Abstract
The autophagy proteins (Atg) modulate not only innate but also adaptive immunity against pathogens. We examined the role of dendritic cell Atg5 and Atg7 in the production of IL-2 and IFN-γ by Toxoplasma gondii-reactive CD4+ T cells. T. gondii-reactive mouse CD4+ T cells exhibited unimpaired production of IL-2 and IFN-γ when stimulated with Atg7-deficient mouse dendritic cells that were infected with T. gondii or pulsed with T. gondii lysate antigens. In marked contrast, dendritic cells deficient in Atg5 induced diminished CD4+ T cell production of IL-2 and IFN-γ. This defect was not accompanied by changes in costimulatory ligand expression on dendritic cells or impaired production of IL-12 p70, IL-1β or TNF-α. Knockdown of Irg6a in dendritic cells did not affect CD4+ T cell cytokine production. These results indicate that Atg5 and Atg7 in dendritic cells play differential roles in the modulation of IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
Keywords: toxoplasma, autophagy, cytokine, T cells, dendritic cells
1. INTRODUCTION
Macroautophagy (hereafter referred to as autophagy) is a homeostatic mechanism that delivers bulk cytosol and organelles to lysosomes for degradation [1, 2]. Autophagy is upregulated under starvation and other forms of cellular stress. This process begins with the formation of an isolation membrane that wraps around organelles or portions of cytoplasm enclosing them by a double membrane structure called the autophagosome [1, 2]. The autophagosome fuses with lysosomes leading to the formation of an autolysosome followed by enzymatic degradation of its cargo [1, 2]. Elongation of the isolation membrane is dependent of Atg proteins that include the Atg5-Atg12/Atg16L1 complex and light chain 3 (LC3; Atg8) [2]. The Atg5–Atg12 ubiquitin-like-conjugate is activated by the E1-like enzyme Atg7 and the E2-like enzyme Atg10 [3]. Atg5–Atg12 forms a complex with Atg16L1 and associates with the isolation membrane [4]. The Atg5-Atg12/Atg16L1 targets cytosolic LC3 (LC3 I) to the isolation membrane where it turns into LC3 II by conjugation to phosphatidylethanolamine, an effect driven by the E3-like enzyme activity of Atg5–Atg12 [5].
Autophagy plays an important role in both innate and adaptive immunity against pathogens [6-8]. Ample experimental data indicate that the autophagy machinery can promote killing of a broad variety of pathogens [6-9]. The obligate intracellular protozoan Toxoplasma gondii is an example of a pathogen that can be killed through autophagy proteins [10-12]. The parasite activates EGFR-Akt signaling in infected host cells [12]. Inhibition of EGFR-Akt signaling causes encasement of the parasite by LC3+ structures followed by killing of the parasite that is dependent on Beclin 1, Atg7 and lysosomal enzymes [12]. Similarly, ligation of CD40 triggers encasement of the parasite by LC3 and parasite killing that requires Beclin 1, hvps34, Atg5, Atg7 and lysosomal enzymes [10, 13, 14].
Autophagy regulates various other aspects of immunity including antigen presentation and cytokine production. Autophagy proteins have been reported to enhance MHC class II processing of microbial antigens including Epstein-Barr virus nuclear antigen 1 (EBNA1), the bacterial transposon-derived neomycin phosphotransferase II, influenza matrix protein 1 and Herpes simplex virus (HSV) [15-18]. Depending on the cell type and probably on the receptor engaged, autophagy positively or negatively regulates virus-induced production of type I IFN production [19, 20]. Autophagy also regulates IL-1β secretion. Basal autophagy diminishes the level of inflammasome activation and thus, decreases IL-1β release [21-24]. In contrast, stimulation of autophagy transiently increases IL-1β secretion [25].
Autophagy proteins are traditionally known for their role in the formation of autophagosomes. However, it appears that autophagosomes can be formed independently of Atg5, Atg7, Atg3 and/or Beclin 1 under certain conditions [26-28]. In addition, autophagy proteins can mediate cellular effects independent of autophagosome formation. Upon engagement of several surface receptors, LC3 can be recruited to phagosomes without forming a double membrane structure. This process (LC3-associated phagocytosis) promotes more rapid maturation of the phagosome [29]. Atg5 also exhibits other autophagy independent functions [20, 30, 31]. Atg5 is required for IFN-γ-mediated killing of type II strains of T. gondii [31, 32]. Rather than causing T. gondii killing via autophagosome-mediated parasite degradation, Atg5 promotes disruption of the membrane of the parasite containing vacuole, a process that is dependent on recruitment of various proteins that include Irga6, an Immunity-related GTPase (IRG) [31].
Autophagy proteins can play differential roles in the interaction between host cells and T. gondii. Whereas IFN-γ requires Atg5 to kill type II strains of T. gondii, deficiency of Beclin 1 does not affect parasite killing [13]. In addition, Atg12, Atg16L1, Atg3 and Atg7, but not Atg9a and Atg14, participate in the IFN-γ-induced recruitment of IRG to the parasitophorous vacuole membrane and parasite killing [33, 34]. A study using Atg5-deficient dendritic cells revealed that while Atg5 did not affect presentation of an antigenic peptide, Atg5 deficiency impaired processing through the MHC class II pathway likely by diminishing lysosomal degradation [18]. Little is known on whether Atg proteins in antigen presenting cells play a differential role in promoting T cell responses against microbial antigens. Herein we examine the role of Atg5 and Atg7 in dendritic cells in the production of IL-2 and IFN-γ by T. gondii-reactive CD4+ T cells.
2. MATERIALS AND METHODS
2.1. Animals
C57BL/6 (B6) and mice expressing the Cre recombinase from within the CD11c locus (CD11c-Cre mice) were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the Animal Resource Center (Case Western Reserve University).ATG7flox/flox mice have been described [35]. ATG7flox/flox mice were bred with CD11c-Cre mice. All mice were female, on a B6 background and were 6-8 weeks old when used for the studies. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of Case Western Reserve University School of Medicine.
2.2 Parasites
Tachyzoites of the temperature-sensitive mutant ts4 of T. gondii, the uracil auxotroph carbamoyl phosphate synthetase II (CPSII) KO T. gondii that express cytosolic YFP and the PTG strain of the parasite were maintained in human foreskin fibroblasts (HFF) cell monolayers in DMEM media plus 1% FBS. Culture medium was supplemented with uracil (200 μM) in the case of the uracil auxotroph parasites. ts4 T. gondii was used to immunize B6 mice. Briefly, animals received 1 × 104 tachyzoites i.p. followed by a second i.p. injection of 1 × 105 parasites 4 weeks afterwards, and a final i.p. injection of 1 × 105 parasites 1 week prior to collection of CD4+ T cells. Dendritic cells were infected with tachyzoites of ts4 or CPSII KO T. gondii when used to stimulate CD4+ T cells. Dendritic cells were infected with RH or PTG T. gondii when used to examine CD40-induced stimulation of anti-microbial activity. For these experiments, the load of T. gondii in dendritic cells was assessed as described [10, 13, 14]. T. gondii lysate antigen preparations were made as previously described [36]. Tachyzoite suspensions and TLA were devoid of detectable LPS as assessed by Limulus amebocyte assay (Sigma Chemical Co., St Louis, MO).
2.3. Lentiviral vectors and siRNA
shRNA against mouse Atg5 was previously described [13]. To generate lentivirus, we co-transfected pLL3.7 containing shRNA and packaging vectors VSV-G, RSV-REV, pMDL-g/p RRE into 293T cells [13]. Supernatants were collected at 24 and 48 h, passed through a 0.45 μm filter, concentrated by ultracentrifugation and stored at −80°C. Control siRNA, Atg5 siRNA [37], Atg7 siRNA [38] or Irga6 siRNA [39] have been previously described.
2.4. Dendritic cells
Bone marrow-derived dendritic cells were obtained by culturing bone marrow cells in RPMI plus 10% fetal bovine serum (FBS) (HyClone, Logan UT) supplemented with GM-CSF (50 ng/ml; Peprotech; Rocky Hill, NJ) for 7 d. The mouse dendritic cell line DC2.4 cells (gift from Dr. Kenneth Rock; Department of Pathology, University of Massachusetts Medical School, MA, USA) was cultured in RPMI plus 10% FBS. When indicated, bone-marrow cells that had been incubated with GM-CSF for 3 days, were transduced with lentiviral vectors encoding shRNA against Atg5 or shRNA luciferase at an MOI of 10:1 in the presence of polybrene (8 μg/ml; Sigma Chemical Co.). After overnight incubation, cells were cultured for 4 additional days in fresh complete medium containing GM-CSF. Thereafter, transduced cells (eGFP+) were sorted by FACS Aria cell sorter on and used for experiments. Efficiency of gene silencing was determined by immunoblot. DC2.4 cells were transfected with control siRNA, Atg5 siRNA, Atg7 siRNA or Irga6 siRNA using TransIT-X2 (Mirus Bio LLC; Madison, WI). In certain experiments, dendritic cells were incubated with Rapamycin (1 μM) for 4 h with or without bafilomycin A (100 nM; both from Sigma Chemical Co).
2.5. ELISA
CD4+ T cells were purified from the spleens of T. gondii-immune animals using magnetic beads (Miltenyi Biotec, San Diego, CA). CD4+ T cells (5 × 105/ml) were incubated with dendritic cells (2.5 × 105/ml) challenged with T. gondii tachyzoites or pulsed with TLA (10 μg/ml). Supernatants were collected at 24 h to measure concentrations of IL-2 and at 72 h to measure concentrations of IFN-γ by ELISA (R & D Systems; Minneapolis, MN). Supernatants collected at 24 h were also used to measure IL-12 p70, IL-1β and TNF-α concentrations by ELISA (eBioscience; San Diego, CA).
2.6. Immunoblot
Cell lysates were separated electrophoretically on SDS polyacrylamide gels (Bio-Rad; Hercules, CA), and then transferred to PVDF membranes. Membranes were probed with either antibody to Atg5 (ProteinTech Group; Chicago, IL), Atg7 (Cell Signaling; Danvers, MA), LC3 (MBL International; Woburn, MA), Irga6 (Santa Cruz Biotechnologies; Santa Cruz, CA) or actin (Santa Cruz Biotechnologies), followed by incubation with secondary Ab conjugated to horseradish peroxidase (Santa Cruz Biotechnologies). Bands were developed using enhanced chemiluminescence.
2.7. Statistical analysis
Statistical significance was assessed by Student's t test and ANOVA. Differences were considered statistically significant when P was < 0.05.
3. RESULTS
3.1. Deficiency of Atg5 in dendritic cells impairs production of IL-2 and IFN-γ by T. gondii-reactive CD4+ T-cells
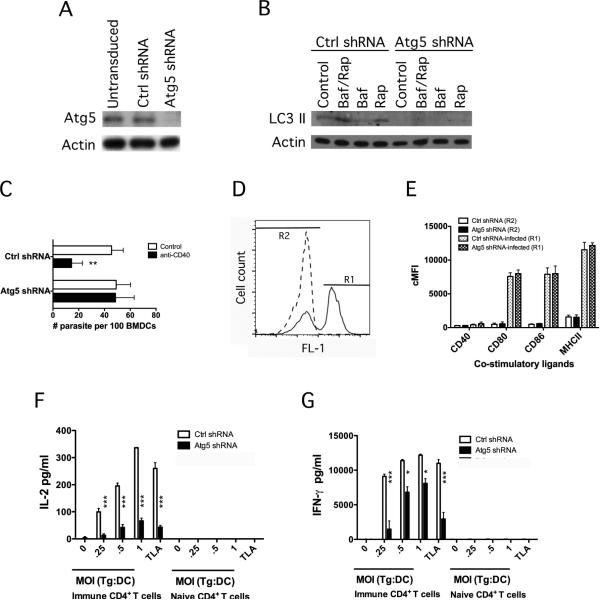
Bone marrow cells from B6 mice were transduced with lentiviral vectors encoding short hairpin RNA (shRNA) directed against Atg5 or control shRNA, or were left untransduced. Bone marrow cells were differentiated to dendritic cells using GM-CSF. Transduction with the lentiviral vector encoding Atg5 shRNA reduced Atg5 protein expression, an effect that persisted for at least 7 days (Figure 1 A and not shown). Next, we determined if Atg5 knockdown affected autophagy. Dendritic cells were incubated with rapamycin (a stimulator of autophagy) with or without bafilomycin A, an inhibitor of the vacuolar ATPase that prevents autophagosome degradation [40], followed by examination of the expression of LC3 II, a molecule associated with the autophagosome membrane [41]. Dendritic cells transduced with the control lentiviral vector exhibited an increase in the expression of LC3 II, an effect that was further enhanced by co-treatment with bafilomycin A (Figure 1B). In contrast, dendritic cells transduced with the Atg5 shRNA-encoding vector exhibit defective LC3 II upregulation indicating that Atg5 knockdown impairs autophagy in dendritic cells (Figure 1B). We tested whether deficiency of Atg5 not only affects autophagy upregulation triggered by rapamycin but also an effector response against T. gondii previously reported to be dependent on autophagy proteins. CD40 ligation causes T. gondii killing that is dependent on the autophagy pathway [10, 13, 14]. Whereas control dendritic cells acquired anti-T. gondii activity when incubated with a stimulatory anti-CD40 mAb, this response was blunted in dendritic cells deficient in Atg5 (Figure 1C).
Figure 1. Bone marrow-derived dendritic cells deficient in Atg5 induce diminished IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
Bone marrow cells from B6 mice were transduced with either control lentiviral vector or lentiviral vector that encodes Atg5 shRNA. Cells were then differentiated into dendritic cells using GMCSF and GFP+ cells were sorted by flow cytometry. A, Atg5 and actin expression were determined by immunoblot analysis. B, Dendritic cells were either left untreated, or treated with Bafilomycin A1 (100 nM) (Baf), Rapamycin (1 μM) (Rap) or both. LC3 II expression was examined by immunoblot. C, Dendritic cells transduced with control or Atg5 shRNA encoding lentiviral vectors were incubated with RH T. gondii followed by stimulation with anti-CD40 mAb. The numbers of tachyzoites per 100 bone marrow-derived dendritic cells (BMDCs) were assessed by light microscopy at 24 h. D, E, Dendritic cells from B6 mice were transduced with lentiviral vector encoding Atg5 shRNA or control shRNA followed by incubation with or without YFP-CPSII knockout T. gondii. D, Cells were sorted by flow cytometry based on green fluorescence and were subsequently examined by light microscopy to determine the percentages of infection. 85% of cells in gate R1 contained intracellular parasites while 10% of cell in gate R2 were infected. Dashed line = Dendritic cells not incubated with T. gondii; Solid line = Dendritic cells challenged with T. gondii. E, Corrected mean fluorescence intensity (cMFI) of CD40, CD80, CD86, and MHCII on uninfected or T. gondii-infected dendritic cells. Infected and uninfected cells were gated as shown in D. cMFI in cells within gate R2 were similar to those in cells not incubated with T. gondii. F, G, Dendritic cells transduced with lentiviral vectors encoding Atg5 shRNA or control shRNA were infected with T. gondii (multiplicity of infection, MOI from 0.25:1 to 1:1 tachyzoites per cell) or were incubated T. gondii lysate antigen (TLA; 10 μg/ml). CD4+ T cells were isolated from T. gondii-immunized or naïve B6 mice and were co-cultured dendritic cells. IL-2 (F) and IFN-γ (G) production were assessed by ELISA using supernatants collected at 24 or 72 h respectively. Results are shown as the mean ± SEM and representative of 3 independent experiments. * p < 0.05; ** < 0.01; *** p < 0.001.
We examined whether Atg5 knockdown affects the expression of CD40, CD80, CD86 and MHC class II in dendritic cells. Infection with T. gondii upregulates expression of these molecules in dendritic cells [42, 43]. Thus, these experiments were conducted using dendritic cells challenged with or without T. gondii. Dendritic cells were incubated with tachyzoites that express cytoplasmic YFP. Levels of green fluorescence by flow cytometry were used to gate on infected dendritic cells (Figure 1D). Sorting of cells with high levels of fluorescence revealed that 85 ± 9% of them contained intracellular tachyzoites whereas 10 ± 3% of the cells with low levels of fluorescence were infected. As was reported with human dendritic cells, T. gondii infection upregulated CD80, CD86 and MHC class II in mouse dendritic cells (Figure 1E). The levels of expression of CD40, CD80, CD86 and MHC class II were similar in dendritic cells transduced with the control or the Atg5 shRNA-encoding vector (Figure 1E). These findings applied to both cells gated on high or low green fluorescence (Figure 1E). Expression of CD40, CD80, CD86 and MHC class II in cells with low green fluorescence were similar to those in cells not challenged with T. gondii (not shown). Thus, Atg5 knockdown does not affect basal expression of CD40, CD80, CD86 and MHC class II or the upregulation of these molecules in response to infection with T. gondii. Next, we examined the effects of Atg5 knockdown on the induction of IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells. Dendritic cells transduced with control or Atg5 shRNA-encoding lentiviral vectors were incubated with either T. gondii tachyzoites or T. gondii lysate antigens (TLA) followed by culture with CD4+ T cells from T. gondii-immune or naïve mice. No stimulation of IL-2 or IFN-γ production was detected when control dendritic cells infected with T. gondii or pulsed with TLA were incubated with naïve CD4+ T cells (Figure 1F, G). Control dendritic cells infected with T. gondii or pulsed with TLA triggered the production of IL-2 and IFN-γ by T. gondii-reactive CD4+ T cells (Figure 1F, G). The production of these cytokines was markedly reduced when CD4+ T cells were incubated with Atg5-deficient dendritic cells. Defective IFN-γ production was not accompanied by impaired IL-12 production. In agreement with studies using HSV [18], Atg5-deficient dendritic cells secreted similar amounts of IL-12 p70 as control cells (Figure 2). Deficiency in Atg5 in dendritic cells did not impair production of IL-1β or TNF-α (Figure 2). Thus, deficiency in Atg5 in dendritic cells inhibits IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells, an effect that does not appear to be caused by defective expression of CD40, CD80, CD86, MHC class II, IL-12 p70, IL-1β or TNF-α.
Figure 2. Deficiency in Atg5 in bone marrow-derived dendritic cells does not diminish IL-12 p70, IL-1β or TNF-α production during incubation with T. gondii-reactive CD4+ T cells.
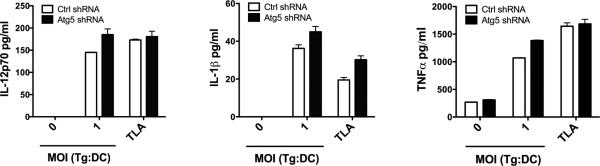
Dendritic cells transduced with lentiviral vectors encoding Atg5 shRNA or control shRNA were infected with T. gondii (MOI 1:1 tachyzoites per cell) or were incubated T. gondii lysate antigen (TLA; 10 μg/ml). CD4+ T cells were isolated from T. gondii-immunized B6 mice and were co-cultured dendritic cells for 24 h. IL-12 p70, IL-1β and TNF-α production were assessed by ELISA. Results are shown as the mean + SEM and representative of 2 independent experiments.
3.2. Deficiency of Atg7 in dendritic cells impairs autophagy in these cells but does not affect production of IL-2 and IFN-γ by T. gondii–reactive CD4+ T cells
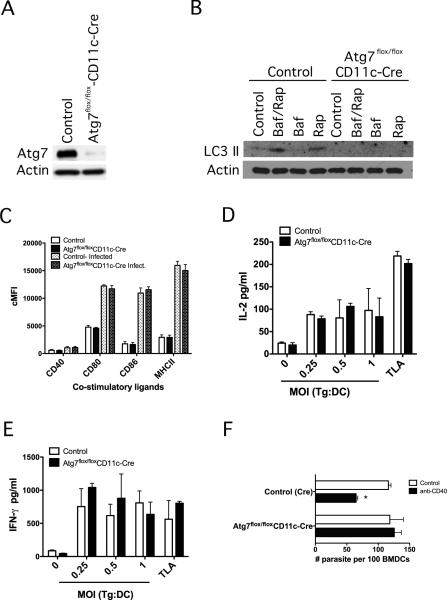
We sought to determine whether the autophagy protein Atg7 played a significant role in the induction of IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells. We used mice where ATG7 was inactivated in CD11c+ cells by breeding of ATG7flox/flox mice [35] with mice that express the Cre recombinase from endogenous CD11c locus (CD11c-Cre). Control mice (ATG7flox/flox) and ATG7flox/flox-CD11c-Cre mice were identified by genotyping. Compared to dendritic cells from control mice, dendritic cells from ATG7flox/flox-CD11c-Cre mice had diminished expression of Atg7 (Figure 3A). In addition, deficiency in Atg7 impaired LC3 II upregulation induced by rapamycin with or without bafilomycin A (Figure 3B). Flow cytometric analysis revealed similar expression of CD40, CD80, CD86 and MHC class II on gated T. gondii-infected or uninfected dendritic cells (Figure 3C). Next, we incubated dendritic cells from control or ATG7flox/flox-CD11c-Cre mice with either T. gondii tachyzoites or with TLA followed by addition of T. gondii-reactive CD4+ cells. Interestingly, production of IL-2 and IFN-γ were similar regardless of the dendritic cells used (Figure 3D, E). We tested whether deficiency of Atg7 affects CD40-induced killing of T. gondii, an effector response against T. gondii dependent on autophagy proteins. Whereas control dendritic cells acquired anti-T. gondii activity when incubated with a stimulatory anti-CD40 mAb, this response was blunted in dendritic cells deficient in Atg7 (Figure 3F). Thus, while dendritic cells from ATG7flox/flox-CD11c-Cre mice exhibit impaired responses that are dependent on the autophagy pathway, these cells do not exhibit defective ability to trigger IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
Figure 3. Bone marrow-derived dendritic cells deficient in Atg7 trigger unimpaired IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
Dendritic cells were obtained from control and ATG7flox/flox-CD11c-Cre mice. A, Atg7 and actin expression were assessed by immunoblot. B, Dendritic cells were either left untreated, or treated with Bafilomycin A1 (100 nM) (Baf), Rapamycin (1 μM) (Rap) or both. LC3 II expression was examined by immunoblot. C. Dendritic cells from ATG7flox/flox-CD11c-Cre mice or control mice were incubated with or without YFP-CPSII knockout T. gondii. cMFI of CD40, CD80, CD86, and MHCII on gated YFP- and YFP+ cells were assessed by flow cytometry. cMFI on gated YFP- cells were similar to those on dendritic cells not challenged with T. gondii. D, E, Dendritic cells from control and ATG7flox/flox-CD11c-Cre mice were incubated with T. gondii (MOI from 0.25:1 to 1:1 tachyzoites per cell) or T. gondii lysate antigen (TLA; 10 μg/ml) followed by addition of CD4+ T cells from T. gondii-immune mice. IL-2 (D) and IFN-γ (E) production were assessed by ELISA. F, Dendritic cells from control and ATG7flox/flox-CD11c-Cre mice were incubated with RH T. gondii followed by stimulation with anti-CD40 mAb. The numbers of tachyzoites per 100 bone marrow-derived dendritic cells (BMDCs) were assessed by light microscopy at 24 h. Results are shown as the mean ± SEM and are representative of 3 independent experiments. * p < 0.05.
3.3. siRNA-mediated knockdown of Atg5 but not of Atg7 in dendritic cells inhibits IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells
The experiments performed thus far compared the effect of shRNA-mediated Atg5 knockdown with Cre-recombinase-mediated knockdown of Atg7. To further test whether Atg5 and Atg7 in dendritic cells play a differential role in the induction of CD4+ T cell cytokine response, we conducted parallel experiments where Atg5 or Atg7 were knocked down using the same methodology. Transfection of the dendritic cell line DC2.4 with Atg5 siRNA or Atg7 siRNA diminished expression of the respective autophagy protein (Figure 4A). Moreover, knockdown of these proteins impaired LC3 II upregulation in response to rapamycin with or without bafilomycin A (Figure 4B). Similar to the results obtained using Atg5 shRNA encoding lentiviral vector, siRNA-induced knockdown of Atg5 in dendritic cells impaired IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells (Figure 4C, D). In contrast, dendritic cells transfected with Atg7 siRNA induced unimpaired production of IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells (Figure 4E, F). Taken together, deficiency of Atg5 but not Atg7 deficiency in dendritic cells impaired IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
Figure 4. siRNA-induced knockdown of Atg5 but not of Atg7 in dendritic cells results in defective IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
DC2.4 cells were transfected with control siRNA or siRNA against Atg5 or Atg7. A, Expression of Atg5, Atg7 and actin were assessed by immunoblot. B, DC2.4 cells were either treated with Rapamycin (1 μM) (Rap), Bafilomycin A1 (100nM) (Baf) or both followed by assessment of LC3II expression by immunoblot. C - F, DC2.4 cells were incubated with T. gondii tachyzoites (MOI 1:1 tachyzoites per cell) or TLA (10 μg/ml) as indicated followed by addition of T. gondii-reactive CD4+ T cells. IL-2 and IFN-γ production were assessed by ELISA. Results are shown as the mean ± SEM and are representative of 3 independent experiments. * p < 0.05; ** p < 0.01.
3.4. siRNA-mediated knockdown of Irga6 in dendritic cells does not affect IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells
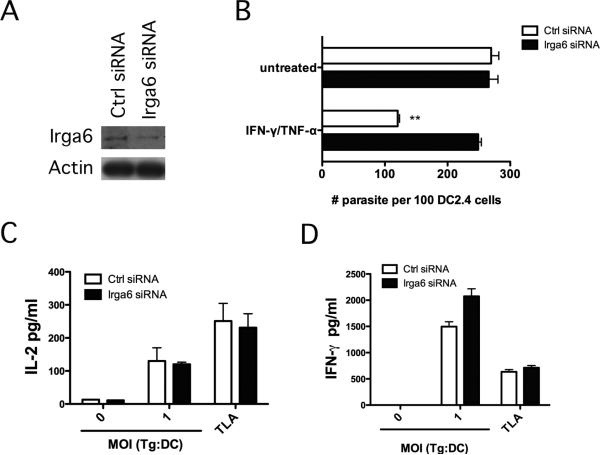
Atg5 mediates the recruitment of IRG to the membrane of the parasitophorous vacuole in IFN-γ-stimulated cells leading to parasite killing [31]. Atg5 in dendritic cells modulated cytokine production even when parasitophorous vacuoles were not expected to be present (dendritic cells pulsed with TLA). This argued against Atg5 enhancing T cell cytokine production through IRG. To more directly test the role of an IRG, we conducted experiments to examine whether IL-2 and IFN-γ production would be affected by knockdown of Irga6, a key IRG downstream of Atg5 [31]. Irga6 was chosen since Atg5 promotes the recruitment and loading of Irga6 to the parasitophorous vacuole membrane, an event that is critical for vesiculation and rupture of the membrane followed by death of type II strains of T. gondii in IFN-γ-treated mouse cells [31]. Transfection of the dendritic cell line DC2.4 with Irga6 siRNA diminished expression of this protein (Figure 5A). Knockdown of Irga6 was functionally relevant since it impaired the anti-T. gondii activity induced by IFN-γ against a type II strain of the parasite (Figure 5B). In contrast, knockdown of Irga6 did not impair IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells (Figure 5C, D). Thus, in contrast to Atg5, Irga6 in dendritic cells does not appear to modulate IL-2 and IFN-γ production by T. gondii-specific CD4+ T cells.
Figure 5. siRNA-induced knockdown of Irga6 in dendritic cells does not affect IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells.
DC2.4 cells were transfected with control siRNA or siRNA against Irga6. A, Expression of Irga6 and actin were assessed by immunoblot. B, DC2.4 cells were incubated with a type II strain of T. gondii followed by stimulation with IFN-γ/TNF-α. The numbers of tachyzoites per 100 DC2.4 cells were assessed by light microscopy at 24 h. C, D, DC2.4 cells were incubated with T. gondii tachyzoites or TLA as indicated followed by addition of T. gondii-reactive CD4+ T cells. IL-2 (C) and IFN-γ (D) production were assessed by ELISA. Results are shown as the mean ± SEM and are representative of 3 independent experiments. ** p < 0.01.
4. DISCUSSION
Autophagy proteins not only regulate innate immunity but can also shape adaptive immune responses. We report that deficiency of Atg5 in dendritic cells impairs the production of IL-2 and IFN-γ by T. gondii-reactive CD4+ T cells. It appears unlikely that canonical autophagy mediates the role of Atg5 in modulation of cytokine production since deficiency in Atg7 had no appreciable effect on secretion of IL-2 and IFN-γ by T. gondii-reactive CD4+ T cells.
Atg5 was previously reported to enhance MHC class II processing of exogenous antigens. While dendritic cells from ATG5flox/flox-CD11c-Cre mice were not defective in presentation of ovalbumin peptides, Atg5 deficiency impaired antigen processing of OVA-expressing HSV-2 and OVA-expressing Listeria monocytogenes likely by diminishing lysosomal degradation [18]. Atg5-deficient dendritic cells were particularly defective in MHC class II processing of soluble and phagocytosed antigens that engage TLR [18]. It has been proposed that these findings likely represent LC3-associated phagocytosis (LAP), a process where engagement of TLR1/2, TLR2/6 or TLR4 is followed by LC3 accumulation around phagosomes and rapid phagosome maturation [44]. Interestingly, this process does not appear to involve the formation of autophagosomes [29, 44]. The reports that T. gondii may engage TLR associated with LAP [45] raise the possibility that Atg5 in dendritic cells may act through LAP to enhance the response of T. gondii-reactive CD4+ T cells. However, silencing of Atg7 revealed an important difference between our studies and those in models of LAP and HSV infection. Whereas Atg7 is required for LAP [29] and Atg7 knockdown in HSV-infected dendritic cells impairs T cell proliferation [18], deficiency of Atg7 had no appreciable effect on IL-2 and IFN-γ production by T. gondii-reactive CD4+ T cells. Thus, it would appear unlikely that Atg5 enhances processing of T. gondii antigens through LAP. A recent report indicates that it is possible for autophagosomes to form in a manner that requires Atg5 but not Atg7 [28]. This raises the possibility that Atg5-dependent but Atg7-independent non-canonical autophagy may enhance antigen processing in dendritic cells.
Increasing evidence points to the differential role of Atg in processes independent of autophagosome formation. In the case of T. gondii, Beclin 1 is not required for toxoplasmacidal activity triggered by IFN-γ against a type II strain of the parasite [13]. Moreover, Atg9a and Atg14 do not participate in the recruitment of Irgb6 around the parasite and the induction of anti-T. gondii activity induced by IFN-γ [33, 34]. In contrast, the complex Atg12-Atg5-Atg16L1, Atg3 and Atg7 are needed for the disruption of the parasitophorous vacuole membrane and parasite killing observed in IFN-γ-treated cells [33, 34]. Our studies indicate that Atg5 in dendritic cells does not function through Irga6 to enhance T cell cytokine production. This work does not rule out a potential role of other IRG. However, the fact that membrane recruitment is a cooperative process among various IRG (for example deficiency of Irga6 reduces Irgb6 loading intensity) [46] and Atg5 regulates cytokine production in conditions where there is no parasitophorous vacuole formation (dendritic cells pulsed with parasite soluble antigens) would argue against dendritic cell IRG playing a major role in Atg5-mediated regulation of T cell cytokine production. Besides regulation of IRG recruitment, other autophagy-independent functions of Atg5 include the interaction with molecules such as retinoic acid-inducible gene I (RIG-I) and interferon-β promoter stimulator 1 (IPS-1) [20]. Future studies that explore whether Atg5 alters the function or trafficking of proteins involved in MHC class II processing in an autophagosome-independent manner may provide important information on the effects of Atg proteins in modulation of adaptive immunity.
Antigen presenting cells have been reported to exhibit autophagosome accumulation associated with the immunological synapse [47], raising the possibility that autophagy may regulate the release of cytokines that modulate T cell effector functions. Autophagy regulates IL-1β secretion, a cytokine that can enhance T cell activation and cytokine production. While basal autophagy diminishes IL-1β release [21, 23, 24], stimulation of autophagy causes a transient increased secretion of this cytokine [25]. Atg5-deficient macrophages secrete reduced amounts of IL-1β upon autophagy stimulation [25]. It appears unlikely that Atg5 in dendritic cells functions through IL-1β to regulate CD4+ T cell production of IL-2 and IFN-γ since deficiency in Atg5 did not impair IL-1β production and addition of recombinant IL-1β failed to restore secretion of these cytokines (Liu et al, unpublished observations). In addition, Atg5-deficient dendritic cells exhibited unimpaired production of IL-12 p70, and TNF-α. Of note, Atg5-deficient dendritic cells have previously been reported to exhibit normal production of IL-12 p40, IL-6 and TNF-α [18]. It remains to be determined whether autophagosomes associated with the immunological synapse mediate transient release of other factors that affect T cell activation, and whether such a phenomenon can take place independently of Atg7.
Our work indicate that Atg proteins in dendritic cells can have distinct effects on the function of CD4+ T cells. These results raise the possibility that Atg5 in dendritic cells may modulate CD4+ T cell cytokine response through mechanisms other than canonical autophagy, LC3-associated phagocytosis or IRG-dependent responses. Further studies may help uncover novel mechanisms by which non-canonical autophagy and/or the autophagy-independent effects of Atg proteins regulate adaptive immunity. These studies may help in the design of strategies to enhance the efficacy of vaccines.
ACKNOWLEDGEMENTS
We thank Kenneth Rock (Univ. of Massachusetts) for providing DC2.4 cells. This work was supported by NIH Grants EY018341 (C.S.S). E.L. and J.V.G. were supported by the Visual Sciences Training Program grant from the National Institutes of Health (T32 EY007157). J.VG is a recipient of a pre-doctoral fellowship from Prevent Blindness Ohio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–32. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395–8. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 4.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–6. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 5.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 6.Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–49. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deretic V. Autophagy: an emerging immunological paradigm. J Immunol. 2012;189:15–20. doi: 10.4049/jimmunol.1102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuballa P, Nolte WM, Castoreno AB, Xavier RJ. Autophagy and the immune system. Annu Rev Immunol. 2012;30:611–46. doi: 10.1146/annurev-immunol-020711-074948. [DOI] [PubMed] [Google Scholar]
- 10.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–77. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–71. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muniz-Feliciano L, Van Grol J, Portillo JA, Liew L, Liu B, Carlin CR, et al. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013;9:e1003809. doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portillo JA, Okenka G, Reed E, Subauste A, Van Grol J, Gentil K, et al. The CD40-autophagy pathway is needed for host protection despite IFN-γ-dependent immunity and CD40 induces autophagy via control of P21 levels. PLoS One. 2010;5:e14472. doi: 10.1371/journal.pone.0014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Grol J, Muniz-Feliciano L, Portillo JA, Bonilha VL, Subauste CS. CD40 induces anti-Toxoplasma gondii activity in nonhematopoietic cells dependent on autophagy proteins. Infect Immun. 2013;81:2002–11. doi: 10.1128/IAI.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–6. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–9. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 17.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–39. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 20.Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci U S A. 2007;104:14050–5. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature. 2008;456:264–8. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 23.Harris J, Hartman M, Roche C, Zeng SG, O'Shea A, Sharp FA, et al. Autophagy controls IL-1β secretion by targeting pro-IL-1β for degradation. J Biol Chem. 2011;286:9587–97. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–63. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. Embo J. 2011;30:4701–11. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–8. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 28.Chang TK, Shravage BV, Hayes SD, Powers CM, Simin RT, Wade Harper J, et al. Uba1 functions in Atg7- and Atg3-independent autophagy. Nat Cell Biol. 2013;15:1067–78. doi: 10.1038/ncb2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 30.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–32. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–69. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konen-Waisman S, Howard JC. Cell-autonomous immunity to Toxoplasma gondii in mouse and man. Microbes Infect. 2007;9:1652–61. doi: 10.1016/j.micinf.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Ohshima J, Lee Y, Sasai M, Saitoh T, Su Ma J, Kamiyama N, et al. Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014;192:3328–35. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, et al. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity. 2014;40:924–35. doi: 10.1016/j.immuni.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subauste CS, de Waal Malefyt R, Fuh F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J Immunol. 1998;160:1831–40. [PubMed] [Google Scholar]
- 37.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–7. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DE, Virok DP, Wood H, Roshick C, Johnson RM, Whitmire WM, et al. Chlamydial IFN-γ immune evasion is linked to host infection tropism. Proc Natl Acad Sci U S A. 2005;102:10658–63. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 41.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subauste CS, Wessendarp M. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-γ. J Immunol. 2000;165:1498–505. doi: 10.4049/jimmunol.165.3.1498. [DOI] [PubMed] [Google Scholar]
- 43.Dupont CD, Christian DA, Selleck EM, Pepper M, Leney-Greene M, Harms Pritchard G, et al. Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to Toxoplasma gondii. PLoS Pathog. 2014;10:e1004047. doi: 10.1371/journal.ppat.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta P, Henault J, Kolbeck R, Sanjuan MA. Noncanonical autophagy: one small step for LC3, one giant leap for immunity. Curr Opin Immunol. 2014;26:69–75. doi: 10.1016/j.coi.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14:109–21. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 46.Khaminets A, Hunn JP, Konen-Waisman S, Zhao YO, Preukschat D, Coers J, et al. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol. 2010;12:939–61. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wildenberg ME, Vos AC, Wolfkamp SC, Duijvestein M, Verhaar AP, Te Velde AA, et al. Autophagy attenuates the adaptive immune response by destabilizing the immunologic synapse. Gastroenterology. 2012;142:1493–503. e6. doi: 10.1053/j.gastro.2012.02.034. [DOI] [PubMed] [Google Scholar]