Abstract
Background
Aortic valve calcification (AVC) without stenosis is common in the elderly, is associated with cardiovascular morbidity and mortality, and may progress to aortic valve stenosis. Arterial stiffness and pulse wave reflection are important components of proximal aortic hemodynamics, but their relationship with AVC is not established.
Methods
To investigate the relationship of arterial wave reflection and stiffness with AVC, pulse wave analysis and AVC evaluation by echocardiography were performed in 867 participants from the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Participants were divided into 4 categories based on the severity and extent of AVC: 1) none or mild focal AVC; 2) mild diffuse AVC; 3) moderate-severe focal AVC; and 4) moderate-severe diffuse AVC. Central blood pressures and pulse pressure, total arterial compliance, augmentation index, and time to wave reflection were assessed using applanation tonometry.
Results
Indicators of arterial stiffness and wave reflection were significantly associated with AVC severity, except for central systolic and diastolic pressures and time to reflection. After adjustment for pertinent covariates (age, sex, race/ethnicity, and eGFR), only augmentation pressure (P = .02) and augmentation index (P = .002) were associated with the severity of AVC. Multivariable logistic regression analysis revealed that augmentation pressure (odds ratio per mmHg = 1.14; 95% confidence interval, 1.02–1.27; P = .02) and augmentation index (odds ratio per percentage point = 1.07; 95% confidence interval, 1.01–1.13; P = .02) were associated with an increase risk of moderate-severe diffuse AVC, even when central blood pressure value was included in the same model.
Conclusions
Arterial wave reflection is associated with AVC severity, independent of blood pressure values. Increased contribution of wave reflection to central blood pressure could be involved in the process leading to AVC.
Keywords: aortic valve calcification, wave reflection, arterial stiffness, blood pressure
Aortic valve calcification (AVC) without concomitant outflow obstruction has been known as aortic valve sclerosis, which is a common abnormality in the elderly.1, 2 AVC is not simply a degenerative process, but is actively modulated and shares many clinical risk factors with atherosclerosis, including age,2, 3 male sex,2 cigarette smoking,2 hypertension,2–5 hypercholesterolemia,4 and diabetes mellitus.4 Aortic valve sclerosis has been reported to be associated with cardiovascular morbidity and mortality, even in the absence of hemodynamically significant obstruction to outflow (stenosis) and independent of traditional cardiovascular risk factors.6 In addition, aortic valve sclerosis may gradually progress to hemodynamically significant aortic valve stenosis and result in poor clinical outcomes.7, 8 Abnormal hemodynamic forces, such as hypertension, high tensile stress, and low shear stress on the aortic leaflets, may result in endothelial injury and disruption, similar to those seen in early atherosclerotic lesions.9, 10
Pulse wave analysis allows the estimation of central blood pressure (BP) non-invasively, as well as of indices of arterial stiffness and wave reflection.11, 12 Increased arterial stiffness and wave reflection have been shown to be strong independent predictors of cardiovascular morbidity and mortality in different patient populations, such as patients with end-stage renal disease,13, 14 hypertension,15, 16 and established coronary artery disease.17 Arterial stiffness and wave reflection are important components of proximal aortic hemodynamics to which the aortic valve is directly exposed. However, the association between AVC and parameters derived from pulse wave analysis has not been investigated in a properly-sized clinical study. The aim of the present study was to investigate in a predominantly elderly cohort the relationship between AVC and the variables derived from pulse wave analysis, including central hemodynamics, indicators of arterial stiffness, and wave reflection.
Methods
Study Population
The study cohort was derived from the Cardiac Abnormalities and Brain Lesion (CABL) study, whose participants were drawn from the Northern Manhattan Study (NOMAS). NOMAS is a population-based study designed to evaluate the incidence, risk factors and clinical outcome of stroke in the population of northern Manhattan. Study design and methodologies of NOMAS have been previously published in detail.18 Briefly, subjects were eligible if they 1) had never been diagnosed with a stroke, 2) were ≥40 years of age, and 3) resided for at least 3 months in a household with a telephone in Northern Manhattan. Beginning September 2005, NOMAS subjects over age 50 years who voluntarily agreed to undergo a brain magnetic resonance imaging study and more extensive cardiovascular assessments were included in the CABL study, which is designed to investigate the relationship between subclinical cardiovascular disease and subclinical brain disease. Participants in CABL who had a complete dataset of pulse wave analysis and evaluation of AVC constitute the cohort of the present study.
The study was approved by the institutional review boards of Columbia University Medical Center and the University of Miami. Written informed consent was obtained from all of the study participants.
Risk Factor Assessment
Hypertension was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg at the time of the visit, or a patient’s self-reported history of hypertension or antihypertensive medication use. Diabetes mellitus was defined by the patient’s self-report, current use of insulin or hypoglycemic agents, or a fasting blood glucose ≥126 mg/dL on ≥2 occasions in each participant. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, a patient’s self-report of hypercholesterolemia or the use of lipid-lowering medication. Smoking status was defined as cigarette smoking at any time in the past or present. Body mass index was calculated as: weight / (height)2 and expressed in kg/m2. The estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease equation.19
Assessment of Aortic Valve Calcification
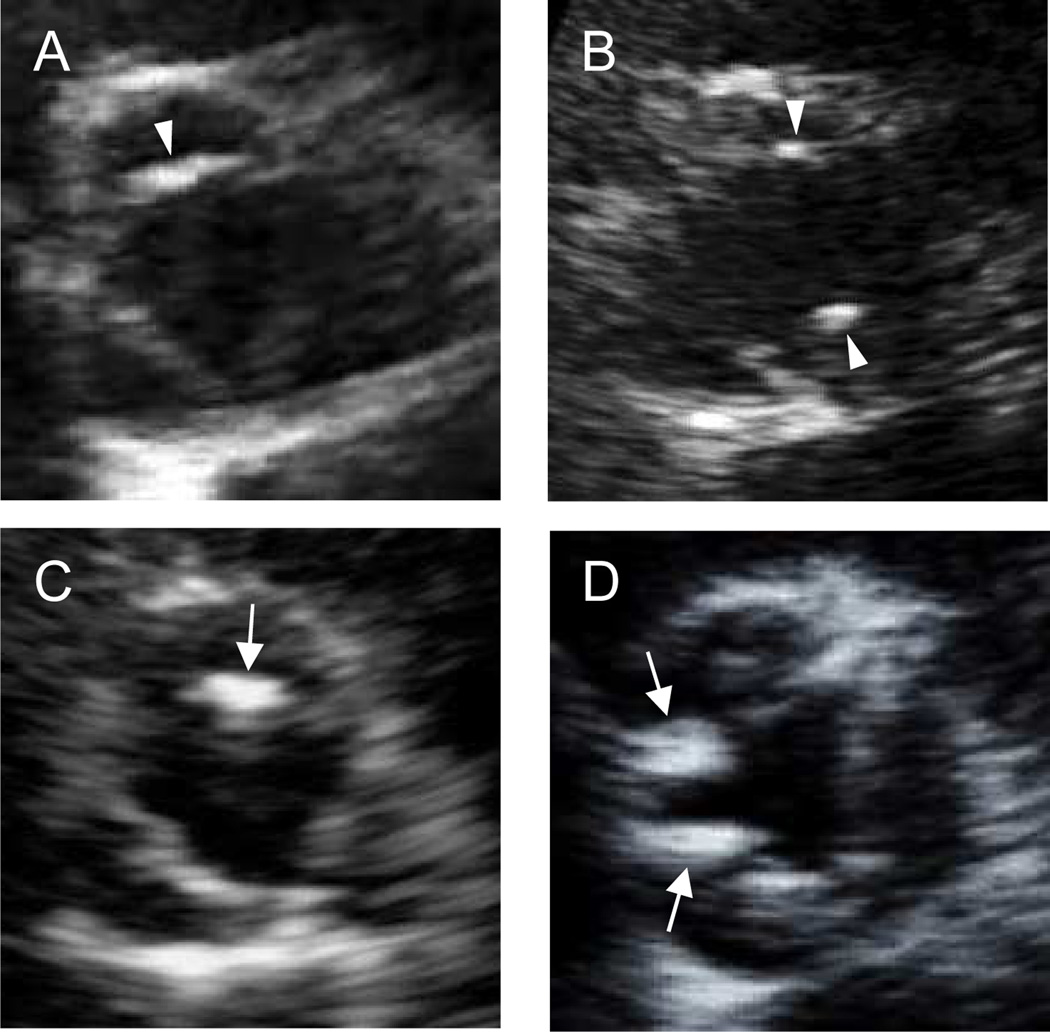
Transthoracic echocardiography was performed by trained registered sonographers following a standardized protocol with a commercially available system (iE33; Philips Medical Systems, Andover, MA, USA) equipped with a 2.5-MHz to 3.5-MHz transducer. Two-dimensional images of the aortic valve were obtained and stored on digital media for subsequent off-line analysis. AVC was defined as bright dense echoes >1 mm in size on ≥1 cusp.20 Each valve leaflet was graded on a scale of 0 (normal) to 3 (severe calcification).2, 5 We excluded 51 subjects with suboptimal images for the assessment and those with bicuspid aortic valve or aortic valve stenosis (defined as peak flow velocity ≥2.0 m/s) and 10 subjects for whom the aortic valve peak flow velocity was not available. Severity of AVC was defined based on the maximum score of calcification among the three leaflets: a maximum score of 0 was considered no AVC; a score of 1, mild AVC; a score of ≥2, moderate-severe AVC. In mild AVC, the presence of calcified deposits of score 1 on 1 cusp only was considered as focal, in ≥2 cusps was defined as diffuse. Likewise, in moderate-severe AVC, the presence of calcified deposits of score of ≥2 on 1 cusp only was considered as focal, in ≥2 cusps was defined as diffuse (Figure 1). Participants were then divided into the following 4 categories of increasing AVC severity: 1) none or mild focal AVC; 2) mild diffuse AVC; 3) moderate-severe focal AVC; and 4) moderate-severe diffuse AVC. All images were interpreted by a single experienced echocardiographer (S.I.) blinded to subject characteristics and risk factors.
Figure 1.
Representative images for each category of aortic valve calcification (AVC) from a parasternal short-axis view. Arrowheads indicate mild calcification, and arrows indicate moderate-severe calcification. (A) mild focal AVC, (B) mild diffuse AVC, (C) moderate-severe focal AVC, and (D) moderate-severe diffuse AVC.
Pulse Wave Analysis
In the same session, after the performance of the echocardiogram, pulse wave analysis of the radial artery by applanation tonometry was performed using a commercially available device (SphygmoCor, Pulse Wave Analysis System, AtCor Medical, Sydney, Australia). A detailed description of the technique and reproducibility data have been previously published.21 Estimated central systolic, diastolic, and pulse pressure (PP) were calculated from the radial pulse wave by a validated generalized transfer function.11, 12 The ratio of central PP over left ventricular stroke volume index (central PP/SVi) was used as an indicator of arterial stiffness.22 Total arterial compliance was calculated using the area method illustrated by Liu et al.23 Aortic augmentation pressure from the reflected wave was measured as the difference between the peak systolic central pressure and the pressure at the onset of the reflected wave from the peripheral reflecting sites. The aortic augmentation index was calculated as the ratio between the augmentation pressure and the central PP and expressed as a percentage. Time to the beginning of the reflected wave (time to reflection) was also measured. Only studies with an acceptable quality score (operator index >80%) were included in the analysis.
Statistical Analysis
Data are presented as mean ± standard deviation or mean (standard error) for continuous variables and as proportions for categorical variables. Differences among groups were assessed by one-way analysis of variance for continuous variables and by Pearson chi-square test for proportions. Analysis of covariance was performed separately for each variable derived from pulse wave analysis to assess differences between the 4 categories of AVC after adjustment for covariates, which were selected based on their univariate association with AVC severity (the threshold for inclusion in the multivariable models was set at a p-value of <.05). The Tukey-Kramer procedure was used for multiple comparisons in one-way analysis of variance and analysis of covariance. In addition, multivariable logistic regression analyses using moderate-diffuse calcification as outcome were carried out. Inter- and intra- observer agreement for the AVC categorization was assessed using kappa statistics. A p-value of <.05 was considered statistically significant. Statistical analyses were performed using SAS software version 9.3 (SAS Inc., Cary, NC, USA).
Results
Study Cohort
Of 1004 participants enrolled in the CABL study, 867 participants (mean age of 71 ± 9 years) who had both AVC assessment and pulse wave analysis available constituted the study sample of the present study, including 339 males (39.1%), 123 non-Hispanic white (14.1%), 141 non-Hispanic black (16.3%), 583 Hispanic (67.2%) and 20 of other ethnicities (2.3%). Of the 867 subjects, 685 had hypertension (79.0%), 248 had diabetes mellitus (28.6%), 582 had hypercholesterolemia (67.2%) and 452 were ever-smokers (52.1%).
Assessment of Aortic Valve Calcification
The demographics and clinical characteristics in the 4 categories of AVC are listed in Table 1. Of the 867 subjects, 155 subjects (17.9%) were classified as none or mild focal AVC, 592 (68.3%) as mild diffuse AVC, 89 (10.3%) as moderate-severe focal AVC, 31 (3.6%) as moderate-severe diffuse AVC. Both of the AVC categories of moderate-severe focal and moderate-severe diffuse AVC showed higher age and higher frequency of male sex compared to the other 2 categories. Race/ethnicity and eGFR were also significantly different among the 4 categories. The moderate-severe diffuse AVC category showed significantly higher aortic valve peak flow velocity compared with any of the other 3 AVC categories.
Table 1.
Demographics and clinical characteristics
| Aortic valve calcification | |||||
|---|---|---|---|---|---|
| None / Mild focal | Mild diffuse | Moderate-severe focal | Moderate-severe diffuse | P value | |
| (n = 155) | (n = 592) | (n = 89) | (n = 31) | ||
| Age, years | 69.3 ± 9.4 | 70.5 ± 8.8 | 77.8 ± 8.9*† | 77.8 ± 7.6*† | <.001 |
| Male | 50 (32.3) | 227 (38.3) | 46 (51.7) | 16 (51.6) | .010 |
| Race/ethnicity | .016 | ||||
| White | 14 (9.0) | 81 (13.7) | 23 (25.8) | 5 (16.1) | |
| Black | 29 (18.7) | 88 (14.9) | 19 (21.4) | 5 (16.1) | |
| Hispanic | 109 (70.4) | 409 (69.0) | 44 (49.4) | 21 (67.8) | |
| Others | 3 (1.9) | 14 (2.4) | 3 (3.4) | 0 (0) | |
| Body mass index, kg/m2 | 28.5 ± 4.5 | 28.0 ± 4.6 | 27.6 ± 5.1 | 27.2 ± 4.9 | .339 |
| Brachial SBP, mmHg | 133.6 ± 22.8 | 129.6 ± 18.5 | 131.0 ± 20.6 | 136.0 ± 20.3 | .059 |
| Brachial DBP, mmHg | 71.6 ± 10.2 | 71.2 ± 10.0 | 69.9 ± 10.2 | 67.0 ± 9.5 | .083 |
| Heart rate, beats/min | 69.3 ± 11.4 | 67.2 ± 10.8 | 66.8 ± 10.0 | 65.5 ± 10.1 | .107 |
| Hypertension | 125 (80.7) | 458 (77.4) | 73 (82.0) | 29 (93.6) | .130 |
| Diabetes mellitus | 46 (29.7) | 165 (27.9) | 26 (29.2) | 11 (35.5) | .807 |
| Hypercholesterolemia | 101 (65.6) | 393 (66.4) | 65 (73.0) | 23 (74.2) | .489 |
| Cigarette smoking (ever) | 74 (47.7) | 314 (53.0) | 44 (49.4) | 20 (64.5) | .314 |
| eGFR, ml/min/1.73m2 | 75.6 ± 17.9 | 73.7 ± 18.7 | 68.4 ± 20.1 *† | 68.3 ± 23.3 | .018 |
| Anti-hypertensive medication use | 116 (74.8) | 409 (69.3) | 71 (79.8) | 26 (83.9) | .057 |
| β-blockers | 42 (27.1) | 163 (27.5) | 22 (24.7) | 8 (25.8) | .953 |
| ACE-inhibitors | 43 (27.7) | 178 (30.1) | 28 (31.5) | 12 (38.7) | .664 |
| Calcium channel blockers | 39 (25.2) | 169 (28.6) | 33 (37.1) | 14 (45.2) | .051 |
| Diuretics | 39 (25.2) | 96 (16.2) | 28 (31.5) | 9 (29.0) | <.001 |
| LV ejection fraction, % | 64.4 ± 7.2 | 63.3 ± 7.2 | 62.5 ± 5.9 | 61.2 ± 8.0 | .056 |
| Aortic jet peak velocity, m/s | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.6 ± 0.2 *†‡ | <.001 |
ACE, Angiotensin converting enzyme; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LV, left ventricular; SBP, systolic blood pressure.
Data are expressed as mean ± SD or as number (percentage).
P < .05 vs. none/mild focal AVC
P < .05 vs. mild diffuse AVC
P < .05 vs. moderate-severe focal AVC.
For the categorization of AVC, the kappa for inter-observer agreement was 0.73 (95% CI, 0.49–0.97) with an 80% agreement (95% CI, 62.4–97.5) and the kappa for intra-observer agreement was 0.87 (95% CI, 0.69–1.0) with a 90% agreement (95% CI, 76.9–100).
Central BP, Arterial Stiffness and Wave Reflection Parameters Associated with AVC
Parameters derived from pulse wave analysis are shown in Table 2. Neither central systolic BP nor central diastolic BP was significantly different among the 4 categories, whereas central PP was higher in more advanced categories of AVC. The ratio of PP to stroke volume index (central PP/SVi), an indicator of arterial stiffness, was higher and total arterial compliance was lower with more advanced AVC. Greater amplitude of wave reflection was observed with more advanced AVC, whereas time to reflection was not significantly different among the 4 categories. After adjusting for pertinent covariates (age, sex, race/ethnicity, and eGFR), significant differences remained in the variables of wave reflection, including augmentation pressure and augmentation index (Table 3). The AVC category of moderate-severe diffuse showed greater amplitude of wave reflection compared with any of the other 3 AVC categories. Central PP, central PP/SVi, and total arterial compliance were no longer significantly associated with the severity of AVC. Even when brachial systolic and diastolic BPs and antihypertensive medication use were added to the model as a covariate, augmentation pressure and augmentation index remained associated with the severity of AVC (P < .01 for both).
Table 2.
Central blood pressure, arterial stiffness and wave reflection parameters in relation to the severity of aortic valve calcification
| Aortic valve calcification | |||||
|---|---|---|---|---|---|
| None / Mild focal | Mild diffuse | Moderate-severe focal | Moderate-severe diffuse | P value | |
| cSBP, mmHg | 121.7 ± 21.5 | 119.3 ± 18.0 | 120.6 ± 20.6 | 125.1 ± 19.0 | .225 |
| cDBP, mmHg | 72.7 ± 10.5 | 72.2 ± 10.1 | 70.8 ± 10.3 | 67.9 ± 9.6 | .069 |
| cPP, mmHg | 49.0 ± 18.4 | 47.1 ± 14.6 | 49.8 ± 16.1 | 57.2 ± 17.3 *†‡ | .003 |
| cPP/SVi, mmHg·m2 /ml | 1.48 ± 0.75 | 1.41 ± 0.53 | 1.59 ± 0.56 | 1.71 ± 0.64† | .003 |
| Total arterial compliance, ml/mmHg | 1.19 ± 0.57 | 1.19 ± 0.55 | 1.06 ± 0.51† | 0.91 ± 0.33 *† | .014 |
| Augmentation pressure, mmHg | 14.2 ± 8.7 | 14.4 ± 7.6 | 15.4 ± 8.2 | 19.9 ± 9.1 *†‡ | .002 |
| Augmentation index, % | 27.4 ± 9.6 | 29.3 ± 9.5* | 29.4 ± 9.5 | 33.6 ± 8.9 *†‡ | .008 |
| Augmentation index at HR 75, % | 24.7 ± 8.0 | 25.5±8.3 | 25.4±9.6 | 29.1±8.1 | .073 |
| Time to reflection, msec | 135.5 ± 10.7 | 135.9 ± 9.9 | 136.6 ± 10.5 | 131.6 ± 10.2 | .112 |
cDBP, Central diastolic blood pressure; cPP, central pulse pressure; cSBP, central systolic blood pressure; HR, heart rate; SVi, stroke volume index.
Data are expressed as mean ± SD.
P < .05 vs. none/mild focal AVC;
P < .05 vs. mild diffuse AVC;
P <05 vs. moderate-severe focal AVC.
Table 3.
Relationship of arterial stiffness and wave reflection parameters with the severity of aortic valve calcification adjusted for demographics and clinical characteristics
| Aortic valve calcification | |||||
|---|---|---|---|---|---|
| None / Mild focal | Mild diffuse | Moderate-severe focal | Moderate-severe diffuse | P value | |
| cSBP, mmHg | 118.8 (1.9) | 116.4 (1.3) | 117.4 (2.3) | 120.5 (3.7) | .39 |
| cDBP, mmHg | 71.3 (1.0) | 71.1 (0.7) | 72.2 (1.2) | 69.8 (1.9) | .68 |
| cPP, mmHg | 47.5 (1.4) | 45.3 (1.0) | 45.2 (1.8) | 50.7 (2.8) | .096 |
| cPP/SVi, mmHg·m2 /ml | 1.45 (0.05) | 1.37 (0.04) | 1.42 (0.07) | 1.52 (0.11) | .18 |
| Total arterial compliance, ml/mmHg | 1.24 (0.05) | 1.24 (0.03) | 1.21 (0.06) | 1.10 (0.09) | .48 |
| Augmentation pressure, mmHg | 13.7 (0.7) | 13.9 (0.5) | 13.9 (0.9) | 18.1 (1.4) *†‡ | .022 |
| Augmentation index, % | 27.2 (0.9) | 29.2 (0.6)* | 29.3 (1.1) | 33.8 (1.7) *†‡ | .0019 |
| Augmentation index at HR 75, % | 23.7 (0.7) | 24.9 (0.5) | 25.0 (0.9) | 28.7 (1.4) *†‡ | .0062 |
| Time to reflection, msec | 136.2 (0.9) | 136.2 (0.6) | 137.2 (1.1) | 132.8 (1.7) | .15 |
cDBP, Central diastolic blood pressure; cPP, central pulse pressure; cSBP, central systolic blood pressure; HR, heart rate; SVi, stroke volume index.
Data are expressed as mean (SE) adjusted for age, sex, race/ethnicity, and eGFR.
P < .05 vs. none/mild focal AVC;
P < .05 vs. mild diffuse AVC;
P < .05 vs. moderate-severe focal AVC.
Relationship of Wave Reflection and Central BP with Moderate-severe Diffuse AVC
Among the 4 categories of AVC, moderate-severe diffuse AVC was the one most strongly associated with wave reflection. We therefore performed a set of multivariable analyses with moderate-severe diffuse AVC as the dependent variable. Augmentation index remained significantly associated with moderate-severe diffuse AVC (Table 4, model 1), even when an indicator of central hemodynamics such as cPP was included in the model (Table 4, model 2). When augmentation pressure was substituted for augmentation index in the model, it also remained significantly associated with moderate-severe diffuse AVC (odds ratio per mmHg =1.14; 95% CI, 1.02–1.27; P = .02 for the fully adjusted model).
Table 4.
Multivariable logistic regression analysis with moderate-severe diffuse AVC as outcome
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Model 1 | ||
| Augmentation index (per each 1%) | 1.08 (1.02, 1.14) | .005 |
| Model 2 | ||
| Augmentation index (per each 1%) | 1.07 (1.01, 1.13) | .018 |
| cPP (per each mmHg) | 1.01 (0.98, 1.03) | .607 |
CI, Confidence interval; cPP, central pulse pressure.
Each model was adjusted for age, sex, race/ethnicity and eGFR.
Discussion
In this study, we found that several variables derived from pulse wave analysis were significantly associated with the severity of AVC in a community-based unselected elderly cohort. Variables of wave reflection remained associated with the severity of AVC after adjustment for pertinent covariates, while none of central hemodynamics and arterial stiffness parameters remained associated with AVC. Furthermore, the association of augmentation pressure and augmentation index with AVC was independent of not only brachial BP but also of central BP, which could have conceivably been a mediator in the association between wave reflection and AVC. Greater contribution of wave reflection to central PP was associated with an increase in risk of moderate-severe diffuse AVC.
A variety of mechanisms have been suggested for AVC, including abnormal hemodynamic forces, atherosclerosis, inflammation, calcium and lipid dysregulation.9, 10, 24 An association between hypertension and calcific aortic valve disease has been consistently reported.2–4, 25 We have also previously reported that diastolic ambulatory BP was independently associated with advanced AVC.5 However, the relationship between hypertension and AVC has been reported only on the basis of brachial BP. Brachial BP, especially systolic BP, is modified by pressure amplification and increases progressively on the way from the aortic valve to the peripheral arteries; therefore, brachial BP cannot be considered identical to the BP measured in the central arteries.26 Central BP more accurately reflects the hemodynamics to which the heart, and especially the aortic valve, are directly exposed. In our cohort, we found that greater central PP as well as increased arterial stiffness and wave reflections were associated with more advanced AVC. However, neither central PP nor arterial stiffness parameters were found to be associated with AVC in a multivariate model. The differences in central PP and arterial stiffness among the 4 categories might be attributed to the differences in background characteristics, such as older age in the more advanced AVC categories, which has been previously shown as the major clinical determinants of increased arterial stiffness.27
A possible mechanism involved in the relationship between arterial wave reflection and AVC might be endothelial dysfunction. The contribution of reflected waves depends on their amplitude and timing, which represent the arterial stiffness of both elastic arteries and muscular arteries. The stiffness of elastic arteries increases progressively with age and increasing blood pressure, whereas the stiffness of muscular arteries is little affected by them, and is instead actively modulated by the smooth muscle tone, in turn largely depending on the endothelial function.26–28 There have been several reports regarding the relationship between endothelial dysfunction, NO signaling and AVC.29, 30 Furthermore, an impairment of platelet NO signaling and elevated augmentation index have been shown to be associated with progression of aortic valve sclerosis.31 Therefore, endothelial dysfunction might be the initial process involved in the development of AVC, which might lead to the increased relative contribution of wave reflection to central PP.
We also found that wave reflection parameters were significantly associated with the moderate-severe diffuse AVC even after adjustment for central PP, which suggests that, for any given pulsatile pressure acting on the aortic valve, a greater contribution of wave reflection might be a promoting factor for AVC. Aortic cusps are exposed to tensile stress (cyclic stretch) to oppose the backward pressure. Elevated cyclic stretch has been reported to cause matrix remodeling in the aortic valve leaflets.10 The central arterial pressure wave is comprised of a forward traveling wave generated by left ventricular contraction and of a reflected wave from the periphery, therefore the same BP and PP can result from different patterns of forward and backward pressure waves. Early return of reflected waves to the heart during systole increases the backward pressure load on the aortic valve, which may interfere with the opening motion of the aortic valve and cause elevated cyclic stretch. This mechanism might promote thickening and calcification of the aortic valve.
Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker (ACE-I/ARB) therapy has been found to be inversely related with augmentation index.32 A negative relationship between therapy with ACE-I/ARB and progression of aortic valve sclerosis has in fact been reported;31 however, whether therapeutic interventions to decrease arterial wave reflection might help delay the progression of AVC and possibly the onset of hemodynamically significant aortic valve stenosis is a hypothesis that deserves further investigation.
Limitations
Our study has limitations. First, the cross-sectional design does not allow us to detect causal relationships. Second, the parameters derived from arterial waveform analysis can provide only indirect indices of arterial stiffness. The measurement of pulse wave velocity, an important method for assessing arterial stiffness, was not performed in our study, as it was one of the evaluations eliminated from the participants’ visit because of time constraints. Third, the present classification of AVC is based on an echocardiographic semi-quantitative scoring, which is less accurate than computed tomography for this purpose. Finally, despite being representative of the multi-ethnic community living in northern Manhattan, our cohort is predominantly elderly and has high frequency of cardiovascular risk factors. Therefore, the results of the present study might not be directly applicable to other populations with different demographics and risk factor distribution.
Conclusions
In our community-based cohort, arterial wave reflection was associated with the severity of AVC independent of confounding cardiovascular risk factors and central BP. Increased contribution of wave reflection to central BP may be associated with the process leading to AVC. The possibility that therapeutic interventions to decrease arterial wave reflection might prevent AVC or its progression toward aortic valve stenosis requires further investigation.
Highlights.
The relation of arterial wave reflection and aortic valve calcification was studied
Wave reflection was associated with moderate-severe aortic valve calcification
Central blood pressures were not associated with aortic valve calcification
Wave reflection may be a therapeutic target to delay aortic valve calcification
Acknowledgements
The authors wish to thank Janet De Rosa, MPH, for the coordination of the study activities and Rui Liu, MD; Rafi Cabral, MD; and Palma Gervasi-Franklin for their help in the collection and management of the data.
Sources of Funding
This study has been funded by the National Institute of Neurological Disorders and Stroke [grant numbers R01 NS36286 to MRDT and R37 NS29993 to RLS/MSVE].
Abbreviations
- AVC
Aortic valve calcification
- BP
Blood pressure
- eGFR
Estimated glomerular filtration rate
- PP
Pulse pressure
- SVi
Stroke volume index
- NO
Nitric oxide
- ACE-I
Angiotensin-converting enzyme inhibitor
- ARB
Angiotensin II receptor blocker
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 3.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 4.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 5.Iwata S, Russo C, Jin Z, Schwartz JE, Homma S, Elkind MS, et al. Higher ambulatory blood pressure is associated with aortic valve calcification in the elderly: a population-based study. Hypertension. 2013;61:55–60. doi: 10.1161/HYPERTENSIONAHA.112.202697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 7.Cosmi JE, Kort S, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, et al. The risk of the development of aortic stenosis in patients with "benign" aortic valve thickening. Arch Intern Med. 2002;162:2345–2347. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 8.Faggiano P, Antonini-Canterin F, Erlicher A, Romeo C, Cervesato E, Pavan D, et al. Progression of aortic valve sclerosis to aortic stenosis. Am J Cardiol. 2003;91:99–101. doi: 10.1016/s0002-9149(02)03011-4. [DOI] [PubMed] [Google Scholar]
- 9.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 10.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296:H756–H764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 12.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 13.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 14.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 16.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the NOrthern MAnhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 20.Wang AY, Woo J, Wang M, Sea MM, Ip R, Li PK, et al. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. 2001;12:1927–1936. doi: 10.1681/ASN.V1291927. [DOI] [PubMed] [Google Scholar]
- 21.Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, et al. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, et al. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–787. doi: 10.1097/00004872-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986;251:H588–H600. doi: 10.1152/ajpheart.1986.251.3.H588. [DOI] [PubMed] [Google Scholar]
- 24.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: A review agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working. Group Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwata S, Walker MD, Di Tullio MR, Hyodo E, Jin Z, Liu R, et al. Aortic valve calcification in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:132–137. doi: 10.1210/jc.2011-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 27.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 28.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini LA, Picano E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J Am Coll Cardiol. 2003;41:136–141. doi: 10.1016/s0735-1097(02)02622-0. [DOI] [PubMed] [Google Scholar]
- 30.Ngo DT, Sverdlov AL, Willoughby SR, Nightingale AK, Chirkov YY, McNeil JJ, et al. Determinants of occurrence of aortic sclerosis in an aging population. J Am Coll Cardiol Img. 2009;2:919–927. doi: 10.1016/j.jcmg.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Sverdlov AL, Ngo DT, Chan WP, Chirkov YY, Gersh BJ, McNeil JJ, et al. Determinants of aortic sclerosis progression: implications regarding impairment of nitric oxide signalling and potential therapeutics. Eur Heart J. 2012;33:2419–2425. doi: 10.1093/eurheartj/ehs171. [DOI] [PubMed] [Google Scholar]
- 32.Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Correlates of arterial stiffness in an ageing population: role of asymmetric dimethylarginine. Pharmacol Res. 2009;60:503–507. doi: 10.1016/j.phrs.2009.06.006. [DOI] [PubMed] [Google Scholar]