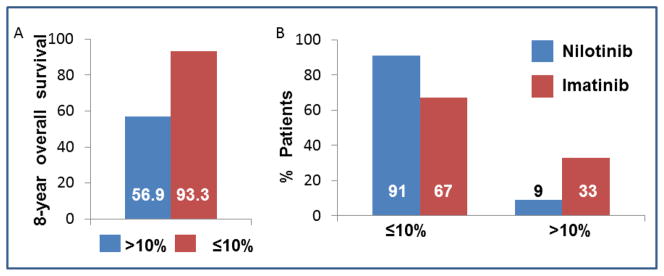
The effect of BCR/ABL1 message measured by International Standard scale at 3 months. Panel A, Overall 8 year survival for patients who achieved molecular response at 3 months from the IRIS. Patients with BCR/ABL1 transcript levels of >10% (n = 68) at 3 months had significantly lower 8-year overall survival (56.9%, p<0.001) compared to patients with transcript levels <10% who had overall survival of 93.3% over 8 years (p<0.001). Panel B demonstrates achievement of BCR/ABL1 message level at three-month time point from ENESTnd.
Two randomized studies, ENESTnd and dasatinib vs. imatinib (DASISION), have taught us that patients at low risk by either Sokal or Hasford prognostic systems are less likely to progress to accelerated/blastic phase when treated with either imatinib or the second generation TKIs. However, patients at the intermediate- and high-risk groups (Table 2) are less likely to benefit from imatinib.23, 24 These prognostic systems (Table 1) should therefore be calculated on all newly diagnosed CML patients.7–9 Such applications currently exist for free on the web (e.g., http://bloodref.com/myeloid/cml/sokal-hasford).
Adherence and Compliance to Therapy
Adherence to treatment is a challenge for many of the patients who are on chronic therapy for any medical condition. The ADAGIO study25 was the first to demonstrate that only 14.2% of CML patients were perfectly adherent with 100% of prescribed imatinib. That study covered only 90 days. A study covering a two-year period in patients with CML or gastrointestinal stromal tumors showed 78% adherence to imatinib.26 Furthermore, this study showed that adherence decreased with imatinib doses above 400 mg daily.26 In the United Kingdom, a study was carried out on 87 patients diagnosed with chronic phase CML and treated with imatinib 400 mg/day for a median of 59.7 months.27 This study reported a median imatinib adherence rate of 98%, however about 26% of the patients had <90% adherence and 14% had <80% adherence.27 Several studies have shown similar rates of non-adherence in patients treated with nilotinib and dasatinib. Santoleri et al28 have shown that adherence to nilotinib was at 0.93 compared to 0.83 with imatinib and 0.85 with dasatinib; however decreased adherence was seen in all three drug treatment cohorts over time. Important factors contributing to non-adherence include drug interactions, adverse reactions, and mental burden of the disease along with forgetfulness of daily dosing. Non-adherence to treatment leads to reduced drug levels resulting in reduced efficacy and potential for loss of response.
Telemonitoring or remote monitoring is a valuable tool currently in development by several pharmaceutical companies. There are two concepts behind the technology. The first is a medication with an implanted ingestible sensor. The medication is ingested by the patient and becomes activated in the stomach and the data are transmitted to a receiver patch on the patient’s abdomen. That data can be visualized on a Smartphone or a computer. In case of missed dosages, the patient can be contacted directly by the provider’s office for reminders.
The second strategy measures adherence based on the patient opening his/her pill bottle, e.g., GlowCap© by Vitality™ (available through different vendors) or MEMS® medication bottle with TrackCap™. These are pill bottles which have an embedded sensor in the bottle cap that sends a signal to a nightlight sensor which has a reminder light and plays a ring tone. The sensor senses and sends a signal to the nightlight receiver each time the bottle has been opened signifying patients administering their pills and thus indicating compliance. The reminder light flashes for an hour from the time of dosing. If the bottle is not opened within this window, the receiver will play a ring tone every few minutes until the bottle has been opened. If the bottle is not opened, a phone call from a monitoring unit will advise the patient or his/her designee about the “missed dosage.” Clearly, patients can open the bottle and not take the medication. A small study performed in February of 2009 by Vitality™, where GlowCaps were used as an intervention and adherence was measured over 3 months, showed improved average adherence rate of 86% compared to 50%. Another trial is currently in progress (NCT01490983). GlowCapc also has a “press to refill” capability which is targeted to improve patient compliance by sending a request for a medication refill to the patient’s pharmacy with just the press of a button on the pill bottle.
With increasing technological progress to simplify complex treatment regimens, medication adherence is likely to improve; however it cannot completely replace the encouragement patients receive from physical interaction. The ADAGIO study25 clearly showed that the duration of the visits, number of patients followed by that center, etc., which characterize the physician/patient relationship, are the most important factors modifying medication adherence. In addition, a team approach, consisting of a social worker, nurse and pharmacist assisting the provider, has the potential to increase adherence.29 For patients with CML where adherence translates into the difference between long term cancer control and refractory disease or progression to a blastic phase, efforts to increase adherence through novel ideas is as important as the physician/patient relationship.
Intolerance
The question remains what treatment to choose if patients develop intolerance to TKIs that significantly affects their quality of life. We propose to switch the patient to another TKI without hesitation because we are concerned that patients who develop such intolerance will not be compliant with their medication and the immediate corollary will be the development of resistance.
Resistance
Resistance is divided into primary and secondary based on the timing. Primary resistance occurs when goal responses are never achieved with frontline therapy. Secondary resistance develops after a targeted response to a frontline therapy was achieved but subsequently lost. Outcome in the latter case is better. At 60-month follow up from IRIS, 6% progressed to accelerated/blastic phases, 3% had hematologic relapse, 5% lost their cytogenetic response and 2% died of causes unrelated to CML.14 In the three-year follow-up of ENESTnd, 0.7% of patients progressed on nilotinib compared with 3.5% on imatinib.23 In the three-year follow-up of DASISION, 7% of patients progressed on each arm.24 The main mechanism of both primary and secondary resistance to TKIs is the development of mutations, occurring in about 50% of the patients followed by other mechanisms such as overexpression of BCR/ABL1, increased expression of adhesion molecules such as CXCR4, increased expression of multi-drug resistance proteins, and unique to imatinib, decreased expression of influx proteins such as organic cation transporter 1.20
There are currently four TKIs available for imatinib failures. Choice of another TKI following development of resistance to imatinib is largely based on side effect profile. For example, a patient with history of pancreatitis or with increased risk for arterial thrombosis (e.g., diabetic patient with intermittent claudication) will not be an optimal candidate for nilotinib. Other side effects associated with nilotinib, are myelosuppression, QT prolongation, and hyperglycemia. Similarly, most physicians would be reluctant to initiate dasatinib in a patient with a history of pulmonary hypertension. Other side effects associated with dasatinib include myelosuppression, bleeding related events, fluid retention, QT prolongation and congestive heart disease. While nilotinib and dasatinib have been used by the community for a long time, bosutinib and ponatinib were approved in 2013. Bosutinib is associated with temporary and self-limiting diarrhea, myelosuppression, hepatic toxicity, and fluid retention, but of note, has not been reported to be associated with only minimal cardiac side effects.30
Ponatinib is unique in that it is the only BCR/ABL1 inhibitor with demonstrated efficacy against all ABL1 mutations,31 although resistance with compound mutations may arise.32 Ponatinib was recently linked to an increased rate of thromboembolic phenomena.33 We expect that the mechanism(s) of these phenomena and ways to mitigate them will be described within the next year and those will lead to potential broader use of this drug. Other side effects associated with ponatinib include heart failure, hepatotoxicity, hypertension, pancreatitis, neuropathy, ocular toxicity, bleeding events, fluid retention, arrhythmias and myelosuppression. At this point, given the significant side effect profile, treatment with ponatinib is restricted to patients with the T315I mutation and for those for whom no other TKI is indicated.
The question will remain what to offer a patient who has failed imatinib as well as a second TKI therapy. The dilemma would be whether to offer the patient treatment with ponatinib, abandon all TKIs and switch to a non-TKI approach with omacetaxine, or proceed onto an allogeneic stem cell transplantation (allo-SCT).
Omacetaxine mepesuccinate, a plant alkaloid and protein synthesis inhibitor, is a novel molecule which has shown activity against cells with T315I mutation harboring cells in vitro.34, 35 A phase II trial involving a total of 62 patients showed CHR achievement in 77% of patients with median response duration of 9.1 months.35 Furthermore, the trial demonstrated a median progression free-survival of 7.7 months. Twenty three percent of patients achieved a major cytogenetic response, and 16% complete cytogenetic response.35 Non-hematologic adverse reactions included infection, diarrhea, and nausea which were Grade 1/2.35 Thus, omacetaxine is a non-TKI therapeutic agent with significant activity in CML.
Allo-SCT remains the only curative treatment in CML. Its mechanism of action is immunologically mediated and can induce eradication of the leukemic stem cells. In the pre-TKI era, allo-SCT was the frontline treatment of choice for young patients with CML with good performance status.36 However since the establishment of imatinib as frontline therapy, the three main indications for allo-SCT in patients with CML remain those in blastic phase, patients with multiple TKI failures or (rarely) intolerance, and patients with TKI-resistance mutations.37 A study by Jabbour et al demonstrated that allo-SCT was an important salvage treatment alternative for patients with TKI-resistant CML.38 Ten patients with imatinib resistance (nine with CML, one with acute lymphoblastic leukemia) underwent engraftment following allo-SCT.38 Three patients died of relapse post-allo-SCT whereas seven patients were alive for at least 19 months.38 For the imatinib-resistant mutants and even the challenging T315I mutants, allo-SCT remains a viable treatment option and it should be considered.
Faster and deeper response and treatment discontinuation
The imatinib discontinuation trials demonstrated approximately 50% rate of disease relapse.39–41 The hypothesis is that faster and deeper response would result in significantly less disease relapse after second generation TKI discontinuation. This is currently being tested in several clinical trials (NCT01698905, NCT01744665, NCT01627132, NCT01850004, NCT01804985 and NCT01887561). If this hypothesis is substantiated, there will be significant clinical, financial and quality of life merit to the use of second generation TKIs upfront for newly diagnosed patients.
Conclusions
With medical progress, CML has been made into a truly chronic disease. With the recent approval of newer and more efficacious TKIs and the expected availability of generic imatinib in 2016, treatment decisions for newly diagnosed chronic phase CML will become increasingly complex. We propose here that all newly diagnosed chronic phase CML patients be stratified based on their risk scores to receive either upfront imatinib versus second generation TKI therapy. While patients with low Sokal/Hasford/EUTOS appear to benefit from imatinib, close attention will have to be paid to those in the intermediate/high risk scores for whom the risk of development of primary resistance is higher. In addition, close attention will have to be paid to compliance, using currently available technology to reduce the risk of developing resistance. Finally, decisions about second and third line approaches will need to be weighed against possible resistant mechanism(s) with due consideration of the use of ponatinib, non-TKI based therapy with omacetaxine and/or allo-SCT, in the appropriate clinical scenarios.
Acknowledgments
Supported partially by grants from the National Cancer Institute Grant CA16056 (CT, EPO, EAG, ESW, MW), the Szefel Foundation, Roswell Park Cancer Institute, the Leonard S. LuVullo Endowment for Leukemia Research, the Nancy C. Cully Endowment for Leukemia Research, the Babcock Family Endowment and the Heidi Leukemia Research Fund, Buffalo, NY. EW is also supported by Cancer Clinical Investigator Team Leadership Award (CCITLA) awarded by National Cancer Institute through a supplement to P30CA016056.
Footnotes
Conflict of Interest
Drs. Talati, Ontiveros, Griffiths and Wang have no conflict of interest. Dr. Wetzler served as consultant for Novartis, Ariad and Teva. He is the Principal Investigator of clinical trials with Bristol Myers Squibb and Teva.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwetz BA. From the Food and Drug Administration. JAMA: the journal of the American Medical Association. 2001;286:35. doi: 10.1001/jama.286.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–9. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. The New England journal of medicine. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 4.Radich JP, Kopecky KJ, Appelbaum FR, Kamel-Reid S, Stock W, Malnassy G, et al. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia. Blood. 2012;120:3898–905. doi: 10.1182/blood-2012-02-410688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 6.Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–30. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99. [PubMed] [Google Scholar]
- 8.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–8. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 9.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–92. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 10.Experts in Chronic Myeloid L. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121:4439–42. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien S, Radich JP, Abboud CN, Akhtari M, Altman JK, Berman E, et al. Chronic Myelogenous Leukemia, Version 1.2014. J Natl Compr Canc Netw. 2013;11:1327–40. doi: 10.6004/jnccn.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigil CE, Griffiths EA, Wang ES, Wetzler M. Interpretation of cytogenetic and molecular results in patients treated for CML. Blood Rev. 2011;25:139–46. doi: 10.1016/j.blre.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 15.Kaeda J, O’Shea D, Szydlo RM, Olavarria E, Dazzi F, Marin D, et al. Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: an attempt to define patients who may not require further therapy. Blood. 2006;107:4171–6. doi: 10.1182/blood-2005-08-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpinati M, Tolomelli G, Bochicchio MT, Castagnetti F, Amabile M, Bandini G, et al. Molecular monitoring of BCR-ABL transcripts after allogeneic stem cell transplantation for chronic myeloid leukemia. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19:735–40. doi: 10.1016/j.bbmt.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116:3758–65. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:232–8. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branford S, Yeung DT, Parker WT, Roberts ND, Purins L, Braley JA, et al. Prognosis for patients with CML and >10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline. Blood. 2014;124:511–8. doi: 10.1182/blood-2014-03-566323. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian H, O’Brien S, Jabbour E, Shan J, Ravandi F, Kadia T, et al. Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:3173–8. doi: 10.1200/JCO.2010.33.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochhaus A, Guilhot F, Al-Ali HK, Rosti G, Nakaseko C, De Souza C, et al. Early BCR-ABL transcript levels predict future molecular response and long-term outcomes in newly-diagnosed patients with chronic myeloid leukemia in chronic phase: Analysis of ENESTND 3-year data. Haematologica. 2012;97:237. [Google Scholar]
- 22.Hughes TP, Hochhaus A, Kantarjian HM, Cervantes F, Guilhot F, Niederwieser D, et al. Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on front-line imatinib or nilotinib 300 mg twice daily. Haematologica. 2014;99:1204–11. doi: 10.3324/haematol.2013.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 24.Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boque C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noens L, van Lierde MA, De Bock R, Verhoef G, Zachee P, Berneman Z, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–11. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 26.Hochhaus A. Educational session: managing chronic myeloid leukemia as a chronic disease. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2011;2011:128–35. doi: 10.1182/asheducation-2011.1.128. [DOI] [PubMed] [Google Scholar]
- 27.Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2381–8. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoleri F, Sorice P, Lasala R, Rizzo RC, Costantini A. Patient adherence and persistence with Imatinib, Nilotinib, Dasatinib in clinical practice. PloS one. 2013;8:e56813. doi: 10.1371/journal.pone.0056813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holloway S, Lord K, Bethelmie-Bryan B, Shepard MW, Neely J, McLemore M, et al. Managing chronic myeloid leukemia: a coordinated team care perspective. Clin Lymphoma Myeloma Leuk. 2012;12:88–93. doi: 10.1016/j.clml.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian HM, Cortes JE, Kim DW, Khoury HJ, Brummendorf TH, Porkka K, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2014;123:1309–18. doi: 10.1182/blood-2013-07-513937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 Compound Mutations Combining Key Kinase Domain Positions Confer Clinical Resistance to Ponatinib in Ph Chromosome-Positive Leukemia. Cancer Cell. 2014;26:428–42. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller GD, Bruno BJ, Lim CS. Resistant mutations in CML and Ph(+)ALL - role of ponatinib. Biologics: targets & therapy. 2014;8:243–54. doi: 10.2147/BTT.S50734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahadeo KM, Cole PD. Successful treatment using omacetaxine for a patient with CML and BCR-ABL1 [corrected] 35INS. Blood. 2010;115:3852. doi: 10.1182/blood-2010-02-269233. [DOI] [PubMed] [Google Scholar]
- 35.Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–80. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah NP. Loss of response to imatinib: mechanisms and management. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2005:183–7. doi: 10.1182/asheducation-2005.1.183. [DOI] [PubMed] [Google Scholar]
- 37.Jain N, van Besien K. Chronic myelogenous leukemia: role of stem cell transplant in the imatinib era. Hematology/oncology clinics of North America. 2011;25:1025–48. vi. doi: 10.1016/j.hoc.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jabbour E, Cortes J, Kantarjian HM, Giralt S, Jones D, Jones R, et al. Allogeneic stem cell transplantation for patients with chronic myeloid leukemia and acute lymphocytic leukemia after Bcr-Abl kinase mutation-related imatinib failure. Blood. 2006;108:1421–3. doi: 10.1182/blood-2006-02-001933. [DOI] [PubMed] [Google Scholar]
- 39.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. The lancet oncology. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 40.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–22. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 41.Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424–30. doi: 10.1200/JCO.2012.48.5797. [DOI] [PubMed] [Google Scholar]