Abstract
Cilia are microtubule-based structures that project into the extracellular space. Ciliary defects are associated with several human diseases, including polycystic kidney disease, primary ciliary dyskinesia, left-right axis patterning, hydrocephalus and retinal degeneration. However, the genetic and cellular biological control of ciliogenesis remains poorly understood. The IFT46 is one of the highly conserved intraflagellar transport complex B proteins. In zebrafish, ift46 is expressed in various ciliated tissues such as Kupffer’s vesicle, pronephric ducts, ears and spinal cord. We show that ift46 is localized to the basal body. Knockdown of ift46 gene results in multiple phenotypes associated with various ciliopathies including kidney cysts, pericardial edema and ventral axis curvature. In ift46 morphants, cilia in kidney and spinal canal are shortened and abnormal. Similar ciliary defects are observed in otic vesicles, lateral line hair cells, olfactory pits, but not in Kupffer’s vesicle. To explore the functions of Ift46 during mouse development, we have generated Ift46 knock-out mice. The Ift46 mutants have developmental defects in brain, neural tube and heart. In particular Ift46(−/−) homozygotes displays randomization of the embryo heart looping, which is a hallmark of defective left-right (L/R) axis patterning. Taken together, our results demonstrated that IFT46 has an essential role in vertebrate ciliary development.
Keywords: cilia, ciliopathy, IFT, intraflagellar transport, IFT46, KO mouse, L/R defect, zebrafish
Introduction
The cilia are microtubule-based organelles evolutionarily conserved from protozoans to vertebrates. Based on their motility, cilia are generally categorized as motile cilia or primary (sensory, non-motile) cilia. A single primary cilium is found on most types of cells, but the number of motile cilia varies in different cells: some, such as those of the embryonic node, have a single motile cilium, while others (ependymal cells in the adult brain and the bronchial epithelium) have multiple motile cilia (Eggenschwiler and Anderson, 2007; Goetz and Anderson, 2010). The importance of primary and motile cilia in embryonic development and in adult physiological processes is underscored by a broad class of human genetic diseases collectively known as “Ciliopathy”, which manifest a broad range of phenotypic abnormalities due to ciliary dysfunction, including obesity, diabetes, skeletal defects, situs inversus, hydrocephalus and polycystic kidney disease (PKD) (Badano et al., 2006; Fliegauf et al., 2007). Recent investigation into the role of cilia during early vertebrate development has connected cilia to multiple signaling pathways and many developmental processes ranging from left-right patterning to kidney cystogenesis (Hamada et al., 2002; Hildebrandt and Otto, 2005; Raya et al., 2006).
Cilia can be structurally divided into sub-compartments including basal body, transition zone, axoneme, ciliary membrane and the ciliary tip (Nigg and Raff, 2009). During ciliary growth, the axoneme is assembled by the addition of new axonemal subunits to its distal tip. Since cilia lack the machinery necessary for protein synthesis, the site of assembly of the axoneme is far from the site of synthesis of axonemal proteins in the cell body (Cole and Snell, 2009; Silverman and Leroux, 2009). This demands the delivery of new axonemal building blocks to their assembly site through the intraflagellar transport (IFT), a conserved process in eukaryotes that assembles, maintains, and disassembles cilia, as well as transduces of cilium-generated signaling. IFT is essential for the development and maintenance of both motile and non-motile sensory cilia. IFT trafficking from the base to the tip of the cilium depends on the microtubules and is associated with two IFT protein complexes, termed IFT-A and IFT-B, which consist of at least 6 and 13 subunits respectively. These IFT proteins are highly conserved across species and are all localized to the cilium, basal body and centrosomes (Rosenbaum and Witman, 2002). In mice, IFT-B is essential for anterograde trafficking, whereas IFT-A is required for retrograde trafficking. Disruption of the kinesin-2 motor or IFT-B blocks cilia formation, while perturbation of retrograde trafficking by disrupting the dynein motor or IFT-A results in short and bulged cilia. These highlight that IFT is indispensable for normal ciliogenesis and maintenance (Scholey, 2003). In addition, IFT has recently attracted intense research interest owing to its association with human disease and developmental abnormalities, including polycystic kidney disease (PKD), hepatic and pancreatic defects, blindness and obesity, skeletal patterning abnormalities as well as situs inversus (Pazour and Rosenbaum, 2002; Pazour, 2004).
IFT46 is a core component of the intraflagellar transport machinery and is required for formation of all cilia. As a mammalian homologue of DYF-6 in Caenorhabditis elegans (C. elegans), which was reported to be an IFT-B subunit in Chlamydomonas reinhardtii (C. reinhardtii), it is specifically required for transporting outer dynein arms into the flagella (Hou et al., 2007). The IFT46 mutants of C. reinhardtii and C. elegans are incapable of assembling cilia, demonstrating that IFT46 plays an essential role in ciliogenesis. IFT46 forms a stable trimetric sub-complex within the IFT-B core complex together with IFT52 and IFT88 (Lucker, 2010; Richey and Qin, 2012).
Here we report the characterization of IFT46 by using two model systems, zebrafish and mouse, to elucidate the expression and function of IFT46 during vertebrate development. We find enriched expression of ift46 in ciliated organs during zebrafish embryonic development. In addition, we show that knockdown of ift46 in zebrafish embryos leads to loss of cilia in various tissues. We also demonstrate that ift46, like other IFT subunits, is localized to the basal body in ciliated cells. We have generated knock-out mouse of Ift46, which are embryonic lethal at E10.5 and exhibit neural tube defects, cardiac edema and randomized heart looping due to the lack of cilia at the node. Taken together, our results indicate the essential role of IFT46 in vertebrate development.
Results
Overexpression of IFT46 induces apoptotic cell death
We originally isolated a new cDNA clone C11orf60 in a large-scale expression screening of human genes in zebrafish embryos. Subsequent sequencing revealed this cDNA encodes the human ortholog of C. reinhardtii intraflagellar transport protein 46 (IFT46).
When human IFT46 was overexpressed following the injection of synthetic mRNA of IFT46 into one cell stage zebrafish at 100 to 200 pg per embryo, we observed increase of apoptosis in the central nervous system of injected embryos in a dose-dependent manner, as determined by acridine orange (AO) staining (Fig. S1A–B). Previous reports have shown that IFT46 in C. reinhardtii is specifically required for transporting outer dynein arms into the flagella (Hou et al., 2007). To better characterize and clarify roles of IFT46 in vertebrate development, we thus performed the functional study of the zebrafish ift46 gene.
The ift46 is expressed in ciliated organs
We identified from the zebrafish genomic and expressed sequence tags (EST) databases the zebrafish ortholog of IFT46, which encodes 355 amino acids containing a characteristic IFT B protein 46 C-terminal domain. The zebrafish ift46 protein shares a high level of similarity in the conserved domain to its counterparts in human, mouse and C. reinhardtii respectively (Fig. 1). RT-PCR revealed both maternal and zygotic expression of ift46 as early as at four-cell stage (Fig. S1C). We then characterized the expression pattern of ift46 during zebrafish early development by whole-mount in situ hybridization (Fig. 2). ift46 was expressed ubiquitously at cleavage, blastula stages and specifically in ciliated tissues at later stages. The ift46 expression was initially detected in dorsal forerunner cells (DFC), which are the precursors of Kupffer’s vesicle (KV) and later in KV at 3-somite stage. By 20–24 hours post fertilization (hpf), widespread expression of ift46 was evident within the brain, spinal cord and pronephros. In the pronephric ducts, ift46 was expressed in a salt and pepper pattern at 24 hpf, which resembles the distribution of multi-ciliated epithelial cells in the pronephros. At 54 hpf, ift46 expression was detected throughout the olfactory placodes and lateral line organs. Thus ift46 is specifically expressed in ciliated tissues, suggesting its involvement in ciliogenesis during zebrafish embryonic development.
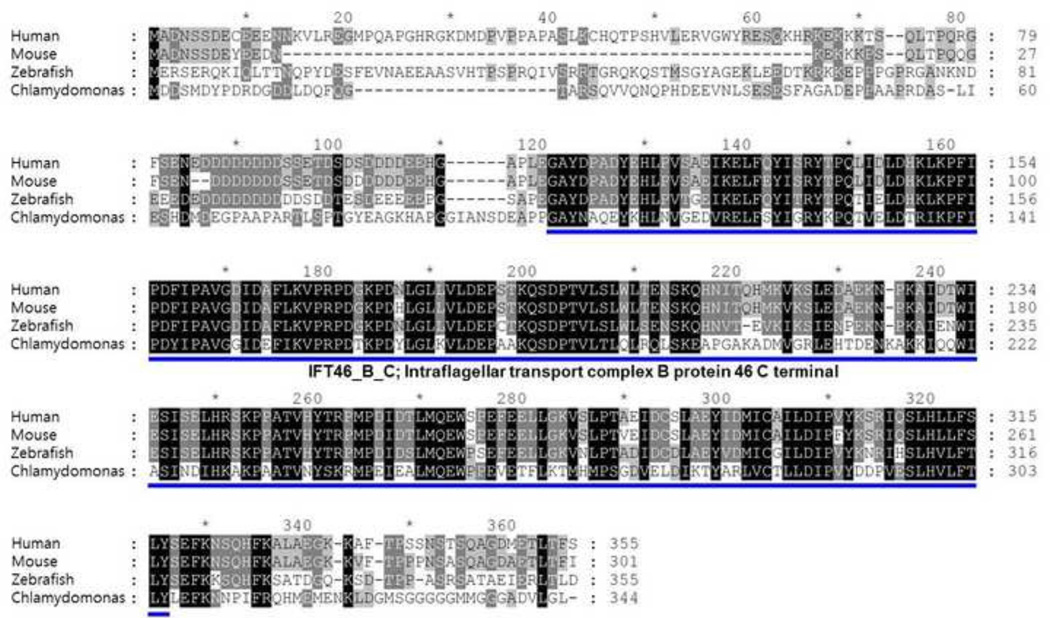
Fig 1. Alignment of IFT46 protein sequences using GeneDoc.
The IFT46 protein sequences from human, mouse, zebrafish and Chlamydomonas are aligned using GeneDoc program. The intraflagellar transport complex B protein 46 C terminal domain is marked with blue bar, The zebrafish ift46 has 59%, 69% similarity to human IFT46 and mouse Ift46 and 69% similarity to Chlamydomonas, respectively.
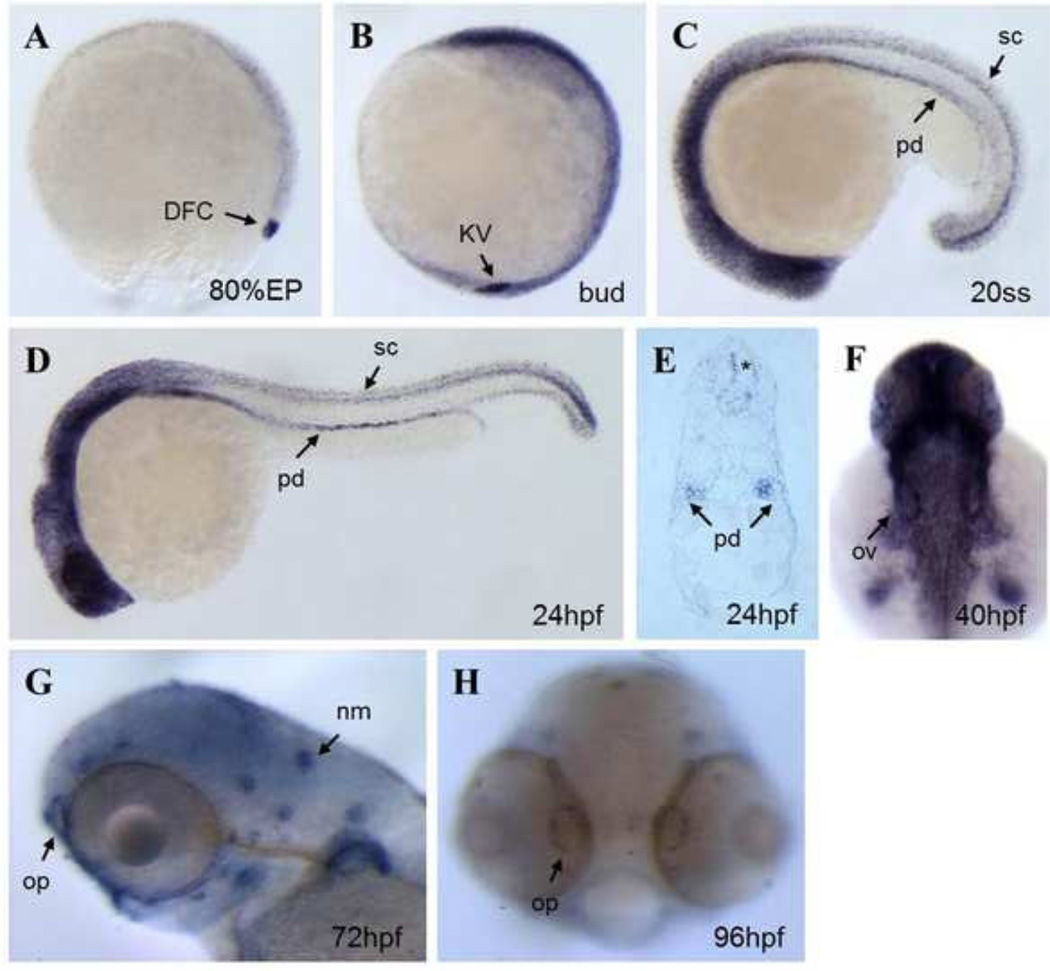
Fig 2. Expression pattern of ift46 in zebrafish embryos.
(A) Whole-mount in situ hybridization shows ift46 expression at 80% epiboly in the dorsal forerunner cells (DFC, arrow). (B) ift46 is strongly expressed in Kupffer’s vesicle (KV, arrow) at bud stage, (C, D) At 20-somite stage and 24 hpf ift46 expression is detected in pronephric ducts (pd), eyes, ears, spinal cord (sc) and diffusely in the brain. (E) Cross-section showing ift46 expression in the pronephric ducts (arrow) and spinal cord (asterisk). (F) At 40 hpf, ift46 is widely expressed in brain, eyes, otic vesicles (arrow) and pectoral fins. (G, H) At 72 and 96 hpf, ift46 is expressed in the olfactory pits (op) and neuromast (nm) hair cells (arrow).
ift46 morphants develop ciliopathy-related defects
To investigate ift46 function in ciliogenesis and embryonic development, we used morpholino oligo (MO) targeting the translational start site of ift46 mRNA to knockdown ift46 expression in zebrafish embryos. The ift46 ATG-MO is highly effective in blocking the translation of ift46 mRNA, as shown by co-injection of the MO and GFP-tagged ift46 into zebrafish embryos (Fig. S1D–E). When ift46 was knocked down with this MO, we observed a series of ciliopathy-related phenotypes, including ventral body curvature, pronephric cysts and retinal dysplasia (Fig. 3A–F’). These are typical phenotypes seen in zebrafish defective in cilium motility or assembly, such as a number of zebrafish IFT mutants (Kramer-Zucker et al., 2005; Zhao, 2007). The pronephric cysts developed progressively in ift46 morphant embryos from 2 dpf to 5 dpf. We examined the histology of kidney defects in ift46 morphants (Fig. 3C–D’). The kidney cysts are located in the glomerular tubular region slightly anterior and medial to the pectoral fin. Cross section of ift46 morphants at 72 hpf revealed large bilateral cysts in the glomerular-tubular region (Fig. 3C and C’). In addition, ift46 morphants kidney ducts are grossly dilated compared to control embryos (Fig. 3D and D’).
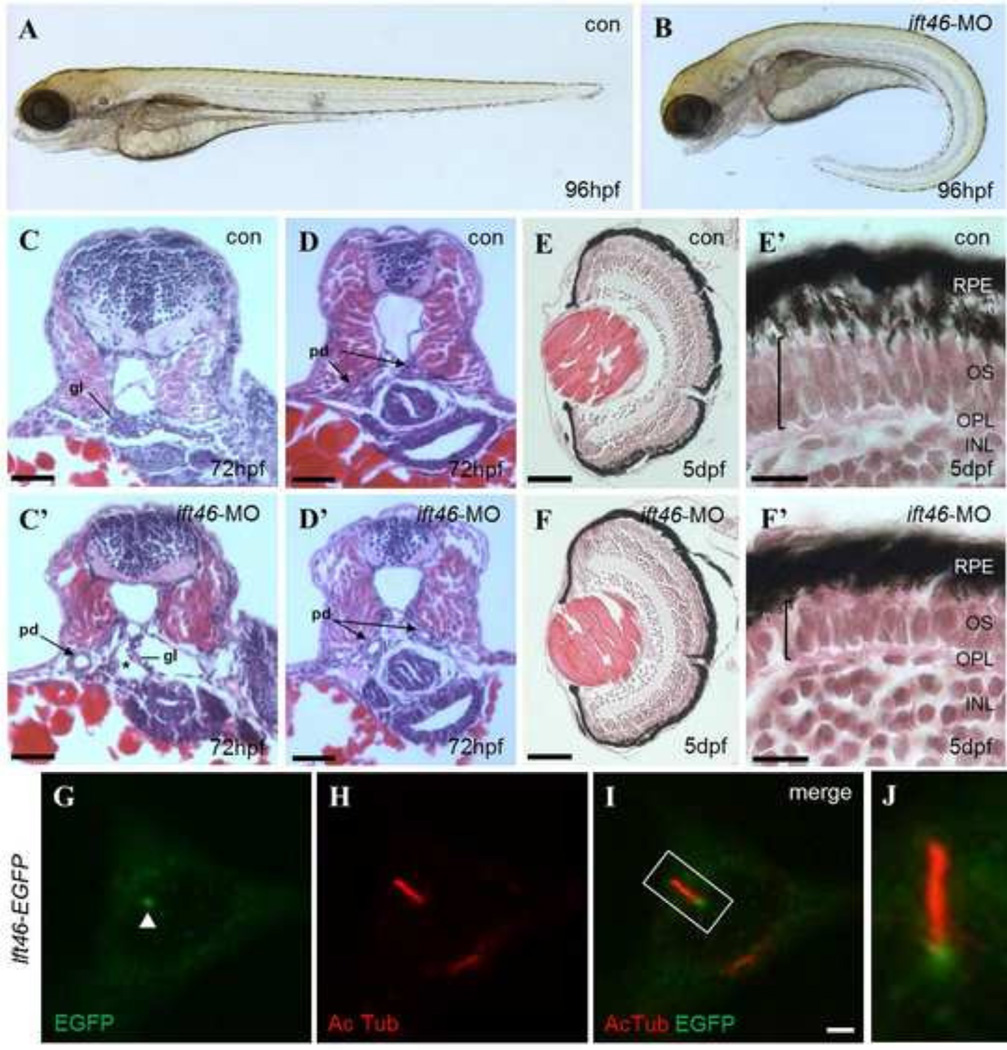
Fig 3. ift46 morphants develop phenotypes associated with ciliary dysfunction.
(A) Wild-type embryos show normal morphology. (B) ift46 morphants are characterized by curved body axis, the development of pronephric cyst and pericardiac edema at 96 hpf. (C–D) Cross-sections of wild-type zebrafish at the glomerular-tubular region of pronephros at 72 hpf show normal morphology of pronephric glomeruli and ducts. (C’–D’) ift46 morphant embryos display a grossly distended cyst (asterisk) in place of glomeruli (gl) and dilated pronephric ducts (pd) (arrow). Scale bar, 50 µm. (E-F’) Histological sections of the eyes of wild-type and ift46 morphants at 5 dpf. The retina in wild-type control (E and E’) has normal laminated structures, including retinal pigment epithelium (RPE), outer segment (OS), outer plexiform layer (OPL) and inner nuclear layer (INL). (E) and (F) scale bar, 200 µm. The outer segment (OS) (bracket) is thinner in ift46 morphants (F’) compared to the control (E’). Scale bar, 100 µm. (G–J) The EGFP-tagged ift46 (green) is localized to the base of primary cilia (red) in hTERT-RPE1 cells. Merged imaged are shown in (I). Arrowhead indicates the base of cilia. Scale bar, 3 µm. (J) Higher magnification view of the boxed area in (I).
We also examined the retinal histology of ift46 morphants. The photoreceptor outer segment is a specialized primary cilium consisting of microtubule-based axoneme and regular stacks of disks containing opsin for the photo-transduction cascade (Sukumaran, 2009; Tsujikawa, 2004). Although the overall size of the eye was unaffected under light microscopy, histological sections revealed that the photoreceptor outer segment is thinner than wild-type control in ift46 morphants (Fig. 3E– F’).
To demonstrate the specificity of the knockdown, we performed rescue experiments for the ift46 morphants by co-injection of ift46 mRNA. Co-injection of zebrafish ift46 mRNA with ATG-MO completely rescued the injected embryos, with embryos showing a straight body axis, substantially reduced cyst formation and cardiac edema (Fig. S2). In addition, injection of human IFT46 mRNA also partially rescued ift46 morphants (Fig. S2C–F), indicating that zebrafish and human orthologs of IFT46 are functionally conserved and IFT46 is likely to have an evolutionarily conserved role in ciliary function.
The IFT46 protein is predicted to have an intraflagellar transport complex B protein 46 c-terminal domain that is highly conserved in mouse and human (Fig. 1). To delineate the functional significance of this structural feature of ift46, we generated a series of deletion mutants of ift46 and tested their activity to rescue ift46 morphant phenotypes (Fig. S2A). The expression of the C-terminal portion of ift46 resulted in a significant rescue of the morphant phenotypes, i.e. kidney cysts and pericardial edema (Fig. S2G), but the expression of the C-terminal deletion mutant of ift46 failed to rescue (Fig. S2H). In addition, the overexpression of ift46 ΔN but not ift46 ΔC in zebrafish embryos led to elevated numbers of apoptotic cells in the brain region. Therefore, we conclude that the C-terminal domain is necessary for ift46 function.
The ift46 localizes to the basal body in ciliated cell and controls the number and the length of cilia during zebrafish development
So far, all IFT proteins characterized have been localized to the cilium, basal body and centrosomes (Kunitomo and Iino, 2008). We expressed GFP-tagged zebrafish ift46 in hTERTRPE1 cells to examine its subcellular localization. Double immunostaining against GFP and acetylated α-tubulin, a marker for cilia, showed that GFP-tagged ift46 was primarily localized at the basal body of cilia but not in the cilium (Fig. 3G–J), consistent with previous reports on IFT46 localization in cultured cells and C. reinhardtii (Gouttenoire et al., 2007; Hou et al., 2007). To investigate whether ift46 is required for cilia assembly like the other IFT-B complex subunits, we first examined cilia morphology using acetylated α-tubulin, a cilia-specific marker, in pronephros, otic vesicle, olfactory pits and lateral line of ift46 morphant embryos. By confocal microscopy we observed a reduction of cilium length in all of these organs in morphants (Fig. 4A–D’). In the pronephric duct, motile cilia in multi-ciliated cells were shorter and disorganized in morphants compared to the controls (Fig. 4A-A’). Presumably the motility of these cilia was abnormal because the same ciliary defects are accompanied by motility defects in other IFT mutant zebrafish, and hence lead to pronephric cysts (Follit et al., 2009; Gerdes et al., 2009; Zariwala et al., 2007). In the otic vesicles, there are two populations of cilia: tether cilia located at the anterior and posterior ends of the ear and a transient population of short cilia that is distributed throughout the otic vesicle lumen (Stooke-Vaughan et al., 2012). In ift46 morphant embryos, both types of cilia in the otic vesicle are defective in morphant embryos: the tether cilia were shorter and the short cilia were missing (Fig. 4B-B’). The cilia in the olfactory pits were shorter compared to the controls as revealed by immunofluorescence (Fig. 4C-C’) as well as scanning electron microscopy (Fig. 4E–F’). The mechanosensory kinocilia of the lateral line hair cells in ift46 morphant embryos were consistently shorter than those of the controls (Fig. 4D-D’). However, although the number of cilia was greatly reduced in the pronephric ducts, the “9+2” microtubule doublet pattern within these cilia was not affected by the loss of ift46 function, as shown by transmission electron microscopy (TEM) (Fig. 4G–H’). Taken together, these results suggest that ift46 plays an essential role in controlling the cilium number and length, but is not required for the structural assembly of the cilium.
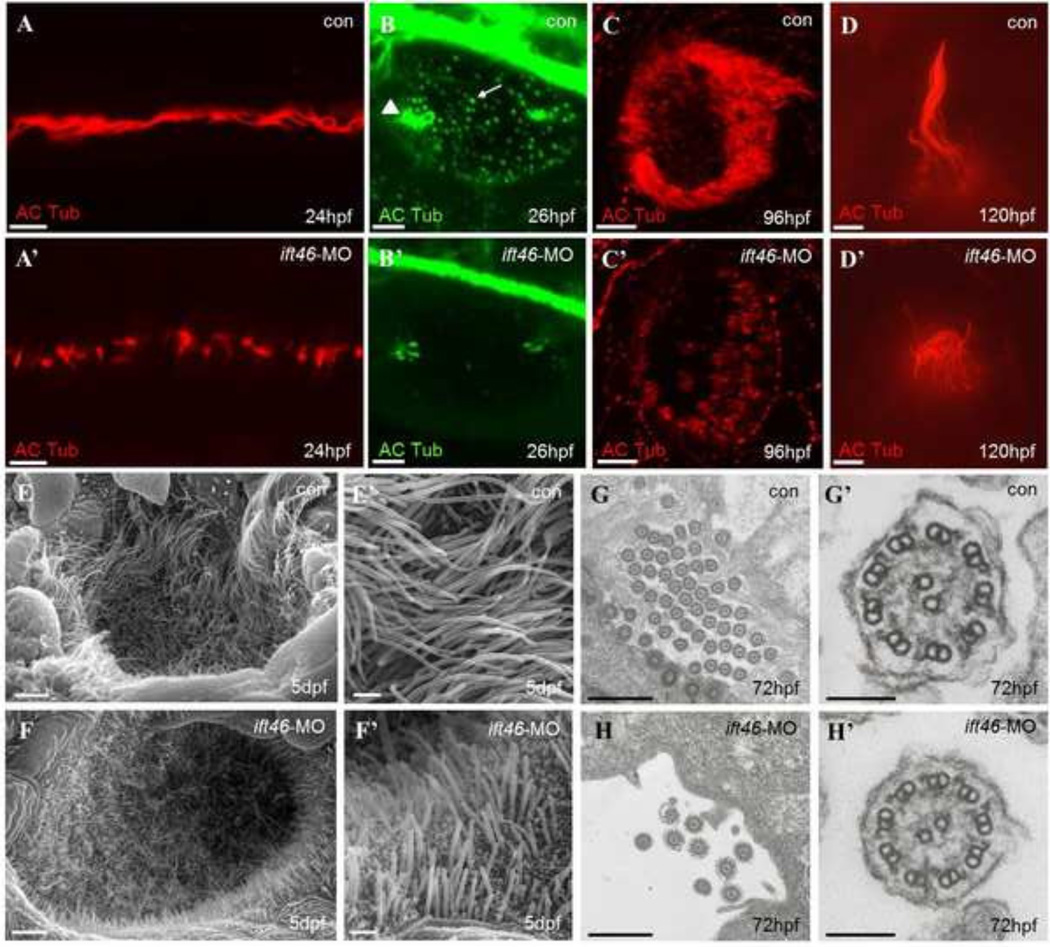
Fig 4. Ciliary defects in zebraifh ift46 morphants.
Immunofluorescence with antibody against acetylated α-tubulin showing cilia in the pronephric ducts, (scale bar, 10 µm) (A-A’), otic vesicle, (scale bar, 10 µm) (B-B’), olfactory placode, (scale bar, 10 µm) (C-C’) and a lateral line hair cell, (scale bar, 5 µm) (D-D’). The ift46 morphants exhibit shortened or fewer cilia compared with the control fish in all of these organs. Arrow head: tether cilia. Arrow: short cilia. (E–F’) SEM images showing the cilia in the olfactory placode of control fish (E, E’) and ift46 morphants (F, F’) at 5 dpf. Scale bar, 40 µm (E) and (F), 4 µm (E’) and (F’) (G-H’) TEM images of cilia in zebrafish pronephric ducts at 72 hpf. Ultrastructure of normal pronephric cilia shows 9+2 microtubule architecture at 72 hpf which is intact in ift46 morphants. (G’, H’) Representative views of individual cilium at higher magnification. Scale bar, 5 µm (G) and (H), 100 µm (G’) and (H’).
In vertebrate embryos, cilia are required for the left-right (LR) axis formation. The Kupffer’s vesicle, a fluid-filled structure, is located at the tail bud in the zebrafish embryo and is the functional counterpart of the mammalian node (Essner et al., 2005; Hirokawa et al., 2009; Kreiling et al., 2007; Oteíza et al., 2008). The KV cells contain motile cilia that generate the fluid flow critical for the establishment of laterality in zebrafish. The absence of these cilia or their lack of motility results in LR patterning defects of various visceral organs such as heart, liver and pancreas (Lenhart et al., 2011). We examined a number of asymmetric markers, including left dorsal diencephalon and left heart primordium marker lefty1, heart primordium marker lefty2, visceral organ marker foxA3 but did not observe any LR defects in ift46 morphants (Fig. S3A–E’). We further examined organogenesis of the KV, and cilia formation in the KV in morphants at the 4-somite stage. The size and shape of the KV, revealed by the expression of charon, a marker of KV and antagonist of Nodal signaling for LR patterning, are normal in ift46 morphants (Fig. S3F-F’) and so are the length and number of cilia in the KV (Fig. S3J–K). Thus ift46 knockdown resulted in neither ciliary defects in the KV nor abnormal development of the organ laterality, presumably due to the maternal expression of ift46.
Left–right axis patterning is defective in Ift46 knock-out mice
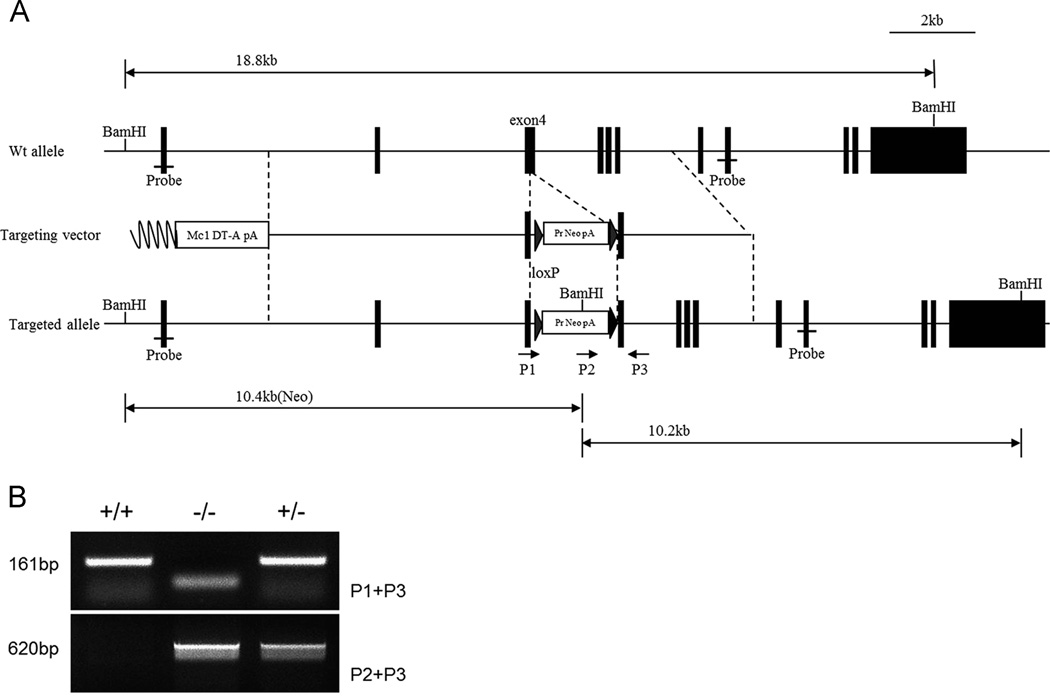
To further assess the functional importance of IFT46 during mammalian development, we generated a knock-out mouse model for Ift46 by homologous recombination strategy. The targeting vector was generated to delete exon 4 of Ift46 (Fig. 5A). Correctly targeted ES cell clones obtained with the gene-targeting construct were verified by PCR (Fig. 5B). The Ift46 homozygous mutants could not be detected among new-born mice of Ift46 heterozygote incrosses, suggesting embryonic lethality of the homozygotes. By genotyping embryos during gestation, we found that homozygous embryos died between E10.0 and E10.5. In fact, the Ift46 homozygotes at E9.5 were phenotypically abnormal with severe developmental defects including growth retardation, abnormal neural tube morphology, abnormal cardiac morphology and hemorrhage (Fig. 6A). Their neural tube failed to close and severe cardiac edema was observed at E10.5. In situ hybridization revealed that Ift46 was widely expressed in whole embryo at E10.5 with strong expression especially in the forebrain and the neural tube (Fig. S4D). These expression patterns are in accordance with the mutant phenotypes, which suggest an important role of Ift46 in neural tube development.
Fig 5. Ift46 mutant mice were generated by homologous recombination.
(A) Schematic diagram indicating the structure of the mouse Ift46 gene and the gene-targeting strategy. The Ift46 mutant mice were generated by targeting exon 4. Genotyping primers are shown in the map P1–P3 (arrows). (B) Genotyping of Ift46 mutants at E9.5. The wild-type allele, Ift46 WT, produces a 161 bp fragment and the mutated allele, Ift46 MT, produces a 620 bp PCR fragment.
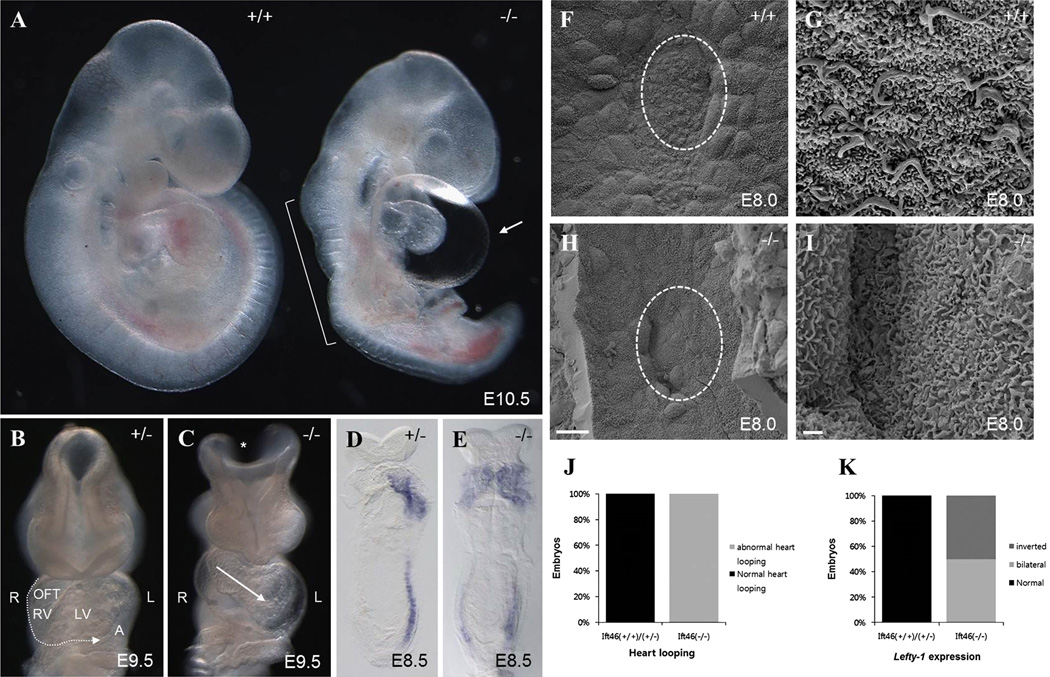
Fig 6. Phenotypic characterization of Ift46 knock-out mouse embryos.
(A) Ift46 homozygotes exhibit anterior defects, kinks of the neural tube (bracket) and pericardial edema (arrow) at E10.5. (B) At E9.5, normal heart-looping and neural tube closure are seen in heterozygous mice. (C) The cranial neural tube (asterisk) fails to close and the heart looping is abnormal in Ift46 homozygous (−/−) (arrow). LV, left ventricle; OFT, outflow tract; RV, right ventricle; A, atrial. (D–E) Whole-mount in situ hybridization of Ift46 heterozygous and homozygous mutant embryos for Lefty-1 detects abnormal bilateral expression of Lefty-1 in homozygotes. (F–I) Scanning electron microscopy of the embryonic nodal cilia in wild-type (F, G) and Ift46 homozygous (−/−) embryos (H, I). Ventral view of the E8.0 embryos at 1,500X (F, H) and 10,000X (G, I) magnifications. Anterior to the top. Circle in (F, H) indicates magnified area in (G, I). Scales bars: 20 µm (F, H), 1 µm (G, I). (J) Analysis of mouse heart looping in Ift46(+/+) /Ift46(+/−) (n=52) and Ift46(−/−) (n=9). (K) Analysis of Lefty-1 expression in E8.5 mouse embryos (Ift46(+/+) /Ift46(+/−), n=9) (Ift46(−/−), n=2).
The randomized turning process of the heart tube and abnormal heart looping are observed in the Ift57 and Ift88 mutant embryos (Follit et al., 2009; Gorivodsky et al., 2009; Houde et al., 2006; Murcia et al., 2000; Taulman et al., 2001). We examined heart looping orientation in Ift46 mutant embryos. A various degree of pericardial edema was observed in all Ift46 homozygous embryos with inverted heart looping. When a clutch of mouse fetus was examined at E9.5, two out of nine showed heart looping defects: one had a linear heart tube and the other showed left-right reversed heart looping (situs ambiguous). This suggests that the Ift46 mutants are defective in left-right axis patterning (Fig. 6B–C). The left-right axis formation is established after the node formation in early-stage embryos. We examined the left-right patterning defect in early embryonic stages by whole-mount in situ hybridization of Lefty-1, the target of Nodal signaling. In wild-type, Lefty-1 is expressed on the left side of the lateral plate mesoderm (LPM) at E8.5 (Fig. 6D). By contrast, Ift46 homozygous mutants showed ectopic bilateral expression of Lefty-1 (Fig. 6E). These results indicated that Ift46 is important for LR axis patterning specification in mouse development. The break in LR asymmetry in the mouse is related to the fluid flow towards the left side of the embryo generated by the beating cilia at the node (Houde et al., 2006). To determine whether ciliogenesis is abolished in Ift46 mutants, we investigated the integrity of the mouse nodal cilia. Scanning electron microscopy of E8.0 embryos showed that Ift46 homozygous mutants exhibited a depression with similar location and gross morphology to the wild-type node (Fig. 6F and H). At higher magnification, however, we could not observe any monocilia in mutant node cells (Fig. 6G and I). Therefore, absence of nodal flow due to the loss of nodal cilia is the cause of the severe LR patterning defects seen in Ift46 homozygous mutants.
Discussion
Recent studies of ciliopathy highlight the critical role of cilia in the pathogenesis of multiple ciliated organs such as kidney, respiratory tracts, heart, retina and brain (Fliegauf et al., 2007; Novarino et al., 2011). However, it is still largely unclear how cilia play such an indispensible role in regulating normal organ development and function. Studies of IFT complexes in many model systems such as zebrafish and mice have shown that these proteins are essential for ciliary development. Previous studies about IFT46, an IFT-B complex protein, primarily focused on ciliogenesis in C. reinhardtii and C. elegans. Here, we described for the first time the functional role of IFT46 in ciliogenesis by using vertebrate models. We isolated zebrafish ortholog of ift46 and showed that its expression is highly restricted in multiple ciliated tissues, which suggests the diverse functions of ift46 in embryonic development and organogenesis.
By overexpressing ift46-GFP construct in hTERT-RPE1 ciliated cells, we have confirmed the localization of ift46 at the basal body of primary cilia. Consistently, mouse Ift46 has been localized to the base of the primary cilia of mouse chondrocytes with a polyclonal antibody against Ift46 (Gouttenoire et al., 2007; Hou et al., 2007). Proteins required for intraflagellar transport concentrate at the basal body of cilia, where they assemble into protein complexes called IFT particles (Rosenbaum and Witman, 2002). The subcellular localization of ift46 in basal body suggests that IFT46 is indeed involved in IFT process. It is notable that the ift46-GFP fusing protein was not detected within the cilia, differing from the previously report for mouse Ift46, which was detected in primary cilium by an antibody (Gouttenoire et al., 2007). Presumably, the GFP moiety may have interfered with the transport of ift46-GFP into the cilia.
Loss of other IFT B complexes subunits, such as ift57, ift88 and ift172, by mutations or morpholino knockdown has been reported to affect ciliogenesis in zebrafish. In these animal models, both sensory cilia in neurons and motile cilia in the pronephric duct are disrupted (Essner et al., 2005; Lunt et al., 2009). ift46 morphant zebrafish indeed display most of these cilia biogenesis defects and the morphology of cilia is broadly affected by the loss of IFT46, which confirms that IFT46 is required for the formation of cilia. This notion is corroborated by the consistent ciliary defects discovered in the KO mice of Ift46 that we have generated. It is noteworthy that the phenotype of ift46 morphant we observed is different from what was previously published. Gouttenoire et al. reported knockdown of ift46 resulted in dorsalization of developing zebrafish embryos without mentioning any cilium-specific phenotype (Gouttenoire et al., 2007). The discrepancy could be due to the different ift46 cDNA sequence they isolated, which is shorter than the ift46 full-length cDNA that we identified using human IFT46 full-length cDNA. As a consequence, we used different ift46 morpholino target sites to knock down the gene. Thus whether any alternative coding sequences of ift46 exist in zebrafish deserves further investigation.
In the absence of IFT, cilia become significantly shorter and have defects in their axonemal microtubules (Kramer-Zucker et al., 2005; Sukumaran, 2009; Tsujikawa, 2004). Indeed, we observed loss of ift46 led to dramatic shortening or loss of cilia in many ciliated organs including olfactory placode, pronephric duct, ear and lateral line organs, as revealed by immunohistochemistry and TEM. Despite the reduced ciliary length in various organs, the motile pronephric cilia in ift46 morphant maintained a normal 9+2 microtubule doublet structures. These results suggest that ift46 is required for ciliogenesis in ciliated organs but is not essential for axonemal dynein assembly in zebrafish.
Similar to other cilium associated proteins, IFT46 protein domain structure reveals little regarding its function. The only predicted domain in IFT46 is a C-terminal domain conserved in many organisms. Through serial deletion analysis, we showed that C-terminal domain is necessary for ift46 function in normal heart and kidney development, while N-terminal region is dispensable. This result is consistent with our observation that the C-terminal region is highly conserved between multiple species, whereas the N-terminal region is less conserved. Identification of proteins that directly interact with the C-terminal region of IFT46 will provide critical insights to the molecular mechanisms underlying IFT46 functions.
The left-right asymmetry defect is commonly associated with ciliary mutant zebrafish (Essner et al., 2005). Although we observed strong expression of ift46 in KV, neither the number nor the length of cilia was affected in ift46 morphants and hence no L-R asymmetry defect. It is likely that the maternal contribution of ift46 transcript delivers sufficient ift46 protein in the KV. This hypothesis is supported by previous studies of ift88/ovl mutants, which exhibit only partial or late loss of cilia, owing to the maternal contribution of this ciliary component. To completely eliminate ift88 activity, maternal-zygotic ovl mutants (MZovl) was generated using the germline replacement, which showed complete lack of cilia in developing embryos (Huang et al., 2009). Similarly maternal zygotic mutants of talpid3 also exhibited a curled body and disruption of primary ciliogenesis phenotypes (Ben et al., 2011). The generation and analysis of maternal zygotic ift46 mutants in the future should provide definitive answer to this possibility. Indeed, we found that the KO mice of Ift46 displayed severe laterality defects and the ciliary signaling at the node was abnormal, confirming that Ift46 is indispensible for nodal signaling and laterality control in mouse embryonic development.
Cilia dysfunction has long been associated with cyst formation and ciliopathies. Kidney cysts are thought to be derived from cell over-proliferation with abnormal cellular rearrangement and defects in ciliogenesis and ciliary function can lead to epithelial over proliferation and eventual cyst formation (Sullivan-Brown et al., 2008; Kishimoto et al., 2008). However, how the ciliary signal is coupled to cell proliferation control is poorly understood. We initially isolated IFT46 from an overexpression screening of a human full-length cDNA library, in which overexpressed of IFT46 in zebrafish embryos caused excessive apoptotic cell death. A similar phenomenon has been previously reported for IFT88, of which over-expression prevents G1-S transition and induces apoptotic cell death (Robert et al., 2007; Delaval et al., 2011). In fact, three of the core B subunits, IFT88, IFT52 and IFT46, interact directly with each other and are capable of forming a ternary complex (Lucker et al., 2010). It is notable that IFT46 protein level is high throughout S/M phase and IFT46 regulates the cell cycle progression in together with IFT27 (Wood et al., 2012). Therefore our results suggest that IFT46 might play a potential role in the cell cycle regulation along with IFT88 and other IFT components. Further work is required to test whether the IFT46 is involved in cell cycle regulation and cytogenesis.
Recently, genome engineering tools (such as ZFN, TALEN and CRISPR) have been successfully developed for targeted mutagenesis (Cho et al., 2013; Sung et al., 2014). This will facilitate the generation of ift46 and other ciliary mutant lines in zebrafish, which will benefit the functional study of ciliary proteins, such as IFT subunits, and provide animal models for ciliopathy disease genes. Furthermore, characterization of the ift46 mutations and its interactions with other proteins involved in ciliary formation in development and cell cycle control will deepen our understanding of its function within and outside cilia.
Materials & Methods
Zebrafish maintenance
Zebrafish were maintained at 28.5°C with a 14h light/10h dark condition. Wild-type embryos were cultured in Ringer’s solution (116 mM NaCl, 2.9 mM KCL, 1.8 mM CaCl2, 5 mM HEPES, pH 7.2) and treated with PTU (phenylthiocarbamide, 1-Phenyl-2-thiourea) (Sigma) to suppress pigmentation. Embryonic stages were determined by the hours post-fertilization (hpf) and microscopic observation of gross morphology.
Overexpression of IFT46 in zebrafish embryos
The human IFT46 clone was a gift from Korea Human Gene Bank, KRIBB. To transcribe the mRNA, the clone was linearized with NotI restriction enzyme and in vitro transcribed by SP6 MESSAGE MACHINE kit (Ambion). The integrity of synthetic mRNA was tested on 1% agarose gel and then dissolved in nuclease-free water. 0.2% phenol red was added immediately before microinjection.
Detection of apoptotic cells
For detection of apoptotic cells, embryos were placed in 10µg/ml acridine orange (Sigma) diluted in egg water for 30 min in dark condition and then washed in egg water.
Cloning of ift46
To clone zebrafish ift46, a cDNA fragment from 24 hpf zebrafish cDNA was amplified by RT-PCR based on the sequence information on the NCBI sequence (GeneBank ID:XM_003199365.1). The primers were 5’-TCCATGGAGAGGTCCAGACGAC-3’ (forward) and 5’-GTCTAGAAATAGGGCAAAAGGGCGACC-3’ (reverse). The PCR products were cloned into the pGEM-T easy vector (Promega) and then subcloned into the EcoRI site in pCS2. To construct the ift46-GFP fusion reporter into pCS2+ GFP vector, ift46 was amplified by PCR and the PCR products were subcloned into the NcoI site in pCS2+ GFP vector. The ift46 ΔN, ΔC constructs were generated using PCR-mediated deletion.
Whole-mount in situ hybridization
To make an anti-sense RNA probe (ift46, foxA3, cmlc2, lefty1, lefty2 and charon), the cDNAs were subcloned into the pGEM T-easy vector (Promega), linearized with a restriction enzyme, and then transcribed in vitro using SP6 or T7 polymerase and digoxigenin-labeled UTP (Roche). Whole-mount in situ hybridization was performed using standard protocols.
Whole-mount immunostaining
Embryos at the designated stages were fixed in 4% paraformaldehyde. After several washes with PBS, embryos were dehydrated with methanol. Whole-mount immunostaining was carried out as previously described using primary antibodies against mouse acetylated α-tubulin (Sigma). To stain nuclei, the embryos were fixed in 4% paraformaldehyde, stained for 10 min with Hoechst 33342 (Sigma) and washed in PBS.
Microinjection of Morpholino and mRNA
ift46 ATG-MO (Gene Tools) used to blocking translation of ift46 is 5’-CTTTTGTCGCTCGGACCTCTCCATG-3’. ATG-MO was resuspended in 1X Danieau buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6mM Ca(NO3)2, 5.0 mM HEPES, pH 7.6) with 0.1% phenol red and microinjected into zebrafish embryos at one to four-cell stage (1ng/embryo) using (WPI). Injected embryos were incubated until the indicated stage and analyzed by whole-mount in situ hybridization or immunostaining. Capped sense RNA encoding ift46 (lack of MO target sites) was synthesized with SP6 RNA polymerase (Ambion) after linearization of pCS2+ift46 with NotI. The synthesized capped RNA was dissolved in nuclease-free water containing 0.2% phenol red as a tracking dye.
Histology
Embryos fixed at 4 dpf in 4% paraformaldehyde were dehydrated with a graded ethanol series up to 100%, processed in xylene and embedded in paraffin. Transverse sections (6µm) were stained with hematoxylin and eosin (H&E) using a standard protocol.
Cell culture and transfection
hTERT-RPE1 cells were grown in DMEM/F12 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin at 37°C in 5% CO2, transfected with Lipofectamine™ Reagent (Invitrogen) and serum-starved for 48h to induce ciliogenesis.
Immunostaining and microscopy
hTERT-RPE1 cells grown on glass coverslips were fixed with 4% PFA, permeabilized with 0.1% Triton X-100 in PBS and blocked with 2% horse serum in PBS. Cells were incubated in primary antibody (acetylated α-tubulin, 1/1000) for 1 hour at room temperature and washed three times with PBS and were incubated in fluorescent-conjugated secondary antibodies (1/500) for 1 hour, washed three times with PBS and mounted using DABCO. Confocal imaging was carried out on LSM5 LIVE CONFIGURATION VARIOTWO VRGB confocal microscope.
Scanning electron microscopy (SEM) and Transmission electron microscopy (TEM)
Embryos were fixed with 2.5% glutaraldehyde for 4°C overnight, post-fixed in 1% OsO4 for 2 hours, dehydrated in a graded series of ethanol. Zebrafish embryos were prepared for TEM and SEM using a standard protocol.
Generation of Ift46 mutant mice and genotyping
Ift46 mutant mice (Acc. No. CDB0892K: http://www.clst.riken.jp/arg/mutant%20mice%20list.html) were generated as described (http://www.cdb.riken.jp/arg/Methods.html). The targeting vector contains two loxP sites and a neomycin resistance cassette for selection in embryonic stem (ES) cells. For gene targeting, ES cell screening and chimera production were carried out. The chimeric mice were mated with C57BL/6 mice to generate F1 heterozygotes. We obtained F1 hetero mice (female 5, male 2 and female 3, male 2) from #91 and #31 ES clones. Genomic DNA was isolated from the mouse ear punched tissues and yolk sac for genotyping. Ift46 mutant mouse alleles were genotyped by allele-specific PCR with the following primers: Ift46 (reverse primer) 5’-TGCTACGATCTACTCCCAGACATGC-3’. Ift46 (WT primer) 5’-AACGATGATGATGACGACGA-3’, Ift46 (MT primer) 5’-CTGACCGCTTCCTCGTGCTTTACG-3‘. Primer set of Ift46 (reverse) and Ift46 (WT) yields a product of 161 bp and primer set of Ift46 (reverse), Ift46 (MT) yields a product of 620 bp. All mouse work conforms to the regulatory standards for experimental animal work as set out by the legal authorities.
Phenotyping of mice
To analyze mouse embryonic phenotypes, crosses of heterozygous mice are set up and vaginal plugs are checked each morning to establish the day of conception. After dissection of female mice, the thin amniotic membrane surrounding embryos was removed, and embryos were fixed in 4% paraformaldehyde. Whole-mount in situ hybridizations was performed using standard protocols.
Histology of mouse embryos
Mouse embryonic brains were fixed in 4% paraformaldehyde solution at room temperature overnight. Specimens were dehydrated and embedded in paraffin. Serial sections (thickness of 6 µm) were prepared.
Scanning Electron Microscopy (SEM) of mouse embryos
Embryos at E8.0 were dissected in PBS with 0.05% Triton X-100 and fixed overnight in 2.5% PFA, 2.5% glutaraldehyde in 0.075M sodium cacodylate buffer, pH 7.4. Embryos are placed in 100% ethanol for critical point drying using E3000 (Polaron) critical point dryer. Critical point dried embryos were placed on a piece of carbon tape and sputter coated with gold in a SC7640 Sputter Coater. Specimens were imaged on a Quanta 250 Field Emission Scanning Electron microscope (FEI).
Supplementary Material
Fig S1. Overexpression of IFT46 induces apoptosis in zebrafish embryos. (A, B) Control and human IFT46 mRNA (100 pg/embryos) injected embryo stained with acridine orange at 24 hpf. overexpression of IFT46 leads to increase of apoptosis in developing embryo. (C) Temporal expression profile of ift46 by RT-PCR. The ift46 transcript is detected from 4-cell stage to 72 hpf in zebrafish. Both maternal and zygotic expression of ift46 is detected. β-actin is a loading control. (D–E) ift46-GFP expression is completely inhibited when co-injection with ift46 translation blocking morpholino in zebrafish embryos, showing the efficacy of the ift46-MO.
Fig S2. The ift46 intraflagellar transport complex B protein 46 C-terminal domain is sufficient for ift46 function. (A) Diagram of ift46 and deletion mutant structures. Numbers indicate the amino acid positions in ift46 protein. (B) Quantification of the rescue of ift46 morpholino phenotypes by co-injection of human and zebrafish mRNA. Control (n=144), ift46-MO (n=180), IFT46 mRNA+ift46-MO (n=120), zebrafish ift46 mRNA+ift46-MO (n=108). Statistical significance of pairwise comparisons are shown (***P<0.001; Student’s t-test). (C–H) Representative images of zebrafish larvae following co- injection of ift46-MO and ift46 encoding mRNAs.
Fig S3. The organ laterality defect is not disrupted in zebrafish ift46 morphants. (A–C’) The ift46 morphant embryos have no left-right patterning defect visualized by whole-mount in situ hybridization for lft1 and lft2 (D–E’). The ift46 morphants does not have any abnormal heart looping and gut laterality with whole-mount in situ hybridization for cmlc and foxA3. (A, artery; I, intestine; L, liver; P, pancreas; V, vein). (F-F’) Expression of charon in Kupffer’s vesicle in the control and the ift46 morphants are comparable. (G-G’) Visualization of cilia with immunostaining of acetylated α-tubulin in the Kupffer’s vesicle in both control and the ift46 morphants at 6-somite stage. Scale bar, 20 µm. (H–I) Organ asymmetry scored by cmlc2 (Control (n=56) and ift46 morphants (n=85)) and foxA3 (Control (n=68) and ift46 morphants (n=81)) expression. (J–K) Quantification of the number and length of the KV cilia shows no statistical differences between the control and the ift46 morphant embryos.
Fig S4. Ift46 expression in mouse embryos and adult mouse brain. (A) Ift46 expression profile of early mouse embryos at E8.5 to E11.5. (B) RT-PCR of Ift46 in various adult mouse tissues including brain, heart, kidney and liver. β-actin is a loading control. (C-D) in situ hybridization of Ift46 in a section of E10.5 mouse embryo. The sense probe is used as a negative control. Ift46 expression is restricted to the notochord (nt), otic vesicle (ov) and forebrain (fb). (E) in situ hybridization of Ift46 in the adult mouse brain. Ift46 is expressed specifically in the brain regions containing ependymal cells. (alv; alveus, hbc; habenular commissure, cp; caudoputamen, cpd; cerebal peduncle)
Highlights.
IFT46 over-expression induces apoptosis in developing zebrafish embryos.
Loss of IFT46 displays typical phenotypes of ciliary defects in both zebrafish and mice.
IFT46 C-terminal domain is required for IFT46 function.
IFT46 is not essential for axonemal dynein assembly in zebrafish.
Acknowledgments
Funding
This research was supported by the Basic Science Research Program (20100024645), the KRIBB Research Initiative Program (KGM4611411) and the Bio & Medical Technology Development Program (2012M3A9D1054519 for J.-H.P. and 2014M3A9A5034156 for N.-S. K.) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning. W. Z. is supported by NIH grants (R00DK091405 and P30DK081943) and Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badano J, Mitsuma N, Beales P, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Ben J, Elworthy S, Ng AS, van Eeden F, Ingham PW. Targeted mutation of the talpid3 gene in zebrafish reveals its conserved requirement for ciliogenesis and Hedgehog signalling across the vertebrates. Development. 2011;138:4969–4978. doi: 10.1242/dev.070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Kim S, Kim J, Kim J-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Cole D, Snell W. SnapShot: Intraflagellar transport. Cell. 2009;137:784–784.e1. doi: 10.1016/j.cell.2009.04.053. [DOI] [PubMed] [Google Scholar]
- Delaval B, Bright A, Lawson N, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 2011;13:461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler J, Anderson K. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner J, Amack J, Nyholm M, Harris E, Yost H. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motil. Cytoskeleton. 2009;66:457–468. doi: 10.1002/cm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Davis E, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S, Anderson K. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev. Biol. 2009;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouttenoire J, Valcourt U, Bougault C, Aubert-Foucher E, Arnaud E, Giraud L, Mallein-Gerin F. Knockdown of the intraflagellar transport protein IFT46 stimulates selective gene expression in mouse chondrocytes and affects early development in zebrafish. J. Biol. Chem. 2007;282:30960–30973. doi: 10.1074/jbc.M705730200. [DOI] [PubMed] [Google Scholar]
- Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 2002;3:103–113. doi: 10.1038/nrg732. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat. Rev. Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y. Left-right determination: involvement of molecular motor KIF3, cilia, and nodal flow. Cold Spring Harb Perspect Biol. 2009;1:a000802. doi: 10.1101/cshperspect.a000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Qin H, Follit J, Pazour G, Rosenbaum J, Witman G. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde C, Dickinson R, Houtzager V, Cullum R, Montpetit R, Metzler M, Simpson E, Roy S, Hayden M, Hoodless P, Nicholson D. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev. Biol. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev. Cell. 2008;14:954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker A, Olale F, Haycraft C, Yoder B, Schier A, Drummond I. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Kreiling J, Williams G, Creton R. Analysis of Kupffer’s vesicle in zebrafish embryos using a cave automated virtual environment. Dev. Dyn. 2007;236:1963–1969. doi: 10.1002/dvdy.21191. [DOI] [PubMed] [Google Scholar]
- Kunitomo H, Iino Y. Caenorhabditis elegans DYF-11, an orthologue of mammalian Traf3ip1/MIP-T3, is required for sensory cilia formation. Genes Cells. 2008;13:13–25. doi: 10.1111/j.1365-2443.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- Lenhart K, Lin S-Y, Titus T, Postlethwait J, Burdine R. Two additional midline barriers function with midline lefty1 expression to maintain asymmetric Nodal signaling during left-right axis specification in zebrafish. Development. 2011;138:4405–4410. doi: 10.1242/dev.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker B, Miller M, Dziedzic S, Blackmarr P, Cole D. Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46. J. Biol. Chem. 2010;285:21508–21518. doi: 10.1074/jbc.M110.106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SC, Haynes T, Perkins BD. Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling. Dev Dyn. 2009;238:1744–1759. doi: 10.1002/dvdy.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia N, Richards W, Yoder B, Mucenski M, Dunlap J, Woychik R. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Nigg E, Raff J. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Novarino G, Akizu N, Gleeson J. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–79. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteíza P, Köppen M, Concha M, Heisenberg C-P. Origin and shaping of the laterality organ in zebrafish. Development. 2008;135:2807–2813. doi: 10.1242/dev.022228. [DOI] [PubMed] [Google Scholar]
- Pazour G, Rosenbaum J. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- Pazour G. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2004;15:2528–2536. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- Raya A, Izpisúa, Belmonte J. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat. Rev. Genet. 2006;7:283–293. doi: 10.1038/nrg1830. [DOI] [PubMed] [Google Scholar]
- Richey E, Qin H. Dissecting the sequential assembly and localization of intraflagellar transport particle complex B in Chlamydomonas. PLoS One. 2012;7:e43118. doi: 10.1371/journal.pone.0043118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Margall-Ducos G, Guidotti JE, Brégerie O, Celati C, Bréchot C, Desdouets C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 2007;120:628–637. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J, Witman G. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Silverman M, Leroux M. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–316. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Scholey J. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- Stooke-Vaughan G, Huang P, Hammond K, Schier A, Whitfield T. The role of hair cells, cilia and ciliary motility in otolith formation in the zebrafish otic vesicle. Development. 2012;139:1777–1787. doi: 10.1242/dev.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Perkins B. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res. 2009;49:479–489. doi: 10.1016/j.visres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter C, Serluca F, Thiberge S, Burdine R. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev. Biol. 2008;314:261–275. doi: 10.1016/j.ydbio.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y, Kim J, Kim H-T, Lee J, Jeon J, Jin Y, Choi J-H, Ban Y, Ha S-J, Kim C-H, Lee H-W, Kim J-S. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 2014;24:125–131. doi: 10.1101/gr.163394.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulman P, Haycraft C, Balkovetz D, Yoder B. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Wood C, Wang Z, Diener D, Zones J, Rosenbaum J, Umen J. IFT proteins accumulate during cell division and localize to the cleavage furrow in Chlamydomonas. PLoS One. 2012;7:e30729. doi: 10.1371/journal.pone.0030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala M, Knowles M, Omran H. Genetic defects in ciliary structure and function. Annu. Rev. Physiol. 2007;69:423–450. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- Zhao C, Malicki J. Genetic defects of pronephric cilia in zebrafish. Mech. Dev. 2007;124:605–616. doi: 10.1016/j.mod.2007.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Overexpression of IFT46 induces apoptosis in zebrafish embryos. (A, B) Control and human IFT46 mRNA (100 pg/embryos) injected embryo stained with acridine orange at 24 hpf. overexpression of IFT46 leads to increase of apoptosis in developing embryo. (C) Temporal expression profile of ift46 by RT-PCR. The ift46 transcript is detected from 4-cell stage to 72 hpf in zebrafish. Both maternal and zygotic expression of ift46 is detected. β-actin is a loading control. (D–E) ift46-GFP expression is completely inhibited when co-injection with ift46 translation blocking morpholino in zebrafish embryos, showing the efficacy of the ift46-MO.
Fig S2. The ift46 intraflagellar transport complex B protein 46 C-terminal domain is sufficient for ift46 function. (A) Diagram of ift46 and deletion mutant structures. Numbers indicate the amino acid positions in ift46 protein. (B) Quantification of the rescue of ift46 morpholino phenotypes by co-injection of human and zebrafish mRNA. Control (n=144), ift46-MO (n=180), IFT46 mRNA+ift46-MO (n=120), zebrafish ift46 mRNA+ift46-MO (n=108). Statistical significance of pairwise comparisons are shown (***P<0.001; Student’s t-test). (C–H) Representative images of zebrafish larvae following co- injection of ift46-MO and ift46 encoding mRNAs.
Fig S3. The organ laterality defect is not disrupted in zebrafish ift46 morphants. (A–C’) The ift46 morphant embryos have no left-right patterning defect visualized by whole-mount in situ hybridization for lft1 and lft2 (D–E’). The ift46 morphants does not have any abnormal heart looping and gut laterality with whole-mount in situ hybridization for cmlc and foxA3. (A, artery; I, intestine; L, liver; P, pancreas; V, vein). (F-F’) Expression of charon in Kupffer’s vesicle in the control and the ift46 morphants are comparable. (G-G’) Visualization of cilia with immunostaining of acetylated α-tubulin in the Kupffer’s vesicle in both control and the ift46 morphants at 6-somite stage. Scale bar, 20 µm. (H–I) Organ asymmetry scored by cmlc2 (Control (n=56) and ift46 morphants (n=85)) and foxA3 (Control (n=68) and ift46 morphants (n=81)) expression. (J–K) Quantification of the number and length of the KV cilia shows no statistical differences between the control and the ift46 morphant embryos.
Fig S4. Ift46 expression in mouse embryos and adult mouse brain. (A) Ift46 expression profile of early mouse embryos at E8.5 to E11.5. (B) RT-PCR of Ift46 in various adult mouse tissues including brain, heart, kidney and liver. β-actin is a loading control. (C-D) in situ hybridization of Ift46 in a section of E10.5 mouse embryo. The sense probe is used as a negative control. Ift46 expression is restricted to the notochord (nt), otic vesicle (ov) and forebrain (fb). (E) in situ hybridization of Ift46 in the adult mouse brain. Ift46 is expressed specifically in the brain regions containing ependymal cells. (alv; alveus, hbc; habenular commissure, cp; caudoputamen, cpd; cerebal peduncle)