Abstract
Background
Prenatal exposure to p,p′-DDE is associated with impairments in motor development during the first year of life, with no related repercussions on mental or motor development at 12 to 30 months and with impairments in cognitive areas, but not in perceptual and motor areas at preschool age. However, its association with particular psychomotor factors, such as establishment of lateralization and spatial orientation, essential elements to the overall learning and specifically reading, writing and spelling in preschoolers, has not been independently evaluated, since cognitive and motor areas have only been explored globally.
Objective
To determine the association between prenatal exposure to p,p′DDE and the establishment of lateralization and spatial orientation in children 5 years of age.
Material and Methods
Establishment of lateralization and spatial orientation was evaluated using the McCarthy Scale of Children's Abilities, with 167 children 5 years of age who participated in a birth cohort in the state of Morelos, Mexico. The information available for each child included: serum concentrations of p,p′-DDE of the mother during at least one trimester of pregnancy, mothers' intelligence quotients, stimulation at home and anthropometry. A logistic regression model was used to calculate the association between prenatal exposure to p,p′-DDE and lateralization and a multiple linear regression model was used for the association with spatial orientation.
Results
A two-fold increase in p,p′DDE in lipid base during the second trimester of pregnancy was associated with a significant reduction, -0.18 points (95%CI -0.41;0.04, in the spatial orientation index, with no impairment in the establishment of hemispheric dominance. Attending preschool and the maternal intelligence quotient were the main determinants of spatial orientation and the establishment of hemispheric dominance.
Conclusions
Prenatal exposure to p,p′-DDE may affect the 5 year old's ability to identify spatial orientation of oneself and surrounding objects. Given the observed role of attending preschool in the functions studied, early attendance in formal education might serve as a stimulation strategy for preschoolers. These preliminary results should be verified and expanded in further prospective studies with DDE.
Keywords: DDE, lateralization, McCarthy Scales, spatial orientation
Introduction
Dichlorodiphenyldichloroethylene (p,p′-DDE), the main metabolite of DDT, is a chemically stable compound which is highly persistent in the environment. It is found in the food chain, accumulates in the organism (Casarett & Doull's, 1986) and easily crosses the placental barrier (Dorea, Cruz-Granja, Lacayo-Romero, & Cuadra-Leal, 2001). DDT metabolites have neurotoxic capacity, they directly affect nerve cells (Casarett & Doull's, 1986) and have endocrine disruption effects in the hypothalamic-hypophysis-thyroid axis (Howdeshell, 2002; Takser et al., 2005).
According to recent epidemiological studies, prenatal exposure to this compound is associated with diminished motor development during the first year of life (Eskenazi et al., 2006; N. Ribas-Fito et al., 2003; Torres-Sanchez et al., 2007). However, studies in preschool and school age children assessing DDE effect on subsequent development and other functions of the central nervous system are rare.
Only three studies have used the McCarthy Scale of Children's Abilities (MSCA) to evaluate the association between prenatal exposure to p,p′-DDE and the neurodevelopment of preschoolers (Gladen & Rogan, 1991; Nuria Ribas-Fito et al., 2006; Torres-Sanchez et al., 2013), none of which reported changes related to this test's motor index, but only an effect on cognitive performance (Torres-Sánchez et al., 2013).
Similar to other tests used before 3 years of age, the MSCA motor scale is primarily aimed at evaluating processes such as gross and fine motor coordination in children. Nevertheless, other complex components are included, such as lateralization, evaluating hemispheric dominance and spatial orientation, which determines knowledge of the left-right concept in relation to oneself and surrounding objects (McCarthy, 2004). These two components overlap with the general cognitive index and the perceptual index, since they are psychomotor aspects that are included in the motor area.
Establishment of lateralization, or hemispheric dominance, is one of the last stages in psychomotor development (Bottini, 2000), which together with visual-motor coordination, spatial organization, body schema and spatial perception are essential for proper learning process generally and specifically reading, writing and spelling for children (Cady, 2009; Ozbic & Filipcic, 2010). To-date, no study has separately evaluated the association between prenatal exposure to p,p′-DDE and the establishment of hemispheric dominance and spatial orientation.
In 2001, a cohort study was initiated in four municipalities in the state of Morelos, Mexico in a formerly malaria-endemic area, where DDT was used as part of anti-malaria campaign until 1998. The median values of maternal blood p,p′DDE during pregnancy were 7.7 ng/ml (wet base) and 1020.4 ng/g (lipid base). In previous results with this cohort a two-fold increase in p,p′-DDE serum concentration during the first trimester of pregnancy was negatively associated (β= -0.52 points) with motor development only during the first year of life and, not with mental development (Torres-Sanchez et al., 2007).
In this same cohort no associations between maternal p,p′DDE serum concentrations and mental and motor development of children were found between 12 to 30 months (Torres-Sanchez et al., 2009); at 3.5 to 5 years, two-fold increased concentrations of p,p′DDE were associated with a significant reduction in the general cognitive index (-1.37 points), the quantitative index (-0.88 points), the verbal index (−0.84 points) and the memory index (-0.80 points), with no repercussions for motor and perceptual indexes, measured by the McCarthy Scale (Torres-Sanchez et al., 2013).
Preliminary results suggest that this metabolite has its main effect on later rather than earlier developmental ages. The effects measured to date were in the overall development of the child and not on particular aspects that may be masked by an overall assessment, such as the establishment of laterality and spatial orientation.
The objective of this study was to explore the association between prenatal exposure to p,p′-DDE and the establishment of lateralization, and the quality of spatial orientation at 60 months of age, during which laterality and spatial orientation are determined as development gains in the McCarthy Scales of Abilities (MSCA); this study was conducted in the same cohorts as those previously published and we re-analyzed the already published data, focusing on two specific subscales.
Materials and Methods
A prospective cohort was created from January 2001 to June 2005 in order to evaluate the association between prenatal exposure to organochlorines compounds and neurodevelopment in children. The details about the creation of the cohort and the neurobehavioral evaluation were reported previously (Torres-Sanchez et al., 2007; Torres-Sanchez et al., 2013).
In summary, the participants included 990 women of reproductive age with no history of chronic diseases, who were not receiving treatment with anticonvulsants and who resided in one of four municipalities in the state of Morelos, Mexico. The women were identified during premarital talks that are required by law for civil marriages in the state. The objective of the study was explained to each person and written consent was requested to visit them before, during and after pregnancy. The study was approved by the ethics committee of the Mexico National Institute of Public Health and the National Institute of Perinatology of Mexico.
During the pre-pregnancy stage, a direct interview was held through which sociodemographic and reproductive information was obtained as well as information about work history, smoking, diet and alcohol use. This was followed by a phone call every 8 weeks for the early detection of pregnancy, and when confirmed a visit was scheduled for each trimester. During these visits the women were asked about the evolution of their pregnancy and diet, and a blood sample was requested to determine organochlorines compounds.
Visits after birth were scheduled at 1, 3 and 6 months of age and every 6 months thereafter until 60 months. The Bayley test was used for the neurobehavioral evaluation up to 30 months of age and the MSCA was used from 42 months to 60 months. During the first visit, women were asked about the conditions of the child's birth, including weight and size. The following postnatal evaluations also included anthropometric measurements (weight, height and cephalic perimeter) as well as a questionnaire about the child's diet, general health status, type of family and preschool attendance. The weight and height of the children were transformed into z-scores for analysis.
Of a total of 404 children from uncomplicated pregnancies, with birth weight >2kg, no history of perinatal asphyxia or congenital defects, 30% were lost to follow-up between 1 and 42 months of age. Of the remaining children, 167 had information about lateralization and spatial orientation at 5 years of age and p,p′-DDE concentrations in mother's serum during at least one trimester of pregnancy.
McCarthy Scale of Children's Abilities
The MSCA (McCarthy, 2004) was used to evaluate establishment of lateralization and spatial orientation at 60 months of age. This test measures cognitive and motor abilities and is designed to evaluate children from 2 years and 6 months of age up to 8 years and 6 months. It consists of five sub-tests: verbal, quantitative, executive perceptual, memory and motor. The combination of the first three subtests make up the general cognitive index (GCI), which is considered equivalent to the intelligence quotient, measured by some intelligence tests (Goldstein & Hersen, 1984). The motor subtest evaluates legs, arms and hand movements according to 4 blocks of tasks: leg coordination, arm coordination, imitative action and drawing. While performing the tasks, the hand preference when hitting a ball, picking up and throwing an object, drawing and the preferred eye with which the child looked through a tube were observed and noted. When the tasks involved in the 4 blocks were performed with the same hand and eye, the child was considered to have an established hemispheric dominance (right- or left-handed). When some of the tasks were performed with the right hand and others with the left hand or both, or when using the eye opposite to the preferred hand, the child was considered not to have had an established dominance.
The tests for spatial orientation in the perceptual subscale evaluated the concepts of right and left in relation to the child's own body by asking the child to indicate some part of his or her body that was located on the left or right side. To evaluate the inverse of the right-left concept, a picture of a child was shown facing the child and he or she was asked to indicate some part of the body on the left or right of the child in the drawing. A minimum score of 0 represented the lack of right-left concepts and a maximum of 12 points represented mastery of this concept.
The test was administered by 3 psychologists with experience in the field, with reproducibility among them of 0.99.
Maternal Intelligence Quotient
The intelligence quotient of the mothers was evaluated using the Wechsler Intelligence Scale for Adults (Wechsler, 1981). The test was administered to each of the mothers during the visit at 3 months of age. The scale provides a verbal intelligence quotient, an execution quotient and a total; the latter was used for the analyses.
Environmental Stimulation at Home
Stimulation at home was evaluated at 6 months of age using the HOME (Home Observation for Measurement of the Environment) test (Caldwell & Bradley, 1984). This was performed in the child's house through an interview while observing the interaction of the child with his or her primary caregiver (typically the mother). Materials for games and the space for play activities were also evaluated. The scale is divided into 8 subscales; the sum of each of these indicates the stimulation index at home, ranging from 0 to 45 points.
Prenatal Exposure to p,p′-DDE
p,p′-DDE was determined from serum samples taken from the mothers (1-2 ml) during the first, second and third trimesters of pregnancy using gas chromatography with electron capture detector (model 3400; Varian, Inc., Palo Alto, CA, USA) according to the protocol recommended by the U.S. Environmental Protection Agency (US-EPA, 1980). The total serum lipid concentrations were determined using colorimetry (Randox Laboratories Ltd., Antrim, United Kingdom). The results were expressed in ng/g (lipid base). For internal quality control, each sample was fortified with aldrin; the average recovery was 98.15 ± 8.8%. For every 10 samples, one sample of bovine serum was analyzed which contained known quantities of β-hexaclorociclohexano (β-HCH), aldrin, hexachlorobenzene (HCB), p,p′-DDE and p,p′-DDD, with an average recovery percentage of 100.8, 100.01, 100.91, 103.4, and 104.1%, respectively. In addition, for each group of 10 randomly-selected serum samples, a duplicate analysis was performed, resulting in an average variation coefficient of 4.37%. External quality control was conducted in the laboratory of Dr. Mary Wolf, Mount Sinai School of Medicine, New York City, resulting in a correlation coefficient of 0.98.
Due to financial limitations, the blood lead levels of mothers during each trimester of pregnancy were determined in a subsample of women. A duplicate analysis was conducted in the Environmental Science Associates Laboratories, Inc. (ESA), Chelmsford, MA, USA, using anodic voltammetry as a separation method. Samples with average concentrations <5μg/dl were analyzed again using atomic absorption spectrometry (model 3000; Perkin-Elmer, Inc., Norwalk, CT, USA). Samples for external quality control were supplied by the laboratories of the Center for Disease Control and Prevention (Atlanta, GA, USA) and the Pennsylvania State Blood Lead Proficiency Testing Program (Exton,PA, USA).
Statistical Analysis
Univariate and bivariate descriptive statistics of maternal and child characteristic associations with establishment of hemispheric dominance and spatial orientation were analyzed with the t-test or chi-squared test, ANOVA or correlation coefficient (according to the variables).
Serum concentrations of p,p′DDE in lipid base for each trimester of pregnancy were right skewed and were natural logarithm transformed to normalize the distributions and residuals. Spearman correlation was used with a total of 84 subjects with results from all trimesters of pregnancy to evaluate the correlation of p,p′-DDE concentrations among trimesters. To assist the interpretation of the results, betas corresponding to natural log p,p′DDE were transformed to base 2 logarithms by multiplying by 0.69, and interpreted as the observed change per each two-fold increase in p,p′-DDE concentrations.
We used separate logistic regression models estimate the association between prenatal exposure to p,p′DDE and the establishment of hemispheric dominance, for each trimester of pregnancy. The association with spatial orientation was estimated using multiple linear regression models. Potential confounders were maternal age, maternal intelligence quotient, sex of the preschooler, birth weight, type of birth, breastfeeding, type of family, attendance in preschool, birth order of the child, maternal use of tobacco during pregnancy and HOME scale.
A sensitivity analysis was conducted for establishment of lateralization as well as spatial orientation, for which each one of the final models was executed and only 84 of the children who had information regarding p,p′-DDE for all trimesters were included. To evaluate the role of lead as a potential confounder, the same analysis was performed in a subsample of 65, 73 and 83 subjects who had maternal blood lead level results during the first, second and third trimesters, respectively.
The interactions between p,p′-DDE with the sex of the child and attendance in preschool were evaluated using the corresponding interaction terms, with a criterion significance level of 0.10. The diagnostics of the model included the analysis of residuals, the evaluation of collinearity, and identification of influential observations to determine the validity of the models. Due to the modest sample size we consider this study an exploratory pilot study of the effects of DDE on these aspects of child development. Thus, we establish an alpha value for statistical significance of p≤0.10.
The statistical analysis was performed with the SPSS version 17.0 statistical package.
Results
The median serum p,p′-DDE concentration in mothers during the first and third trimesters of pregnancy ranged from 1331.1 ng/g to 826.3 ng/g in lipid base. The correlation between the trimesters was statistically significant and no significant changes were observed over the course of the pregnancy. There were no significant differences between boys and girls in mean levels of maternal serum p,p′-DDE. For the subsample in which lead blood levels during pregnancy were determined, the geometric mean was 6.9, 6.2 and 7.4μg/dl in the first, second and third trimesters, respectively. Concentrations over 5μg/dl were detected in 53.2% of the samples (Table1).
Table 1. Concentration of p,p′-DDE and Lead Blood Level in Maternal Serum Between the Trimesters of Pregnancy.
| Contaminants | Trimester of pregnancy | ||
|---|---|---|---|
| 1st (n=146) | 2nd (n=123) | 3rd (n=125) | |
| p,p′-DDE (ng/g)* | |||
| P10 | 260.8 | 152.7 | 149.0 |
| P50 | 1331.1 | 1138.1 | 826.3 |
| P90 | 4253.9 | 2983.4 | 2767.6 |
| Sex of Child ** | Mean ± SD | Mean ± SD | Mean ± SD |
| Female | 1624 ± 1376 | 1297 ± 1194 | 1434 ± 1457 |
| Male | 1997 ± 2266 | 1424 ± 1494 | 1121 ± 1280 |
| Blood Lead levels (μg/dl) | 6.9 (n=81) | 6.2 (n=75) | 7.4 (n=94) |
| Blood Lead levels > 5μg/dl (%) | 55.6 | 50.7 | 53.2 |
: p,p′-DDE (ng/g) correlation: 1st vs. 2nd trimester= 0.58; 1st vs.3rd trimester= 0.50; 2nd vs. 3rd trimester= 0.52; p<0.05
: t test to compare means between boys and girls.
There were no significant differences between boys and girls in any subscale of the McCarthy Scales. However, when analyzing each of the components of the motor subscale boys have better arm coordination than girls (9.9 vs 8.0 p=0.002), while girls score better on the subtest for drawing of child (10.1 vs 9.1 p=0.02), There were no significant sex differences on the subtest of spatial orientation (pertaining to the perceptual subscale) (data not shown).
Seventy-five percent of the preschoolers had established hemispheric dominance at 5 years of age. We found no significant differences for established hemispheric dominance for age, schooling, smoking or mother's intelligence coefficient, nor for p,p′DDE concentrations in wet and lipid basis. Age, schooling and intelligence quotient of the mother were positively and significantly associated with better spatial orientation, while p,p′DDE concentrations on a lipid basis on second trimester were negatively and significantly associated with spatial orientation (Table 2). The average spatial orientation index value was 5.7 ± 2.3, with a minimum of 0 and a maximum of 12. Ten percent of the children had a score below 2 points.
Table 2. Maternal Characteristics According to the Establishment of Laterality and Spatial Orientation.
| Characteristics | Establishment of laterality | Spatial orientation | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes n = 125 | No n = 42 | “p”value ** | β*** | 95% CI | “p”value | |
| Age (years) | 0.10 | 0.01; 0.20 | 0.02 | |||
| Mean ± SD | 21.5 ± 3.6 | 21.6 ± 3.7 | 0.87 | |||
| Min-Max | 16 - 31 | 16 - 31 | ||||
| Education (years) | 0.20 | 0.08; 0.32 | 0.00 | |||
| Mean ± SD | 10.6 ± 2.9 | 10.2 ± 2.8 | 0.46 | |||
| Min-Max | 1 - 19 | 5 - 17 | ||||
| Intelligence quotient | 0.06 | 0.03; 0.09 | 0.00 | |||
| Mean ± SD | 86.1 ± 11.7 | 87.2 ± 10.2 | 0.59 | |||
| Min-Max | 58 - 123 | 57 - 102 | ||||
| Smoking (%) | ||||||
| (Yes vs No) | 5(4) | 6(15) | 0.31 | 0.94 | -0.46;2.34 | 0.18 |
| Prenatal exposure to p, p′DDE wet basis (ng/ml) | ||||||
| Trimester 1 (n = 146) | -.011 | -0.36; 0.13 | 0.37 | |||
| Mean ± SD* | 6.05 ± 2.7 | 6.05 ± 2.6 | 0.94 | |||
| Min-Max | 0.30 - 47.0 | 0.8 - 73.7 | ||||
| Trimester 2 (n = 123) | -0.20 | - 0.44; 0.03 | 0.08 | |||
| Mean± SD * | 6.6 ± 3.0 | 5.4 ± 3.0 | 0.32 | |||
| Min-Max | 0.4 - 115.5 | 0.4 - 33.1 | ||||
| Trimester 3 (n = 125) | -0.19 | - 0.44; 0.07 | 0.15 | |||
| Mean± SD * | 8.1 ± 2.4 | 7.3 ± 3.3 | 0.87 | |||
| Min-Max | 0.6 99.4 | 0.4-49.4 | ||||
| Prenatal exposure to p, p′DDE lipid basis (ng/g) | ||||||
| Trimester 1 (n = 146) | -0.16 | - 0.41; 0.08 | 0.20 | |||
| Mean± SD * | 1192.7 ± 2.8 | 1070.0± 2.8 | 0.59 | |||
| Min-Max | 69.4 - 10829.2 | 179.4 - 11047.9 | ||||
| Trimester 2 (n = 123) | -0.22 | - 0.46; 0.004 | 0.05 | |||
| Mean ± SD * | 893.9 ± 3.0 | 679.6 ± 3.1 | 0.25 | |||
| Min-Max | 36.9 - 8955.2 | 84.7 - 5431.6 | ||||
| Trimester 3 (n = 125) | -0.06 | - 0.30; 0.17 | 0.57 | |||
| Mean± SD * | 735.4 ± 2.7 | 749.7 ± 4.0 | 0.93 | |||
| Min-Max | 46.9 8518.5 | 36.2 6310.6 | ||||
Geometric mean and standard deviation.
t-test or Chi2
Change per two-fold increase of prenatal p,p′DDE concentrations.
Among the children's characteristics, only being first born (90 vs. 76%) and attending preschool (95 vs. 86%) were significantly associated with the establishment of hemispheric dominance. Caesarean births, attending preschool and better stimulation at home were positively and significantly associated with spatial orientation (Table 3).
Table 3. Infant Characteristics According to Establishment of Laterality.
| Characteristics | Establishment of laterality | Spatial orientation | |||
|---|---|---|---|---|---|
|
| |||||
| Yes n = 125 | Not n = 42 | “p”value * | β 95% CI | “p”value | |
| CHILDREN'S | |||||
| Sex (%) | |||||
| (Boy vs. Girl) | 76 (61.0). | 22 (52.4). | 0.34 | -0.41 - 1.11; 0.29 | 0.25 |
| Type of birth (%) | |||||
| (Vaginal vs. Caesarean) | 67 (53.6). | 25 (59.5). | 0.50 | 0.78 0.10; 1.48 | 0.02 |
| Birth order of the child (%) | |||||
| (First-born vs. Second or more) | 113 (90.4). | 32 (76.2). | 0.02 | 0.10 - 0.95, 1.15 | 0.85 |
| Breastfeeding (%) | |||||
| (Yes vs. No) | 116 (92.8). | 38 (90.5). | 0.63 | -0.18 - 1.48; 1.11 | 0.78 |
| Type of family (%) | |||||
| (Nuclear vs. Extended) | 52 (41.6). | 16 (38.1). | 0.69 | 0.52 - 0.18; 1.23 | 0.14 |
| Attendance in preschool (%) | |||||
| (Yes vs. No) | 119 (95.2). | 36 (85.7). | 0.04 | 2.42 1.13; 3.73 | 0.000 |
| HOME Scale ** | |||||
| Mean ± SD | 30.3±4.4 | 30.3±5.2 | 0.94 | 0.10 0.03; 0.18 | 0.006 |
| Min-Max | 19 40 | 21 39 | |||
| < 25 points (%) | 14 (11.2). | 9 (21.4). | 0.10 | ||
| Z-score, weight for age | |||||
| Mea± SD | 0.29 ± 1.4 | 0.41 ±1.1 | 0.62 | -0.03 - 0.30; 0.23 | 0.81 |
| Min-Max | -2.3 -4.1 | -1.3 - 4.2 | |||
| Z-score, height-for-age | |||||
| Mean± SD | -0.01±1.1 | 0.09±1.0 | 0.58 | 0.02 - 0.29; 0.33 | 0.91 |
| Min-Max | -2.2 - 3.2 | -1.7 - 3.0 | |||
t- test or Chi2
At six months of age.
Since no significant differences were observed between p,p′DDE concentrations in wet and lipid basis and the establishment of laterality and spatial orientation, subsequent results are explained only in terms of lipid based p,p′DDE concentrations.
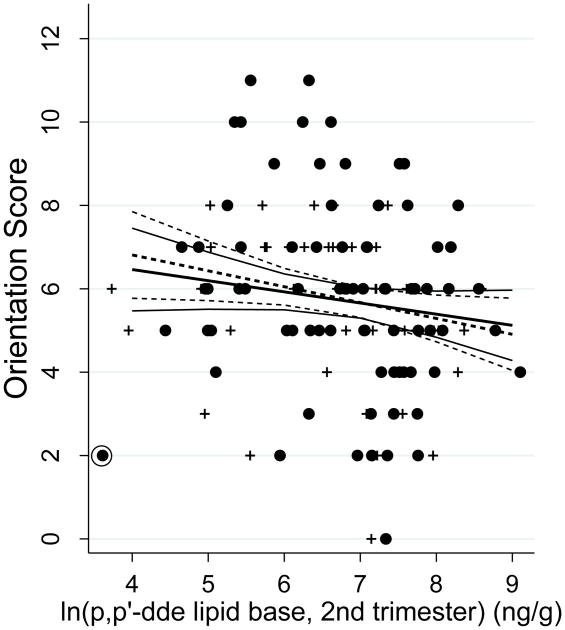
There was no crude association between p,p′DDE concentrations and establishment of laterality at any trimester. In contrast, prenatal p,p′-DDE concentration during the second trimester of pregnancy was negatively associated with spatial orientation. After adjustment for all potential confounders included in the models, a two-fold increase in p,p′-DDE concentrations was associated with a reduction in only the spatial orientation index (β= -0.18; 95% CI -0.41;0.04), (Table 4 and Figure 1). One observation in the orientation model was identified as highly influential. In a supplementary analysis (Appendix Table A1), removing this observation resulted in a 0.68 standard deviation increase in the coefficient for DDE (β= -0.26; 95% CI -0.51; -0.02) (see Figure 1).
Table 4. Logistic regression of the establishment of the laterality and linear regression of spatial orientation and prenatal p,p′DDE exposure in the second trimester of pregnancy (adjusted analysis).
| Study variables | Establishment of laterality | Spatial orientation | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| OR* | 95% CI | “P”value | β* | 95% CI | “P”value | |
| Maternal Intelligence quotient | 1.00 | 0.96; 1.05 | 0.73 | 0.04 | 0.01, 0.07 | 0.01 |
| Sex (Male vs. Female) | 0.53 | 0.19; 1.49 | 0.22 | -0.24 | -1.02;0.54 | 0.55 |
| Birth weight | 0.79 | 0.24; 2.54 | 0.69 | 0.11 | -0.74; 0.96 | 0.79 |
| Type of birth | 0.78 | 0.29;2.11 | 0.63 | 0.52 | -0.21; 1.26 | 0.16 |
| Maternal age | 1.01 | 0.87; 1.17 | 0.89 | 0.08 | -0.02; 0.18 | 0.12 |
| Breastfeeding (Yes vs. No) | 1.97 | 0.42; 9.14 | 0.38 | -0.27 | -1.50; 0.96 | 0.67 |
| Type of family | 0.81 | 0.30; 2.20 | 0.68 | 0.46 | -0.28; 1.20 | 0.22 |
| Birth order of the child (First vs. other) | 2.17 | 0.52; 9.06 | 0.29 | 0.14 | -1.07; 1.36 | 0.81 |
| HOME scale | 1.04 | 0.93; 1.16 | 0.50 | 0.02 | -0.06; 0.10 | 0.67 |
| Preschool Attendance (Yes vs. No) | 3.43 | 0.60; 19.51 | 0.16 | 1.98 | 0.38; 3.57 | 0.01 |
| Smoking (Yes vs No) | 0.07 | 0.009;0.55 | 0.01 | 0.29 | -1.41;2.00 | 0.72 |
| Prenatal exposure to p, p′ DDE (ng/g) (n = 122) | 1.13 | -1.21; 1.53 | 0.44 | -0.18 | -0.41; 0.04 | 0.12 |
: Change per two-fold increase of prenatal p,p′DDE concentrations.
Figure 1.
Marginal plot of effects of second trimester maternal p-p′DDE lipid base concentration on orientation at 60 months from the model in Table 4. Circles are males, pluses are females but only a single regression line is fit for both sexes since neither sex by itself nor in interaction with maternal p-p′DDE was significant in the models. Solid lines are the predictions and 95% CI of the model with all available subjects, dashed lines represent the predictions and 95% CI of the model removing the highly influential subject (circled observation in lower left of graph) with high dfbeta statistic (see Appendix Table A1). Note that the DDE values in the graph are represented in natural logs while the table coefficients are in log base 2.
In the multivariate analysis, being the first-born and preschool attendance were associated with around three times the odds of established hemispheric dominance at 5 years of age (p=0.10). Meanwhile, preschool attendance, mother's intelligence quotient and maternal age were the main determinants of spatial orientation (Table 4). No significant interactions were observed with sex.
Similar association was observed between prenatal p,p′-DDE exposure during second trimester of pregnancy and hemispheric establishment and spatial orientation after maternal lead exposure was included in the final model (OR= 1.39; 95%CI 0.72;2.69; p = 0.32; β= - 0.13; 95% CI -0.42;0.14; p = 0.33 respectively) (data not included in tables).
Finally an analysis performed with only those subjects for whom DDE concentrations were available for all trimesters (n=84) showed similar significance between prenatal p,p′-DDE concentrations in lipid base on second trimester of pregnancy and spatial orientation (β= - 0.25; 95% CI -0.55;0.04 p=0.09) and a lack of association with establishment of lateralization (data not included in tables).
Discussion
This is the first study in preschoolers to evaluate the association between prenatal exposure to p,p′-DDE and specific psychomotor factors. The results indicate that prenatal exposure to p,p′-DDE may be associated with a small reduction in the spatial orientation of children in reference to themselves and others, whereas no association was observed with the establishment of hemispheric dominance.
Previous studies with preschoolers (Gladen & Rogan, 1991; Nuria Ribas-Fito et al., 2006; Torres-Sanchez et al., 2013) did not find an association between prenatal exposure to p,p′-DDE and the MSCA motor and perceptual index. A possible explanation for this is that p,p′-DDE likely affects only very specific psychomotor functions and not the more global indexes. The motor index includes information about other functions such as arm and leg coordination, visual-motor coordination, balance and muscle strength, so that when all the functions that measure the index are included, no adverse effect is observed on motor in general.
Spatial orientation is one of the complex psychomotor processes included in the perceptual subscale; it involves both cognitive as well as motor factors. Similar to the motor scale, the perceptual scale has not been associated with prenatal exposure to p,p′-DDE. Nevertheless, along with the verbal and quantitative scales it constitutes the general cognitive index, which showed a significant reduction (-1.37 points) for each two-fold increase in p,p′-DDE concentrations in the third trimester of pregnancy (Torres-Sanchez et al., 2013).
The association observed between prenatal exposure to p,p′-DDE and spatial orientation suggests that this compound can affect many areas of child neurodevelopment and that the functions affected in the psychomotor area may be so specific and so highly related with cognitive functioning that they become diluted when evaluating motor function as a whole. The present results suggest that the conclusions of previous work with this cohort demonstrating motor effects up to only 1 year of age (Torres-Sanchez et al., 2007, Torres-Sanchez et al., 2009, Torres-Sanchez et al., 2013) could be modified to include effects detected in subsequent development.
Fetal brain development is a process that depends on maternal thyroid function. A decrease in its functioning affects proliferation, migration, synaptogenesis and myelination (Howdeshell, 2002). Prenatal exposure to p,p′-DDE acts as an endocrine disruptor of thyroid functioning and is associated with a reduction in triiodothyronine resin uptake ratios (similar to those observed in hypothyroidism) in mothers and children (Julvez, Debes, Weihe, Choi, & Grandjean, 2011). During the second trimester of pregnancy (3rd to 5th month), neuronal migration occurs in the frontal or motor region of the cerebral cortex and the deep layers of the temporal lobes, including the hippocampus and other limbic areas (Rhawn, 1982; Rosselli, Matute, & Ardila, 2010). The hippocampus is an area of the brain involved in coding spatial location (Dennis, 2004) and a reduction in thyroid functioning during this stage of pregnancy may affect neuronal migration to the hippocampus and functions related to spatial orientation.
These results are also consistent with other factors regarding the establishment of hemispheric dominance and spatial orientation, such as: being the first born (Ardila & Rosselli, 2007), independent of the sex of the child (Kaufman, Zalma, & Kaufman, 1978), attending school (Cady, 2009) and higher intelligence quotient of the mother.
The likelihood of these results being due to a differential measurement error is small. The determinations of serum p,p′-DDE were performed much earlier than the evaluation of neurodevelopment. The psychologists who administered the McCarthy scale were not aware of the prenatal p,p′-DDE concentrations of the children or that there was an area or test that was particularly affected by this exposure. Nevertheless, we do not reject the possibility of a random error in the administration of the MSCA, the calculation of exposure or another covariable which could have caused our results to be underestimated.
Possibly, the observed association between DDE and spatial orientation could be explained by other neurotoxins. However, a model correcting for lead gave similar results as the main analysis.
In summary, the results indicate that prenatal exposure to p,p′-DDE may affect very specific and complex psychomotor factors that are not evident when analyzing motor and perceptual development with the motor and perceptual index as a whole. According to the results prenatal exposure to p,p′-DDE may affect the child's ability to identify right and left of oneself and surrounding objects. Impairment of spatial organization affects the learning process by altering reading-writing acquisition, positioning of letters and general writing motor skills (Amorapanth, Widick, & Chatterjee, 2010; Cady, 2009; Martínez, García, & Montoro, 1988; Ozbic & Filipcic, 2010).
Since this is the first study to separately evaluate the association between prenatal p,p′-DDE exposure and these psychomotor components; its results should be replicated in prospectively planned studies. The positive and independent association between attending preschool and the establishment of lateralization, and spatial orientation in particular, suggest that early participation in formal education is probably a more powerful determinant than the range of p,p′-DDE exposure in this study on lateralization and spatial orientation. In that case, this academic activity could be used as a stimulation strategy for preschoolers resident in more highly contaminated areas.
Supplementary Material
Highlights for review.
Prenatal exposure to p,p′-DDE may affect the child's ability to identify on self and surrounding objects the right or left.
There were no significant sex differences on the subtest of spatial orientation
Early participation in formal education can be an important determinant of the establishment of lateralization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erika Osorio-Valencia, Email: erikaosorio4@hotmail.com.
Luisa Torres-Sánchez, Email: ltorress@insp.mx.
Lizbeth López-Carrillo, Email: lizbeth@insp.mx.
Mariano E Cebrián, Email: mcebrian@cinvestav.mx.
Stephen J. Rothenberg, Email: drlead@prodigy.net.mx.
María del Carmen Hernández Chávez, Email: karmenhdez@hotmail.com.
References
- Amorapanth PX, Widick P, Chatterjee A. The Neural Basis for Spatial Relations. Journal of Cognitive Neuroscience. 2010;22(8):1739–1753. doi: 10.1162/jocn.2009.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila, Rosselli . Neuropsicología clínica. México: 2007. [Google Scholar]
- Bottini . Psicomotricidad: prácticas y conceptos. Madrid, España: Miño y Dávila editores; 2000. [Google Scholar]
- Cady . Psicosomática y Psicomotricidad. Madrid, España: Editoriales Dossat; 2009. [Google Scholar]
- Caldwell, Bradley . Home Observation for Measurement of the Environment. Littlerock, AK: University of Arkansas; 1984. [Google Scholar]
- Casarett, Doull's . Toxicology. New York: Macmillan Publishing Company; 1986. [Google Scholar]
- Dennis . Principios de neuropsicología humana. México D.F: McGraw-Hill Interamericana Editores S.A de C.V; 2004. [Google Scholar]
- Dorea JG, Cruz-Granja AC, Lacayo-Romero ML, Cuadra-Leal J. Perinatal metabolism of dichlorodiphenyldichloroethylene in Nicaraguan mothers. Environmental Research. 2001;86(3):229–237. doi: 10.1006/enrs.2001.4277. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118(1):233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Rogan WJ. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. The Journal of Pediatrics. 1991;119:58–63. doi: 10.1016/s0022-3476(05)81039-x. [DOI] [PubMed] [Google Scholar]
- Goldstein, Hersen . Handbook of Psychological Assessment. New York: Pergamon; 1984. [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environmental Health Perspectives. 2002;110:337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julvez J, Debes F, Weihe P, Choi AL, Grandjean P. Thyroid Dysfunction as a Mediator of Organochlorine Neurotoxicity in Preschool Children. Environmental Health Perspectives. 2011;119(10):1429–1435. doi: 10.1289/ehp.1003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Zalma R, Kaufman NL. The Relationship of Hand Dominance to the Motor Coordination, Mental Ability, and Right-Left Awareness of Young Normal Children. Child Development. 1978;49(3):885–888. [PubMed] [Google Scholar]
- Martínez García, Montoro . Primeros pasos en psicomotricidad en la educación infantil. Madrid, España: Narcea S.A. ediciones; 1988. [Google Scholar]
- McCarthy . Escalas Mc Carthy de Aptitudes y Psicomotricidad para niños 7a edición, revisada. Madrid: Publicaciones de Psicología Aplicada; 2004. [Google Scholar]
- Ozbic M, Filipcic T. Complex imitation of gestures in school-aged children with learning difficulties. Kinesiology. 2010;42(1):44–55. [Google Scholar]
- Rhawn The neuropsychology of development: Hemispheric laterality, limbic language and the origin of thought. Journal of Clinical Psychology. 1982;38(1):4–33. doi: 10.1002/1097-4679(198201)38:1<4::aid-jclp2270380102>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N, Cardo E, Sala M, de Muga ME, Mazon C, Verdu A, et al. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111(5):E580–E585. doi: 10.1542/peds.111.5.e580. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N, Torrent M, Carrizo D, Munoz-Ortiz L, Julvez J, Grimalt JO, et al. In utero exposure to background concentrations of DDT and cognitive functioning among preschoolers. American Journal of Epidemiology. 2006;164(10):955–962. doi: 10.1093/aje/kwj299. [DOI] [PubMed] [Google Scholar]
- Rosselli Matute, Ardila . Neuropsicología del desarrollo infantil. México, D.F: Editorial El Manual Moderno, S.A. de C.V; 2010. [Google Scholar]
- Takser L, Mergler D, Baldwin M, de Grosbois S, Smargiassi A, Lafond J. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environmental Health Perspectives. 2005;113(8):1039–1045. doi: 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez L, Rothenberg SJ, Schnaas L, Cebrian ME, Osorio E, del Carmen Hernandez M, et al. In utero p,p′-DDE exposure and infant neurodevelopment: A perinatal cohort in Mexico. Environmental Health Perspectives. 2007;115(3):435–439. doi: 10.1289/ehp.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez L, Schnaas L, Cebrian ME, del Carmen Hernandez M, Osorio Valencia E, Garcia Hernandez RM, et al. Prenatal dichlorodiphenyldichloroethylene (DDE) exposure and neurodevelopment: A follow-up from 12 to 30 months of age. Neurotoxicology. 2009;30(6):1162–1165. doi: 10.1016/j.neuro.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez L, Schnaas L, Rothenberg SJ, Cebrian ME, Osorio-Valencia E, del Carmen Hernandez M, et al. Prenatal p,p′-DDE Exposure and Neurodevelopment among Children 3.5-5 Years of Age. Environmental Health Perspectives. 2013;121(2):263–268. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US-EPA. Manual of analytical methods for the analysis of pesticides in humans and environmental samples. Washington, D.C.: US Environmental Protection Agency; 1980. [Google Scholar]
- Wechsler . WAIS-Español Escala de Inteligencia para Adultos. México D.F.: Manual Moderno; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.