Highlights
-
•
Fact recall improved between 6 and 8 years, whereas source recall was comparable.
-
•
Executive function uniquely predicted source recall, controlling for age and language.
-
•
Task-related increases in theta power were observed for fact and source recall.
-
•
Executive processes support memory for context.
-
•
Theta synchronization reflects retrieval-related processes in middle childhood.
Keywords: Source memory, Episodic memory, Memory retrieval, EEG power, Executive function, Middle childhood
Abstract
Source memory involves recollecting the contextual details surrounding a memory episode. When source information is bound together, it makes a memory episodic in nature. Unfortunately, very little is known about the factors that contribute to its formation in early development. This study examined the development of source memory in middle childhood. Measures of executive function were examined as potential sources of variation in fact and source recall. Continuous electroencephalogram (EEG) measures were collected during baseline and fact and source retrieval in order to examine memory-related changes in EEG power. Six and 8-year-old children were taught 10 novel facts from two different sources and recall for fact and source information was later tested. Older children were better on fact recall, but both ages were comparable on source recall. However, source recall performance was poor at both ages, suggesting that this ability continues to develop beyond middle childhood. Regression analyses revealed that executive function uniquely predicted variance in source recall performance. Task-related increases in theta power were observed at frontal, temporal and parietal electrode sites during fact and source retrieval. This investigation contributes to our understanding of age-related differences in source memory processing in middle childhood.
1. Introduction
Episodic memory involves recollection of the central content of information (e.g., fact memory) and its surrounding contextual details, known as source memory (SM). Source monitoring refers to the cognitive processes involved in making judgments about the origin of information and may serve as an important framework in explaining episodic memory development (Johnson, 2005, Johnson et al., 1993). Unfortunately, very little is known about the factors that contribute to SM formation. We investigated (1) age-related differences in fact and source recall in middle childhood, (2) the contribution of higher order executive functions to variation in fact and source recall, and (3) patterns of brain electrical activity exhibited during fact and source recall. In the following sections, we discuss what is known about source memory development and its associations with executive function skills and then review related psychophysiological investigations examining the neural correlates of SM.
1.1. Developmental investigations of source memory
Children have difficulty recollecting the contextual details associated with an event (Drummey and Newcombe, 2002, Lindsay et al., 1991). Drummey and Newcombe (2002) taught 4-, 6-, and 8-year-olds novel facts from one of two sources (experimenter or puppet) and tested children on fact and source recall after a 1-week delay. Fact recall steadily improved from 4 to 8 years. Source recall improved between 4 and 6 years and was equivalent among 6- and 8-year-olds. Four-year-olds committed more extraexperimental errors (i.e., incorrectly attributing a source to outside the experimental setting) than intraexperimental errors (i.e., attributing the wrong source within the experimental setting). The finding that 6-year-olds performed equivalently to 8-year-olds on source recall led the authors to conclude that SM skills are relatively intact by age 8. However, the proportion correct for source recall ranged from .40 to .46, which suggests that SM skills develop beyond early childhood. Indeed, other research has found age-related improvement on source discrimination tasks from childhood to adulthood (Billingsley et al., 2002, Chastelaine et al., 2007, Ghetti et al., 2010, Ofen et al., 2007). It is unclear what accounts for age-related improvement in SM processing. We propose that developmental improvement in SM is linked to individual differences in higher order executive processes.
1.2. Associations with executive function
Executive functions (EF) refer to cognitive processes that organize and coordinate goal-directed actions, and consist of working memory, inhibitory control and set-shifting dimensions (Mikaye et al., 2000). In the aging literature, frontal lobe factor scores on EF tasks are associated with better SM performance (Glisky et al., 1995) and lower error rates (Rubin et al., 1999). Only a few studies have examined the relation between EF and SM in childhood. Ruffman et al. (2001) found that the EF component of working memory was related to children's SM accuracy, whereas inhibitory control negatively predicted false alarm errors (i.e., incorrectly attributing a new item as old). Using a global EF composite, Rajan et al. (2014) found that EF uniquely predicted fact and source recall in 4- and 6-year-olds. In terms of specific contextual features, EF predicted episodic memory for spatial and temporal context in participants aged 4–16 years (Picard et al., 2012). Thus, successful SM may require working memory-dependent strategies for linking content to context and the ability to inhibit feelings of familiarity in favor of relevant information (Raj and Bell, 2010). Given that SM skills continue to develop beyond early childhood, we examined whether EF ability would explain variation in fact and source recall in middle childhood.
1.3. Brain electrical activity during fact and source recall
Improvement in SM has been linked to maturation of prefrontal, medial temporal, and parietal brain regions (Ghetti et al., 2010, Ofen et al., 2007, Raj and Bell, 2010). In adults, the prefrontal cortex is involved in SM retrieval (Nolde et al., 1998), hippocampal activation is increased during SM encoding and retrieval (Davachi et al., 2003), and parietal cortex activation is involved in directing attention toward relevant source features (Vilberg and Rugg, 2008). From childhood to adolescence, age-related increases in dorsolateral prefrontal cortex activation (Ofen, 2012, Ofen et al., 2007) and medial temporal lobe activation (Chai et al., 2010, Ghetti et al., 2010) contribute to age-related improvement in episodic recollection.
Event-related potential (ERP) studies reveal that children display different scalp topographies than adults during source retrieval (Cycowicz et al., 2003, Riggins et al., 2013). In addition, the ERP correlates of strategic recollection during source monitoring have been observed for adolescents and adults, but not young children (Sprondel et al., 2011). We examined the neural correlates of fact and source recall by collecting EEG, which provides a continuous measurement of electrophysiological activity during the course of recall and is advantageous to use in developmental populations (Casey and de Haan, 2002).
Memory-related changes in EEG power, which is thought to reflect the excitability of groups of neurons, have been observed in adults. Specifically, neural activity in the theta frequency range (4–7 Hz) is correlated with episodic memory and likely involves the hippocampal-cortical network (Nyhus and Curran, 2010, Klimesch et al., 2001). For example, theta synchronization (reflected by task-related increases in theta power) is associated with episodic encoding and retrieval (Klimesch et al., 1997). Little is known about the functional role of theta activation in episodic memory during childhood. In two-year-olds, task-related increases in theta band power were observed during memory encoding and retrieval and differentiated high and low memory performance (Cuevas et al., 2012). The present investigation addressed this gap in the literature by examining whether memory-related changes in theta activation would be observed in middle childhood.
1.4. Goal and hypotheses
The purpose of our investigation was to assess age-related differences in fact and source recall in 6- and 8-year-olds. We examined whether age-related variability in fact and source recall could be attributable to individual differences in executive function and whether memory-related changes in theta EEG activation would be evident in middle childhood. The following hypotheses were made:
-
1.
Age-related improvement on fact and source recall will be observed. Given that recall of contextual information continues to improve from childhood to adulthood (Billingsley et al., 2002), we hypothesized that fact and source recall would continue to improve between 6 and 8 years of age.
-
2.
Fact and source recall will depend on EF. We predicted that EF would be associated with fact recall, SM accuracy, and lower rates of false alarms errors. In addition, EF would explain variation in fact and source recall. The SM task was highly dependent on word retrieval and recruited the use of free verbal recall. Rajan et al. (2014) found that fact and source recall were positively correlated with expressive vocabulary. Thus, it was necessary to control for language ability. We predicted that EF would uniquely predict variance in fact and source recall performance, beyond the contribution of age and language.
-
3.
Task-related increases in theta power will be evident during fact and source recall. Based on past research which has linked theta rhythms to cortical-hippocampal episodic memory networks (Klimesch et al., 1997), we hypothesized that task-related increases in theta power at frontal, temporal, and parietal electrode sites would be evident during fact and source recall processing.
2. Method
2.1. Participants
Forty 6-year-olds (range: 5 years 7 months–6 years 8 months; 16 boys, 24 girls; 39 Caucasian, 1 African American) and 39 8-year-olds (range: 7 years 5 months–8 years 8 months; 21 boys, 18 girls; 34 Caucasian, 3 African American, 1 Asian, 1 American Indian/Alaska Native) participated in this study. Following Institutional Review Board (IRB) approval, children were recruited using a database compiled from commercial mailing lists and email contact via a local Working Mother's listserve. Recruitment letters were mailed to parents of eligible participants and subsequent phone conversations took place with those interested in participation, during which specific details of the research design were further explained and a lab visit scheduled.
Children were eligible if they were born within 4 weeks of their expected due date, experienced no prenatal or birth complications, were healthy and medication free at the time of testing, and had no developmental or neurological diagnoses. Seven children were excluded (developmental diagnosis: n = 4; premature birth: n = 2; outside age range: n = 1). Thus, behavioral analyses are reported on 72 children (6-year-olds: n = 35; 8-year-olds: n = 37). With respect to parental education, 100% of mothers and 99% of fathers graduated from high school (4% and 8% technical degree, 44% and 37% bachelor's degree, 48% and 43% graduate degree; respectively). Average maternal and paternal age was 38 and 40 years, respectively. Children received a $10 gift card as compensation and parents were entered into a lottery drawing for one $50 gift certificate.
2.2. Procedure
2.2.1. EEG recording
EEG recordings were collected during baseline and during all task procedures. Recordings were made from 32 left and right scalp sites, referenced to Cz. Hypothesis testing focused on six regions: frontal pole (Fp1/Fp2), medial frontal (F3/F4), lateral frontal (F7/F8), anterior temporal, (T7/T8), anterior parietal (P3/P4), and posterior parietal (P7/P8). Baseline EEG was recorded for a duration of 60s during which children were shown a brief video presentation. This procedure was intended to minimize eye movements and gross motor activity (Wolfe and Bell, 2004). Parents were instructed not to talk to their children during the EEG recording.
EEG was recorded using a stretch cap (Electro Cap, Inc., Eaton, OH). Recommended procedures for EEG data collection with infants and young children were followed (Pivik et al., 1993). Electrode impedances were measured and accepted if below 10 kΩ. The electrical activity from each lead was amplified using separate SA Instrumentation Bioamps (San Diego, CA) and bandpassed from .1 to 100 Hz. Activity for each lead was displayed on the monitor of an acquisition computer. The EEG signal was digitized online at 512 samples/s for each channel so that the data were not affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp., Southfield, MI) and the raw data were stored for later analyses.
2.2.2. EEG analysis
EEG data were examined and analyzed using EEG Analysis System software developed by James Long Company (Caroga Lake, NY). First, the data were re-referenced via software to an average reference configuration (Lehmann, 1987). Average referencing, in effect, weighted all the electrode sites equally and eliminated the need for a noncephalic reference. The average reference EEG data were artifact scored for eye blinks using Fp1 and Fp2 (Myslobodsky et al., 1989) and for gross motor movements. These artifact scored epochs were eliminated from all subsequent analyses. The data were then analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1-s width and 50% overlap. EEG analyses focused on the theta band (4–7 Hz). Power was expressed as mean square microvolts and the data were transformed using the natural log (ln) to normalize the distribution.
2.2.3. Source memory task
This task was developed by Drummey and Newcombe (2002). We modified the task by incorporating two female sources (experimenter and a research assistant), which allowed for a more developmentally appropriate and naturalistic source discrimination. In the encoding phase, children were taught a series of 10 novel and interesting facts, five from each source. Each fact was asked in the form of a question, which allowed the experimenter to gauge each child's prior knowledge of the presented facts. Facts were presented from these sources in a blocked rather than random sequence, and the order of presentation was counterbalanced (Drummey and Newcombe, 2002). After 10 novel facts were presented, each fact was then repeated to the child in a random sequence by the same previous source.
After a 20 min delay, children's memory for the previously taught facts was tested along with their ability to correctly attribute the source of this information. During the test phase, a total of 20 questions (10 old, 10 new) were asked. All facts were presented in a random sequence. For each test question, the procedure was as follows: First, the experimenter tested each child's verbal recall memory (i.e., fact recall) for each of the previously heard facts along with the new facts introduced at test. If a child answered the fact recall question correctly, then the source question was asked. However, if a child failed to demonstrate fact recall a four-alternative forced choice recognition test was given. Children were then asked to recall how this information was learned and identify its source by producing a verbal response (i.e., source recall). If the child failed to provide a source response, a four-alternative forced choice test was given (i.e., experimenter, the research asssistant, a parent, or a teacher). The total administration for the test phase lasted approximately 10 min. This task was videotaped and later scored for accuracy. Proportion correct was calculated as the dependent measure of interest for Fact Recall, Fact Knowledge (fact recall plus fact recognition) and Source Recall. The percentage of agreement between two coders for 28% of the sample was calculated, and interrater reliabilities ranged from .98 to 1.00.
An event marker was placed on the electrophysiological record so that EEG recordings could be synchronized with the fact recall (artifact free dft windows, M = 11.58, SD = 8.48) and source recall (artifact free dft windows, M = 8.55, SD = 7.7) phases of the memory task. Retrieval-related EEG started immediately after the experimenter asked the fact recall and source recall question and continued until the child indicated a response. The artifact-free EEG from correct trials were used in the analyses. The average number of trials from which electrophysiological data was collected was 7.38 (SD = 1.83) and 6.04 (SD = 1.75) for fact and source recall, respectively.
2.2.4. EF tasks
Working memory was assessed using the Forward and Backward Digit Span tasks (Wechsler, 1986). For the Forward Digit Span, children were presented with a series of digits and were instructed to repeat the sequence in the same order. The experimenter lengthened the sequence by adding one extra digit to the series until the child erred on two consecutive trials. For the Backward Digit Span, children were instructed to repeat the sequence in reverse order. The highest span in which the child could repeat the entire digit sequence in correct order/correct reverse order was used as the variable of interest. The percentage of agreement between two coders for 28% of the sample was calculated, and interrater reliability was .97 for the Forward Digit task and 1.00 for the Backward Digit task.
Inhibitory control was assessed using the same Stroop-like task procedure as Ruffman et al. (2001). In the conflict condition, children were instructed to count the number of digits (e.g., 3 3 3 3) and press this corresponding number on the computer keypad. This required children to inhibit the automatic tendency of decoding the presented digit on the screen (e.g., “3”) for the appropriate response (e.g., 4). In the control condition, no such conflict was experienced. Instead, children were instructed to count the number of letters (e.g., B B B) presented on the computer screen and register their response on the keypad. Reaction times for both non-conflict and conflict trials were recorded. The Stroop interference score was used as the dependent variable of interest, and was calculated by subtracting the mean reaction time of the non-conflict condition from the mean reaction time of the conflict condition.
2.2.5. Language assessment
The Expressive Vocabulary Test (EVT; Williams, 1997) was administered to examine expressive vocabulary and word retrieval. The EVT is a nationally standarized instrument that has been normed for ages 2½ through 90+ years and is co-normed with the Peabody Picture Vocabulary Test–III (PPVT; Dunn and Dunn, 1997). Children's raw scores were used in all analyses.
3. Results
Descriptive statistics on the SM, language and EF measures for both 6- and 8-year-old children are presented in Table 1. Independent samples t-tests revealed that 8-year-olds had a higher proportion of correct responses than 6-year-olds on fact recall and fact knowledge. However, 6- and 8-year-olds did not differ on source recall. Children were more likely to commit intraexperimental errors (n = 69) than extraexperimental errors (n = 3) and this latter variable was excluded from analyses. No differences were found between these two age groups on the percentage of intraexperimental errors and false alarms committed during the SM test. For the executive function tasks, 8-year-olds performed better than 6-year-olds on Forward and Backward Digit Span, but no age difference was found for the Stroop interference score. Eight-year-olds also outperformed 6-year-olds on expressive vocabulary.
Table 1.
Descriptive statistics for the source memory, language, and EF tasks as a function of age.
| 6-year-olds |
8-year-olds |
t | p | d | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | M | SD | n | ||||
| Fact recall | .61 | .15 | 35 | .86 | .11 | 37 | −7.81 | <.001 | 1.91 |
| Fact knowledge | .89 | .11 | 35 | .99 | .03 | 37 | −4.97 | <.001 | 1.26 |
| Source recall | .57 | .19 | 35 | .63 | .16 | 37 | −1.47 | .15 | .34 |
| Intraexperimental error | .31 | .15 | 34 | .34 | .12 | 35 | −.88 | .38 | .22 |
| False alarms | .38 | .35 | 20 | .22 | .25 | 13 | 1.43 | .16 | .51 |
| Forward digit | 4.94 | .80 | 35 | 5.42 | .87 | 36 | −2.34 | .02 | .57 |
| Backward digit | 2.68 | .68 | 34 | 3.94 | .98 | 36 | −6.23 | <.001 | 1.49 |
| Stroop interference | 206.35 | 631.77 | 35 | 53.82 | 604.32 | 37 | −1.05 | .30 | .25 |
| EVT | 70.57 | 9.53 | 35 | 89.22 | 13.42 | 37 | −6.76 | <.001 | 1.59 |
Note: All values for the source memory measures represent mean proportions. Forward and Backward Digit Span values represent highest span achieved. For the Stroop task (measured in RT), higher values represent a greater interference score.
Pearson correlations were calculated among the SM, EF, and language measures (Table 2). In general, fact recall, fact knowledge, and source recall were all positively correlated with the Forward and Backward Digit Span EF tasks. The Stroop interference score was negatively correlated with source recall performance, indicating that children with lower interference scores tended to perform better on the SM task. In addition, a trend toward significance was seen for false alarm errors being negatively correlated with Forward and Backward Digit Span performance and positively correlated with children's Stroop interference scores.
Table 2.
Pearson correlations among source memory, EF and language collapsed across age.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Fact recall | – | ||||||||
| 2. Fact knowledge | .701** | – | |||||||
| 3. Source recall | .225+ | .196 | – | ||||||
| 4. Intraexperimental error | .057 | .044 | −.524** | – | |||||
| 5. False alarms | −.232 | −.367* | −.380* | .267 | – | ||||
| 6. Forward digit | .398** | .309** | .302* | −.062 | −.297+ | – | |||
| 7. Backward digit | .545** | .359** | .139 | .173 | −.341+ | .419** | – | ||
| 8. Stroop interference | .043 | .012 | −.312** | .058 | .335+ | −.090 | −.067 | – | |
| 9. EVT | .67*** | .48*** | .36** | .03 | −.47** | .40*** | .59*** | .28* | – |
p < .10.
p < .05.
p < .01
As previously mentioned, measures of working memory and inhibitory control tap into a common EF construct. Therefore, due to the conceptual relations among the EF measures, the Stroop Intereference score, Forward Digit highest span, and Backward Digit highest span scores were aggregated into a single EF composite score, a method which has been used in prior research (Picard et al., 2009). The Stroop Interference score was multiplied by −1, so that lower scores on all variables indicate poorer performance. We then converted the raw scores of these variables into standardized z-scores and took the mean of these z-scores to create a composite EF score. To retain as much data as possible, if children were missing data from one or two EF tasks, their composite score was aggregated based on data from the other available tasks. For 70 children the EF composite score represents an aggregrate of all three EF measures. One child failed to pass the learning criterion for the Backward Digit Span task, and thus the EF composite score for this child represents an aggregate of the remaining two EF measures. Due to experimenter error, one child was not administered the Forward and Backward Digit Span tasks, and thus for this child the EF score is the Stroop interference z-score.
3.1. Predicting fact and source recall performance
Hierarchical regressions were performed to determine whether EF would predict performance on fact and source recall. The first step in the regression analysis incuded age and language as predictor variables. To determine the amount of variance explained in fact and source recall above and beyond age and language, EF was added in the second step of the regression analysis. For fact recall, 57% of the variance in performance was accounted for by age, language and EF ability. The results of model 2 revealed that the inclusion of EF did not contribute unique variance, although age and language retained a significant contribution. For source recall, 20% of the variance in performance was accounted for by age, language and EF ability. The results of model 2 reveal a significant contribution from EF to the explanation of variance in source recall, above and beyond age and language. EF uniquely accounted for 7% of the variance, whereas age and language did not contribute unique variance (Table 3).
Table 3.
Regression analysis investigating predictors of fact and source recall.
| R | R2 | ΔR2 | ΔF | F | β | t | |
|---|---|---|---|---|---|---|---|
| Fact recall | |||||||
| Step 1 | |||||||
| Age | .75 | .57 | 44.14*** | .44 | 4.23*** | ||
| EVT | .40 | 3.87*** | |||||
| Step 2 | |||||||
| Age | .75 | .57 | .00 | .04 | 29.02*** | .43 | 4.16*** |
| EVT | .39 | 3.27** | |||||
| EF | .02 | .18 | |||||
| Source recall | |||||||
| Step 1 | |||||||
| Age | .36 | .13 | 5.13** | −.08 | −.57 | ||
| EVT | .41 | 2.81** | |||||
| Step 2 | |||||||
| Age | .44 | .20 | .07 | 5.47* | 5.47** | −.11 | −.83 |
| EVT | .23 | 1.41 | |||||
| EF | .33 | 2.34* | |||||
p < .05.
p < .01.
p < .001.
3.2. EEG results
With the inclusion of EEG, there was a further reduction in sample size. Sixty-nine children contributed electrophysiological data to the following analyses. The primary reason for missing data was too much movement artifact within the EEG record (n = 3).
3.2.1. Statistical analysis
The EEG data were analyzed using a repeated measures multivariate analysis of variance (MANOVAs) for fact and source recall with region (i.e., frontal pole, medial frontal, lateral frontal, anterior temporal, anterior parietal, posterior parietal), hemisphere (i.e., left, right) and condition (i.e., baseline versus task) as within-subjects factors and age (i.e., 6 or 8 years) as between-subjects factors. For ease in examining any interaction effects among these variables, follow-up MANOVAs were performed. A multivariate approach for examining interaction effects has been suggested by Keselman (1998). In order to limit the family wise Type I error rate, a Bonferonni procedure was adopted. We set the familywise Type I error rate to α = .10 instead of α = .05 because this latter value would be too conservative for highly correlated variables, such as regional EEG power values (Yoder et al., 2004). Of major interest were main effects and interactions involving age and condition factors.
3.2.2. EEG power during fact and source recall
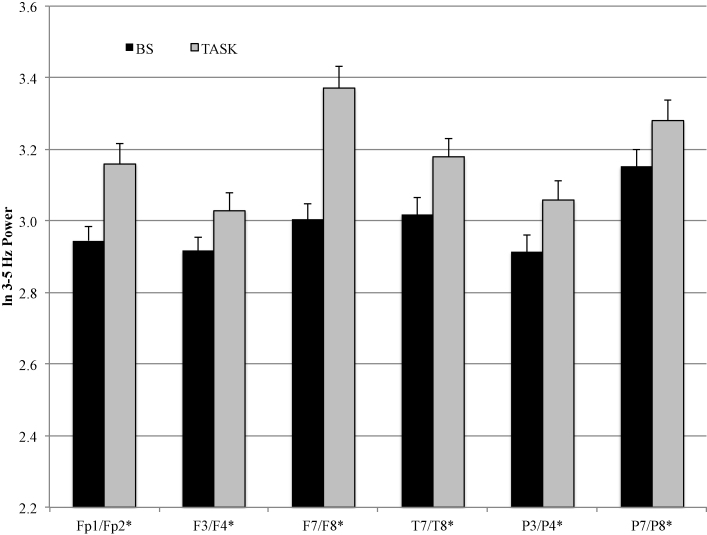
The results of the EEG power MANOVAs are displayed in Table 4. There were main effects for condition, region and hemisphere for fact recall. There was no main effect or interaction effect involving age. These effects were superseded by a Condition × Region and Region × Hemisphere interaction. For ease in examining the Condition × Region interaction, we collapsed across non-significant factors (i.e., age) and completed separate follow-up MANOVAs on the EEG power values for each of the 6 electrode regions. The adjusted p value was ≤.017 (.10/6 = .017). The results of the follow-up regional MANOVAs are displayed in Table 5. There was a main effect involving condition across all 6 electrode regions. Only the main effect of hemisphere at the F7/F8 electrode site reached the adjusted level of significance. All other main effects and interaction effects involving hemisphere were not significant. The means for EEG power (collapsed across hemisphere) during baseline and fact recall are displayed in Fig. 1. Across all 6 electrode regions, EEG power values were higher during fact recall than during baseline.
Table 4.
Multivariate analyses F values for baseline and task activation comparisons.
| Age | Condition | Region | Hemi | A × xC | A × R | A × H | C × R | C × H | R × H | |
|---|---|---|---|---|---|---|---|---|---|---|
| df | 1,59 | 1,59 | 5,55 | 1,59 | 1,59 | 5,55 | 1,59 | 5,55 | 1,59 | 5,55 |
| Item Recall | 25.820*** (.304) | 13.810*** (.557) | 4.050* (.064) | 5.936*** (.351) | 3.362* (.234) | |||||
| df | 1,57 | 1,57 | 5,53 | 1,57 | 1,57 | 5,53 | 1,57 | 5,53 | 1,57 | 5,53 |
| Source Recall | 20.563*** (.265) | 15.223*** (.590) | 4.705* (.076) | 5.849*** (.356) | 3.839** (.266) | |||||
p < .05.
p < .01.
p < .001.
Effect sizes (ηp2)are in parentheses. Three- and four-way interactions were not significant.
Table 5.
Regional multivariate analyses F values for baseline and task activation comparisons.
| Fp1/Fp2 | F3/F4 | F7/F8 | T7/T8 | P3/P4 | P7/P8 | |
|---|---|---|---|---|---|---|
| df | 1,67 | 1,68 | 1,66 | 1,67 | 1,66 | 1,65 |
| Item recall | ||||||
| Condition | 23.592*** (.260) | 8.375** (.110) | 49.729*** (.430) | 12.377** (.156) | 11.802** (.152) | 6.538* (.091) |
| Hemisphere | 4.810* (.067) | 11.422** (.148) | ||||
| C × H | ||||||
| df | 1,64 | 1,66 | 1,64 | 1,66 | 1,64 | 1,64 |
| Source recall | ||||||
| Condition | 23.732*** (.271) | 5.784* (.081) | 37.229*** (.368) | 5.952* (.083) | 6.283* (.089) | |
| Hemisphere | 13.670*** (.176) | 8.873** (.122) | ||||
| C × H | 5.394* (.078) | |||||
p < .05.
p < .01.
p < .001.
Effect sizes (ηp2)are in parentheses.
Fig. 1.
EEG power values for baseline and fact recall at the 4–7 Hz frequency band.
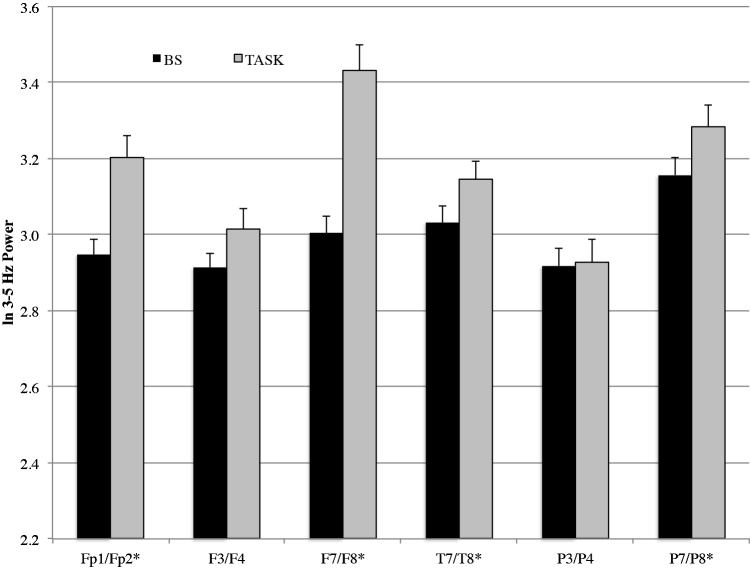
The results of the EEG power MANOVA for source recall revealed main effects for condition, region and hemisphere (Table 4). There was no main effect or interaction effect involving age. These effects were superseded by a Condition × Region and Region × Hemisphere interaction. To examine the Condition × Region interaction, we collapsed across non-significant factors (i.e., age) and completed separate follow-up regional MANOVAs (Table 5). With the exception of the P3/P4 electrode region, there was a main effect involving condition across most electrode regions. The condition main effect at the F3/F4 electrode site failed to reach the adjusted level of significance. There was a main effect of hemisphere at two frontal electrode sites (Fp1/Fp2, F7/F8). However, none of the Condition × Hemipshere interaction effects were significant. The means for EEG power during baseline and source recall (collapsed across hemisphere) are displayed in Fig. 2. EEG power values were higher during source recall than during baseline for select frontal (Fp1/Fp2, F7/F8), temporal (T7/T8) and parietal (P7/P8) electrode regions.
Fig. 2.
EEG power values for baseline and source recall at the 4–7 Hz frequency band.
4. Discussion
The purpose of our study was to examine developmental improvement in the ability to recollect the source-specifying details of a memory episode. We investigated age-related differences in fact and source recall in a sample of 6- and 8-year-old children. In general, our results matched those observed by Drummey and Newcombe (2002). Specifically, 8-year-olds performed better on the measures of fact recall and fact knowledge whereas source recall was comparable between 6- and 8-year-olds. One discrepancy was that children were less likely to experience extraexperimental errors in the present study. This was likely due to the shorter delay imposed. The 1-week delay between encoding and testing phases in Drummey and Newcombe (2002) may have allowed for greater interference and intrusion between multiple sources, thus increasing the occurrence of extraexperimental errors.
Although 6- and 8-year-olds’ level of source recall was comparable, performance was far from ceiling levels, and the proportion of correct responses ranged from .57 to .63, respectively. It is still unclear when SM processes become equated with that of adults. Picard et al. (2012) examined the link between feature-binding abilities and EF skills to the development of three different components of episodic memory (i.e., factual content, spatial context, and temporal context) in 4- to 16-year-old participants. The authors found that memory for factual content showed a pronounced increase during the preschool years, continued to show slight improvement between ages 6 and 9, and reached maturity approximately at 9 years of age. In contrast, children under the age of 8 found it difficult to retrieve spatial and temporal contextual information (Picard et al., 2012). This finding mirrors the results of the present study. It was evident that children did not reach full maturity on their level of source recall by age 8.
It is important for future research investigations to examine SM development during the transitional stages between early childhood, middle childhood, and adolescence. Longitudinal research methodologies should also be incorporated in order to better understand developmental changes in SM ability. To our knowledge, there has only been one longitudinal investigation examining the developmental progression of source monitoring across early and middle childhood. Using the same SM paradigm, Riggins (2014) followed 4-, 6- and 8-year-olds’ performance on fact recall and binding of correct fact/source pairs across 3 years. Similar to the results of cross-sectional studies, fact recall showed steady improvement between 4 and 10 years of age. However, memory for correct fact/source combinations (which required binding of item and context information) showed pronounced improvement specifically between 5 and 7 years of age. In contrast, SM responses that were not conditionalized on item memory (which was examined in the present study) showed steady improvement between 4 and 10 years of age (Riggins, 2014). These results lend further support to our assertion that SM abilities continue to improve across early to middle childhood.
We also sought to determine whether age-related variability in fact and source recall could be attributable to individual differences in executive function. Although EF ability was correlated with fact recall, it failed to uniquely predict fact recall performance beyond the contribution of age and language. In contrast, for SM, EF skills were better predictors of the variance in source recall, uniquely accounting for 7% of the variance in performance. These findings suggest that EF skills are associated with accuracy in SM (Davidson and Glisky, 2002, Glisky et al., 2001) and that monitoring the origin of information may require working memory dependent strategies for linking content to context. Therefore, these results provide some initial insight about the factors that support SM ability in middle childhood.
Lastly, we hypothesized that memory-related changes in theta EEG activation would be observed in middle childhood. Although we failed to find evidence for differential patterns of theta activation in 6- and 8-year-olds, all children exhibited baseline-to-task increases in theta EEG power during fact and source recall. Previous research has found that task-related increases in theta power support different memory processes in adults, such as rehearsal, short-term memory episodic encoding and episodic retrieval (Klimesch, 1999). To elaborate, Klimesch and colleagues found that theta synchronization (at frontal, central, parietal and occipital electrode sites) differentiated between good and bad episodic memory performers (Dopplemayr et al., 1998) and was associated with increased conscious awareness during retrieval (Klimesch et al., 2001). Thus, theta synchronization is related to episodic retrieval and may be detected by scalp-recorded brain electrical activity. The present results suggest that increases in 4–7 Hz theta frequency band power in middle childhood potentially reflects hippocampal-cortical oscillations which support episodic memory processes.
Baseline-to-task increases in theta EEG were observed at frontal, temporal and parietal electrode sites during fact and source recall. These findings are consistent with the adult SM neuroimaging literature (see Spaniol et al., 2009 for a review). To elaborate, hippocampal encoding activation is greater for correctly identified source items compared with incorrectly identified source items (Davachi, 2006, Mayes et al., 2007). Adult fMRI studies have found that, in contrast to old/new item recognition, source retrieval is associated with greater activation in the left prefrontal cortex (Dobbins et al., 2002, Mitchell et al., 2004, Nolde et al., 1998, Ranganath et al., 2000, Rugg et al., 1999).
In addition, the anterior, dorsolateral, and ventrolateral regions of the prefrontal cortex may differentially support source memory (Mitchell and Johnson, 2009). As proposed by Blumenfeld and Ranganath (2007), the ventrolateral prefrontal cortex may be involved in the control processes that select the goal-relevant features of items, whereas dorsolateral prefrontal cortex activation supports elaboration and organization of multiple features (Staresina and Davachi, 2006). Parietal cortex activity has also been linked to encoding and retrieval of source information. Specifically, the parietal cortex is involved in the perceptual binding of feature information (Uncapher et al., 2006) and in directing attention toward relevant source features (Vilberg and Rugg, 2007). These results suggest that the frontal, temporal, and parietal lobe brain regions involved in episodic remembering in adults are also activated during episodic recall in middle childhood.
Although this present investigation focused on patterns of brain electrical activity during retrieval, it would also be worthwhile for future investigations to examine whether increases in theta band power would be observed during encoding of source items subsequently recalled correctly. Additional research is needed to understand the extent to which prefrontal, temporal, and parietal lobe regions interact during encoding and retrieval of item and SM judgments. To elaborate, it would be worthwhile to examine measures of functional connectivity using EEG coherence, defined as the frequency-dependent squared cross correlation between two electrode sites (Nunez, 1981, Thatcher et al., 1987). In adults, evidence suggests that the prefrontal cortex regulates top-down processing of posterior brain activity, and that this regulation supports later SM (Summerfield et al., 2006). Task-related functional connectivity of temporo-parietal memory networks also supports retrieval processes (Hirose et al., 2013). Very little is known about the developmental trends in functional brain connectivity that support early memory formation. Menon et al. (2005) found that the neural organization of memory encoding changes from childhood to adolescence. Specifically, the authors found age-related decreases in medial temporal lobe activation and age-related increases in functional connectivity between medial temporal lobe and prefrontal cortex regions (Menon et al., 2005). Future research should examine whether task-related changes in frontal-parietal and frontal-temporal EEG coherence are evident during SM processing, as previous investigations have documented these changes in early childhood during working memory (Bell and Wolfe, 2007).
EEG is advantageous to use in developmental populations because it is relatively non-invasive. However, this neuroimaging technique does have certain limitations. For instance, it is not possible to localize cortical versus subcortical brain activity using EEG. EEG offers poorer spatial resolution than other neuroimaging techniques that are dependent on changes in blood flow or the metabolic processes of the brain. In addition, the science of mapping the functions of item and SM processes to specific brain regions is still complicated, and interpretations about functional specificity of prefrontal, medial temporal, and parietal cortex brain regions should be made with caution. Therefore, it is necessary for future investigations to document convergent findings across multiple neuroimaging techniques (such as comparisons among fMRI, magnetoencephalography (MEG), ERP, and EEG studies, etc.) in order to increase the generalizability of these findings. With respect to future electrophysiological investigations, measures of EEG coherence can provide an index of the degree of functional connectivity between prefrontal and posterior brain regions and how EM processing functions as an interaction between these brain regions.
4.1. Conclusion
Source memory involves recollecting the contextual details associated with a memory episode. When source-specifying details are bound together, it makes a memory episodic in nature. Unfortunately, very little is known about the factors that contribute to SM formation in early development. In contrast to previous research which stated that the most pronounced improvement in source memory occurs between 4 and 6 years of age, we found that children's recollection of factual information and their ability to monitor the source of this information continues to show improvement during middle childhood and beyond. In addition, individual differences in early executive abilities (i.e., working memory and inhibitory control) support source memory development. We found that EF uniquely accounted for variance in children's source memory performance, even after controlling for age and language. In adults, memory-related changes in EEG power in the theta frequency range are correlated with episodic memory. In the present study, we found retrieval-related increases in theta band power were also evident in middle childhood. Specifically, baseline-to-task increases in EEG power were observed at frontal, temporal, and parietal electrode sites, suggesting that increases in theta EEG may potentially reflect hippocampal cortical oscillations that support episodic memory. Future longitudinal investigations with convergence from multiple neuroimaging methodologies are needed to expand our knowledge about the emergence and development of source monitoring abilities.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
The research reported here represents a portion the first author's dissertation research. She would like to thank her dissertation committee members (Kirby Deater-Deckard, Kurt Hoffman, and Kee Jeong Kim) for their input on this project. We are grateful to the families for their participation. We wish to thank Anjolii Diaz, Candace Hummer and Andrea Ton for assistance with data collection and coding. Data collection and analysis was supported by grant HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Partial support for the preparation of this manuscript was provided by grant R305B130012 from the Institute of Education Sciences (IES). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of IES, NICHD or the National Institutes of Health.
Footnotes
Available online 22 October 2014
References
- Bell M.A., Wolfe C.D. Brain reorganization from infancy to early childhood: evidence from EEG power and coherence during working memory tasks. Dev. Neuropsychol. 2007;31(1):21–38. doi: 10.1207/s15326942dn3101_2. [DOI] [PubMed] [Google Scholar]
- Billingsley R.L., Lou Smith M., McAndrews M.P. Developmental patterns in priming and familiarity in explicit recollection. J. Exp. Child Psychol. 2002;82(3):251–277. doi: 10.1016/s0022-0965(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Blumenfeld R.S., Ranganath C. Prefrontal cortex and long term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Casey B.J., de Haan M. Introduction: new methods in developmental science. Dev. Sci. 2002;5(3):265–267. [Google Scholar]
- Chai X.J., Ofen N., Jacobs L.F., Gabrieli J.D. Scene complexity: influence on perception, memory, and development in the medial temporal lobe. Front. Hum. Neurosci. 2010;4:21. doi: 10.3389/fnhum.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastelaine M., Friedman D., Cycowicz Y.M. The development of control processes supporting source memory discrimination as revealed by event-related potentials. J. Cogn. Neurosci. 2007;19(8):1286–1301. doi: 10.1162/jocn.2007.19.8.1286. [DOI] [PubMed] [Google Scholar]
- Cuevas K., Raj V., Bell M.A. A frequency band analysis of two-year-olds’ memory processes. Int. J. Psychophysiol. 2012;83(3):315–322. doi: 10.1016/j.ijpsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Duff M. Pictures and their colors: what do children remember? J. Cogn. Neurosci. 2003;15(5):759–768. doi: 10.1162/089892903322307465. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L., Mitchell J.P., Wagner A.D. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):2152–2157. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P.S.R., Glisky E.L. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn. Affect. Behav. Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Foley H., Schacter D.L., Wagner A.D. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dopplemayr M., Klimesch W., Schwaiger J., Auinger P., Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci. Lett. 1998;257(1):41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- Drummey A.B., Newcombe N.S. Developmental changes in source memory. Dev. Sci. 2002;5(4):502–513. [Google Scholar]
- Dunn L.M., Dunn L.M. American Guidance Service; Circle Pines, MN: 1997. Peabody Picture Vocabulary Test, Third Edition. [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. J. Neurosci. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky E.L., Polster M.R., Routhieaux B.C. Double dissociation between item and source memory. Neuropsychology. 1995;9(2):229–235. [Google Scholar]
- Glisky E.L., Rubin S.R., Davidson P.S.R. Source memory in older adults: an encoding or retrieval problem? J. Exp. Psychol.: Learn. Mem. Cogn. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Hirose S., Kimura H.M., Jimura K., Kunimatsu A., Abe O., Ohtomo K., Konishi S. Dissociable temporo-parietal memory networks revealed by functional connectivity during episodic retrieval. PLoS ONE. 2013;8(8):e71210. doi: 10.1371/journal.pone.0071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.K. The relation between source memory and episodic memory: comment on Siedlecki et al. (2005) Psychol. Aging. 2005;20(3):529–531. doi: 10.1037/0882-7974.20.3.529. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Hashtroudi S., Lindsay D.S. Source monitoring. Psychol. Bull. 1993;114(1):3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Keselman H.J. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35(4):470–478. [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Doppelmayr M., Schimke H., Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34(2):169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Doppelmayr M., Yonelinas A., Kroll N.E.A., Lazzara M., Röhm D., Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Cogn. Brain Res. 2001;12(1):33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins A.S., Remond A., editors. Rev series, vol. 1. Elsevier; Amsterdam: 1987. pp. 309–354. (Handbook of Electroencephalography and Clinical Neurophysiology. Methods of Analysis of Brain Electrical and Magnetic Signals). [Google Scholar]
- Lindsay D.S., Johnson M.K., Kwon P. Developmental changes in memory source monitoring. J. Exp. Child Psychol. 1991;52(3):297–318. doi: 10.1016/0022-0965(91)90065-z. [DOI] [PubMed] [Google Scholar]
- Mayes A., Montaldi D., Migo E. Associative memory and the medial temporal lobes. Trends Cogn. Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Boyett-Anderson J.M., Reiss A.L. Maturation of medial temporal lobe response and connectivity during memory encoding. Cogn. Brain Res. 2005;25(1):379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mitchell K.J., Johnson M.K. Source monitoring 15 years later: what have we learned about the neural mechanisms of source memory? Psychol. Bull. 2009;135(4):638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K.J., Johnson M.K., Raye C.L., Greene E.J. Prefrontal cortex activity associated with source monitoring in a working memory task. J. Cogn. Neurosci. 2004;16:921–934. doi: 10.1162/0898929041502724. [DOI] [PubMed] [Google Scholar]
- Mikaye A., Friedman N.P., Emerson M.J., Witzki A.J., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit. Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Myslobodsky M.S., Coppola R., Bar-Ziv J., Karson C., Daniel D., van Praag H., Weinberger D.R. EEG asymmetries may be affected by cranial and brain parenchymal asymmetries. Brain Topogr. 1989;1(4):221–228. doi: 10.1007/BF01129599. [DOI] [PubMed] [Google Scholar]
- Nolde S.F., Johnson M.K., D’Esposito M. Left prefrontal activation during episodic remembering: an event-related fMRI study. Neuroreport. 1998;9(15):3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Nunez P. Oxford University Press; New York, NY: 1981. Electrical Fields of the Brain. [Google Scholar]
- Nyhus E., Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci. Biobehav. Rev. 2010;34(7):1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N. The development of neural correlates for memory formation. Neurosci. Biobehav. Rev. 2012;36(7):1708–1717. doi: 10.1016/j.neubiorev.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N., Kao Y., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J.D. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10(9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Picard L., Cousin S., Guillery-Girard B., Eustache F., Piolino P. How do the different components of episodic memory develop? Role of executive functions and short-term feature-binding abilities. Child Dev. 2012;83(3):1037–1050. doi: 10.1111/j.1467-8624.2012.01736.x. [DOI] [PubMed] [Google Scholar]
- Picard L., Reffuveille I., Eustache F., Piolino P. Development of autonoetic autobiographical memory in school-age children: genuine age effect or development of basic cognitive abilities? Conscious. Cogn. 2009;18(4):864–876. doi: 10.1016/j.concog.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Pivik R.T., Broughton R.J., Coppola R., Davidson R.J., Fox N.A., Nuwer M.R. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Raj V., Bell M.A. Cognitive processes supporting episodic memory formation in early childhood: the role of source memory, binding, and executive functioning. Dev. Rev. 2010;30(4):384–402. [Google Scholar]
- Rajan V., Cuevas K., Bell M.A. The contribution of executive function to source memory development in early childhood. J. Cogn. Dev. 2014;15(2):304–324. doi: 10.1080/15248372.2013.763809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Johnson M.K., D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. http://www.jneurosci.org/cgi/content/full/4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Dev. Psychol. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Rollins L., Graham M. Electrophysiological investigation of source memory in early childhood. Dev. Neuropsychol. 2013;38(3):180–196. doi: 10.1080/87565641.2012.762001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S.R., Van Petten C., Glisky E.L., Newberg W.M. Memory conjunction errors in younger and older adults: event-related potential and neuropsychological data. Cogn. Neuropsychol. 1999;16(3–5):459–488. [Google Scholar]
- Ruffman T., Rustin C., Garnham W., Parkin A.J. Source monitoring and false memories in children. Relation to certainty and executive functioning. J. Exp. Child Psychol. 2001;80(2):95–111. doi: 10.1006/jecp.2001.2632. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Fletcher P.C., Chua P., Dolan R.J. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S., Kim A.S., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sprondel V., Kipp K.H., Mecklinger A. Developmental changes in item and source memory: evidence from an ERP recognition memory study with children, adolescents, and adults. Child Dev. 2011;82(6):1938–1953. doi: 10.1111/j.1467-8624.2011.01642.x. [DOI] [PubMed] [Google Scholar]
- Staresina B.P., Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J. Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield Neocortical connectivity during episodic memory formation. PLoS Biol. 2006;4(5):e128. doi: 10.1371/journal.pbio.0040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher R.W., Walker R.A., Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Uncapher M.R., Otten L.J., Rugg M.D. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52(3):542–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg K.L., Rugg M.D. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45(10):2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg K.L., Rugg M.D. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46(7):1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1986. Wechsler Intelligence Scale for Children – Revised (WISC-R) [Google Scholar]
- Williams K.T. American Guidance Service; MN: 1997. Expressive Vocabulary Test Circle Pines. [Google Scholar]
- Wolfe C.D., Bell M.A. Working memory and inhibitory control in early childhood: contributions from electrophysiology, temperament, and language. Dev. Psychobiol. 2004;44(1):68–83. doi: 10.1002/dev.10152. [DOI] [PubMed] [Google Scholar]
- Yoder P.J., Blackford J.U., Waller N.G., Kim G. Enhancing power while controlling family-wise error: an illustration of the issues using electrocortical studies. J. Clin. Exp. Neuropsychol. 2004;26(3):320–331. doi: 10.1080/13803390490510040. [DOI] [PubMed] [Google Scholar]