Abstract
The tumor microenvironment plays a critical role in regulating breast tumor progression. Signaling between preadipocytes and breast cancer cells has been found to promote breast tumor formation and metastasis. Exosomes secreted from preadipocytes are important components of the cancer stem cell niche. Mouse preadipocytes (3T3L1) are treated with the natural antitumor compound shikonin (SK) and exosomes derived from mouse preadipocytes are co-cultured with MCF10DCIS cells. We examine how preadipocyte-derived exosomes can regulate early-stage breast cancer via regulating stem cell renewal, cell migration, and tumor formation. We identify a critical miR-140/SOX2/SOX9 axis that regulates differentiation, stemness, and migration in the tumor microenvironment. Next, we find that the natural antitumor compound SK can inhibit preadipocyte signaling inhibiting nearby ductal carcinoma in situ (DCIS) cells. Through co-culture experiments, we find that SK-treated preadipocytes secrete exosomes with high levels of miR-140, which can impact nearby DCIS cells through targeting SOX9 signaling. Finally, we find that preadipocyte-derived exosomes promote tumorigenesis in vivo, providing strong support for the importance of exosomal signaling in the tumor microenvironment. Our data also show that targeting the tumor microenvironment may assist in blocking tumor progression.
Keywords: DCIS, Preadipocyte, Microenvironment, Exosomes, MicroRNAs
Introduction
Ductal carcinoma in situ (DCIS) is the most common type of non-invasive early-stage breast cancer in women. DCIS is characterized by the abnormal growth of epithelial cells confined within the ductal area, maintenance of the basement membrane, and lack of stromal invasion [1]. Patients diagnosed with DCIS are treated with surgery, radiation, and hormonal therapy. However, they remain at risk to develop invasive ductal carcinoma (IDC) and 15 % of patients demonstrate recurrence and progression [2]. Thus, it is important to understand the mechanisms underlying the development of DCIS and to find novel therapeutics that may prevent DCIS formation.
The link between breast cancer and obesity has been well established and it is for the leading researchers to examine the potential involvement of adipose tissue in the tumor microenvironment. Adipose tissue consists of heterogeneous cells including adipocyte progenitors and adipocytes, which differentiate from mesenchymal stem cells (MSCs) in a process known as adipogenesis [3]. It has been suggested that adipose tissue provides a favorable microenvironment for tumor growth and metastasis through secretion of signaling molecules and acting as a source of energy for cancer cells [4].
Emerging evidence indicates that the tumor microenvironment plays a crucial role in cancer progression. The factors released to the tumor microenvironment from preadipocytes and adipocytes adjacent to the mammary epithelial cells might influence tumorigenesis. Breast cancer cells and adipocytes have been shown to interact and may influence each other’s behavior. Breast cancer cells affect adipocyte differentiation and likewise the adipocyte microenvironment is able to alter the gene expression in breast cancer cells [5]. Adipocytes surrounding breast tumors have been shown to adopt altered phenotypes and promote tumor metastasis [6]. Previous studies from our laboratory and others showed that co-culturing breast cancer cells with adipocytes promotes phenotypic changes and enhances cancer cell migration and tumor formation [7, 8]. Moreover, we found that components of tumor microenvironment including MSCs and adipocytes can regulate breast cancer stem cells [7]. Thus, elucidating the mechanisms underlying the interactions of cancer stem cells with their microenvironment might provide novel targets for inhibiting tumor development and eliminating cancer stem cells.
Exosomal secretion is one of the mechanisms through which tumor cells can communicate with and reprogram their microenvironment [9]. Exosomes are small extracellular vesicles ranging from 30 to 100 nm formed by the endocytic component of the cells, and can be secreted by various types of cells including cancer cells. They contain mRNA, miRNA, proteins, and lipids [10]. Exosomes are involved in intra- and intercellular communications and have the ability to act as drug carriers [11, 12]. It has been suggested that exosomes are able to govern the function of remote cells by secreting their contents into cells distant from their site of origin [13]. Depending on the cells they are secreted from or the status of the cellular microenvironment, exosomes can either promote tumor growth or exert antitumor effects [14]. Recent research showed that exosomes play a major role in the circulation of miRNAs [13]. Exosomes from tumor cells are suggested to be involved in genetic transfer of specific miRNAs capable of signaling within nearby cells. It has been shown that miRNAs secreted from exosomes enhance the invasive potential of several breast cancer cell lines [15]. The capability of exosomes to exert antitumor effects was shown in a recent study where exosomes derived from MSCs had protein and RNA profiles different than the MSCs themselves [16]. Mouse breast cancer cells (4T1) could internalize these exosomes and miR-16 released from exosomes downregulated VEGF expression in cancer cells, suppressing angiogenesis [16]. Previous studies from our laboratory linked cell–cell signaling in DCIS stem-like cells to the exosomal trafficking of miRNAs and identified that epigenetic therapy can alter exosomal miRNA content [17].
Shikonin (SK), the major bioactive component of the herbal plant shikon, is a potential chemopreventive agent for the treatment of many cancers including breast cancer [18]. We have previously shown that NRF2 antioxidant target gene NQO1, which protects against the toxicity and carcinogenicity of estrogen metabolites, is upregulated in MCF7 breast cancer cells following SK treatment, thus suggesting a strategy to prevent development of estrogendependent breast cancer. Additionally, we have shown that SK prevents tumorigenesis in vivo [19]. Moreover, several studies including research from our lab have shown that SK can act as an antitumor agent by targeting multiple signaling pathways rendering cancer cells less invasive and more sensitive to chemotherapy [19, 20]. Thus, it is crucial to elucidate the mechanisms through which SK may exert its antitumor effects.
In this study, using cells representing early-stage basallike breast cancer, we investigated the role of exosomes secreted from preadipocytes in regulating cancer cell behavior and tumor formation. We identified the miR-140/ SOX9 pathway as a key component of preadipocytederived exosomal signaling. Furthermore, we demonstrated that preadipocyte-secreted exosomes enhance tumorigenesis in vivo. We found that the natural compound SK dramatically alters preadipocyte signaling, which could impact nearby cancer cells. This study provides a molecular basis through which preadipocyte-derived exosomes exert their oncogenic effects and finds that SK can target tumor microenvironment signaling, a new mechanism of SK antitumor activity.
Materials and methods
Cell culture
MCF10DCIS (DCIS) cells (Asterand, Detroit, MI) were cultured in DMEM/F12 supplemented with 5 % heat-inactivated horse serum (Invitrogen; Carlsbad, CA), 4 µg/ml insulin (Gibco, Grand Island, NY), 100 ng/ml cholera toxin, 0.5 µg/ml hydrocortisone (Sigma; St Louis, MO), and 20 ng/ml EGF (Life Technologies; Grand Island, NY). Mouse preadipocytes (3T3L1, MBA-1) cells were grown in DMEM supplemented with 5 % FBS (HyClone, Rockford, IL) and 1 % l-glutamine (Invitrogen; Carlsbad, CA). MBA- 1 cells were obtained from the laboratory of Dr. Da-wei Gong (Department of Medicine at University of Maryland School of Medicine, Baltimore, MD). Human breast preadipocytes (ZenBio, Research Triangle Park, NC) were grown in preadipocyte media (ZenBio, Research Triangle Park, NC). Cells were incubated in 5 % CO2 at 37°C.
Transwell migration and co-culture assays
Transwell migration assays were performed using transwell migration chambers with 8-µm pore size (Costar; Cambridge, MA). MCF10DCIS cells (0.5 × 105 cells/ml) were seeded in the upper chamber (1.5 ml). To facilitate migration, the lower chamber contained 1.5 ml DMEM with 10 % FBS. The cells were allowed to migrate toward the 10 % FBS gradient overnight. The migrated cells were stained with 1 % crystal violet in methanol/PBS and counted using light microscopy. Five random fields were selected and counted per experiment. For co-culture assays, MCF10DCIS cells (0.5 × 105 cells) plus 100 µl exosomes from 3T3L1 cells were seeded in the top chamber with inserts of 0.4 µn pore size and the lower chamber contains DMEM with 10 %FBS.
Mammosphere assays
MCF10DCIS single cells were obtained using 40-µm cell strainers (Fisher Scientific; Pittsburgh, PA) and counted. For mammosphere formation, 10,000 cells/ml were seeded in six-well plates coated with 2 % polyhema (Sigma; St Louis, MO) in DMEM/F12 containing 2 % B27, 20 ng/ml EGF, 4 µg/ml insulin, and 0.4 % BSA. After 7 days of culture, spheres larger than 100 µm were quantified by light microscopy.
Quantitative real-time PCR (qRT-PCR)
qRT-PCR analysis of mRNA/miRNA expression was performed as described previously with normalization to either GAPDH or β-actin for mRNAs and to U6 small nuclear RNA for miRNAs [21].
Oil-Red-O and BODIPY staining
Differentiated preadipocytes were rinsed twice with PBS and fixed in 10 % formalin at room temperature for 30 min. The working solution of Oil-Red-O was prepared fresh before staining and after complete drying of the plate, cells were stained for 30 min at room temperature. Lipid droplets were also visualized by fluorescent BODIPY (493/503) staining as described previously [22]. After staining, cells were washed twice with distilled water and images were captured using a Nikon Eclipse fluorescent microscope.
Western blotting and immunofluorescent staining
Total cell lysates (50–100 µg) were separated by SDS-PAGE and blotted onto polyvinylidene difluoride membrane. The membrane was incubated with specific primary antibody overnight at 4 °C, followed by the horseradish peroxidase (HRP)-conjugated secondary antibody, and visualized by the ECL Western blotting detection system (Thermo Scientific; Rockford, IL). β-Actin (Sigma; St Louis, MO, USA) was used as loading control with a 1:5000 dilution. SOX9 (Millipore; Billerica, MA), SOX2 (Santa Cruz Biotechnology; Dallas, TX), and DLK1 (Santa Cruz Biotechnology; Dallas, TX) were used with 1:500, 1:250, and 1:100 dilutions, respectively.
Exosome purification, labeling, and antibody arrays
Exosomes were prepared and purified by the traditional ultracentrifugation method as described [23]. Protein content in the exosomes was measured using a Pierce Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). The average exosome yield was 80–85 µg from 100 ml (1–1.2 × 107 cells) of cell culture supernatant. For in vitro experiments, exosomes were labeled with a fluorescent linker PKH-26 (Cat#MINI26-1KT, Sigma, St. Louis, MO). The Exo-Check antibody array (System Biosciences, Mountain View, CA) was used to detect known exosome markers. Briefly, exosome protein lysates were prepared by adding 600 µl of exosome lysis buffer to 300 µg of exosome protein. The antibody membrane array was placed in distilled water at room temperature for 2 min. Exosome lysate/binding mixture was added to the antibody membrane and then incubated overnight on shaker at 4 °C. After washing with array wash buffer, detection buffer was added to the membrane and incubated at room temperature for 2 h. The membrane was washed twice with wash buffer, developed, and exposed for the final signal analysis.
Exosomes were also screened for growth factors and cytokines using Human Membrane Antibody arrays (Cat#ab169819, Abcam, Cambridge, MA.) according to the manufacturer’s instructions. Briefly, exosome proteins (170 µg) were incubated with array membranes overnight. The array membranes were then incubated with biotin-conjugated anti-adipokines and detected by HRP-conjugated streptavidin. Signals were quantified using imageJ software (http://rsbweb.nih.gov/ij/download.html).
Xenograft studies
In vivo studies were conducted using animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) of University of Maryland School of Medicine in compliance with federal guidelines. Five- to six-week-old immunodeficient Nu/Nu female mice were randomized into two groups of five. Cells were resuspended in matrigel/media suspension (1:1 ratio) and injected to the 5th mammary gland nipple. DCIS cells were injected with or without exosomes derived from preadipocytes. Tumor growth was monitored weekly using digital calipers. The formula ‘(length × width2)/2’ was used to assess the tumor size. Six weeks after injection the mice were sacrificed, tumors were harvested, and fixed in formalin.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism software and data were assessed by the 2-tailed Student t test. Results were considered significant when p < 0.05 (*) and data are presented as mean ± SEM.
Results
Shikonin treatment inhibits preadipocyte differentiation by targeting the SOX9/miR-140 signaling pathway
Tumor microenvironment plays a critical role in the development and progression of breast cancer. Furthermore, it has been reported that signaling between adipocytes and breast cancer cells can promote breast tumor aggressiveness and invasion [24]. A more complete understanding of the mechanisms underlying the role of the tumor microenvironment may prove critical to future therapeutic strategies. We have previously reported that the natural compound SK inhibits tumor growth of estrogen receptor α-positive breast cancer cells and co-treatment with SK increases the sensitivity of breast cancer cells to endocrine therapy [18]. We began our current study by investigating the potential for SK to target the tumor microenvironment and impact cell–cell signaling between preadipocytes and breast cancer cells.
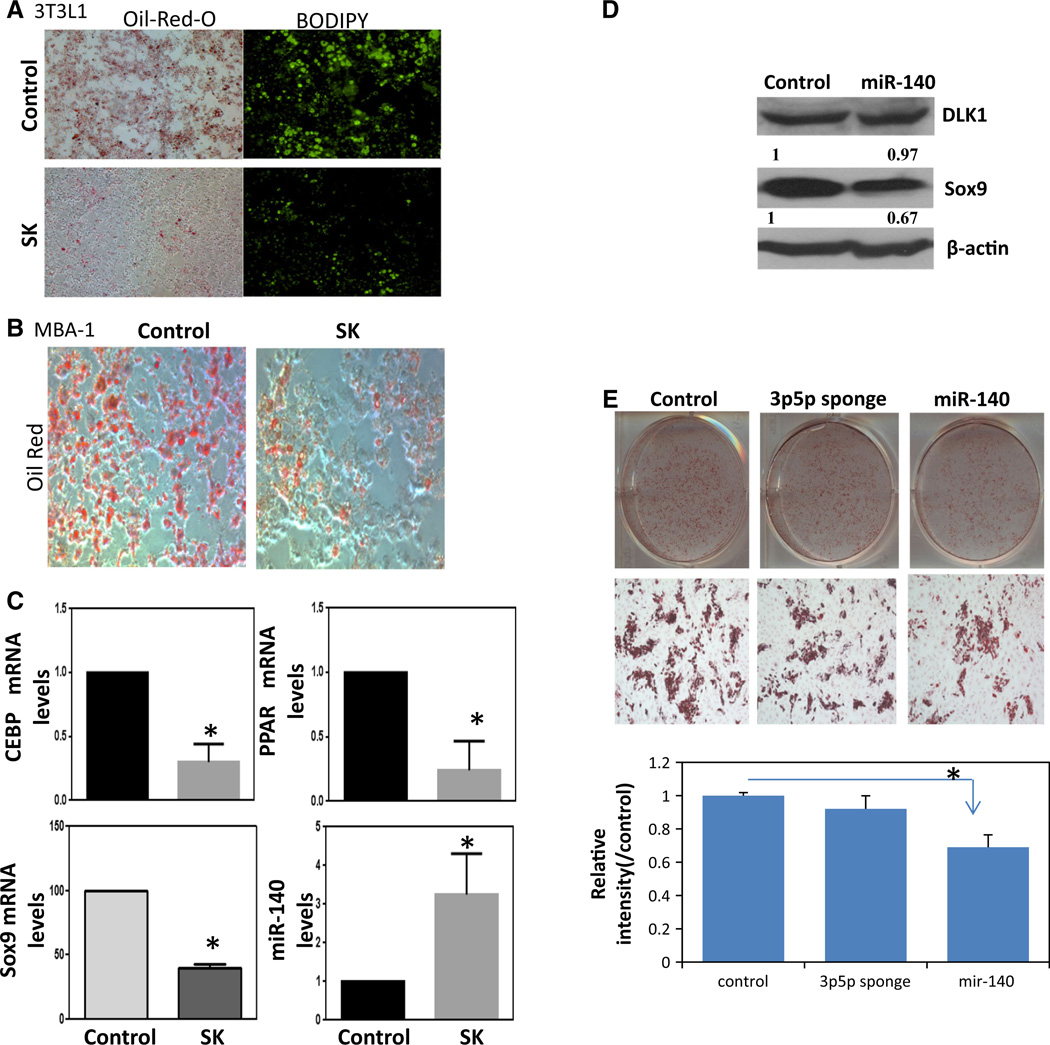
We first investigated the effect of SK on preadipocyte differentiation using the murine preadipocyte cell line 3T3L1. These cells have MSC-like functions and differentiate into mature adipocytes upon stimulation with differentiation cocktail (D-biotin, dexamethasone, insulin, and IBMX) [7]. 3T3L1 cells were stimulated with the differentiation cocktail plus 2 µM SK, and at the 8th day the number of mature adipocytes was visualized using lipid accumulation staining Oil-Red-O (Fig. 1a, left panel), as well as BODIPY, a cell permeable lipophilic fluorescent dye (Fig. 1a, right panel). SK treatment resulted in remarkably lower lipid accumulation and lipophilic fluorescent staining, suggesting that SK treatment can reduce preadipocyte differentiation (Fig. 1a). To further confirm the inhibitory effect of SK in regard to preadipocyte differentiation, we also examined an additional murine preadipocyte cell line MBA-1. MBA-1 cells were similarly stimulated with the adipocyte differentiation cocktail plus SK. Following the 8th day, MBA-1 cells were stained with Oil-Red-O. SK treatment also decreased preadipocyte differentiation of MBA-1 cells further supporting the ability of SK to inhibit preadipocyte differentiation (Fig. 1b).
Fig. 1.
Shikonin (SK) treatment inhibits preadipocyte differentiation by targeting SOX9/miR-140 signaling pathway, a Shikonin (2µM) resulted in a dramatic decrease in differentiation as evidenced by decrease in lipid droplet accumulation. 3T3L1 cells following differentiation (8th day after stimulation with D-biotin, dexamethasone, insulin, and IBMX cocktail) were stained with Oil-Red-O (Fig. 1a, left panel), as well as BODIPY, a cell permeable lipophilic fluorescent dye (Fig. 1a, right panel). b MBA-1 cells were differentiated following adipocyte differentiation cocktail. Oil-Red-O staining again indicated that the shikonin treatment (2 µM) blocked adipocyte differentiation. c Treatment with shikonin altered molecular markers of adipocyte differentiation. (top panel) qRT-PCR measuring CEBPα and PPARγ mRNA levels following 3T3L1 differentiation with or without shikonin treatment (2 µM). Results were normalized to β-actin levels. (bottom panel) qRT-PCR examining SOX9 and miR-140 levels following 3T3L1 differentiation with or without shikonin treatment (2 µM). Results were normalized to β-actin or U6 snRNA, respectively. d 3T3L1 cells were transfected with mouse miR-140 expression plasmids for 48 h. Whole-cell lysates were collected for Western blot analysis using antibodies against DLK1 and SOX9. Actin was used as a loading control. Densitometry for Western blot was performed using UN-SCAN IT Gel program. e Human breast preadipocytes were differentiated following transfection with miR- 140 sponge or miR-140 expression vector or control vector. Oil-Red- O staining was performed and absorbance measurements were taken with spectrophotometer at 492 nm. *p < 0.05 versus control. Data represent the mean ± SE (n = 4)
We confirmed SK inhibition of preadipocyte differentiation at a molecular level using qRT-PCR to examine PPARγ and C/EBPα expression, two well-known transcription factors that are activated in terminal adipocyte differentiation. mRNA levels of both transcription factors were significantly reduced in SK-treated 3T3L1 cells (Fig. 1c, top panel).
The transcription factor SOX9 has been reported to promote preadipocyte differentiation by binding to promoter regions of C/EBP [25]. Our published studies demonstrate that SOX9 is activated in DCIS stem-like cells where it is regulated via miR-140, which is frequently silenced in DCIS [26]. miR-140 is also a key regulator of stromal cell differentiation [27]. Furthermore, we have found that miR-140 can be activated in breast cancer cells following treatment with chemopreventive compounds [7]. Thus, we tested if the SOX9/miR-140 pathway is involved in SKs ability to repress preadipocyte differentiation. qRT-PCR data showed that upon SK treatment of 3T3L1 cells mRNA levels of SOX9 significantly decreased, while miR-140 level significantly increased (Fig. 1c, bottom panel). Since our previous studies already demonstrated that miR-140 can inhibit SOX9 expression in breast cancer cells through targeting the 3′UTR of SOX9 mRNA [28], we decided to investigate if miR-140 also targets SOX9 in preadipocytes. We transfected mouse miR-140 expression plasmids into 3T3L1 preadipocytes and observed that overexpression of miR-140 leads to a decreased SOX9 protein level but has no effect on the protein level of DLK1, a preadipocyte marker, indicating that miR-140 specifically targets SOX9 in preadipocytes (Fig. 1d). Taken together, these results show that SK inhibits preadipocyte differentiation in part by targeting SOX9/miR-140 signaling pathway.
To further examine the impact of miR-140 modulation on adipogenesis, we transfected human breast preadipocytes with a miR-140 inhibitor sponge or with a miR-140 expression vector (Fig. 1e). Whereas inhibition of miR-140 did not affect adipocyte differentiation, miR-140 overexpression significantly inhibited differentiation as evidenced by decreased Oil-Red-O staining. Together these results suggest that SK activation of miR-140 is one mechanism through which SK can inhibit preadipocyte differentiation.
Shikonin inhibits preadipocyte-derived exosomal signaling pathway
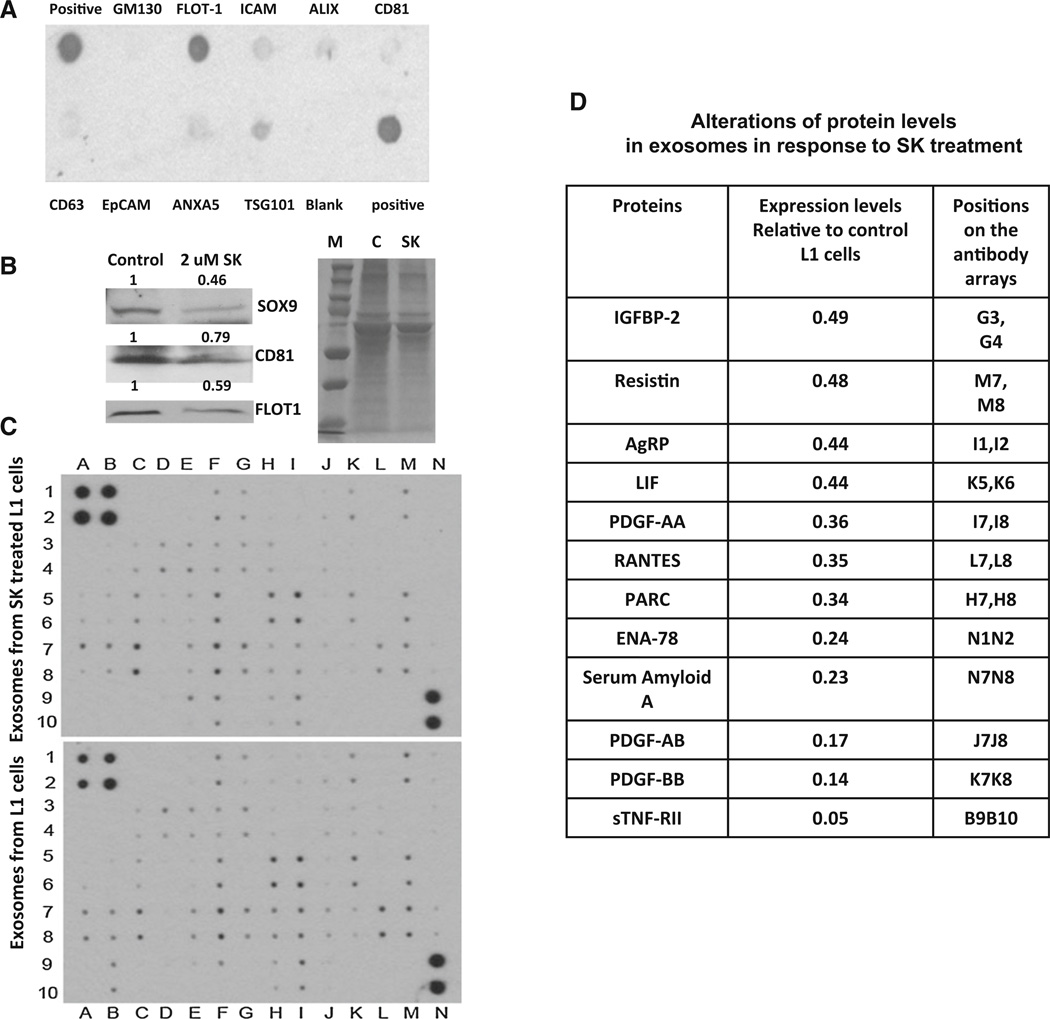
Our observations on the effect of SK on adipogenesis led us to examine the impact of SK on preadipocyte-derived exosomal signaling pathway. As exosomes secreted from preadipocytes can alter the tumor microenvironment and promote tumor invasiveness [29], we postulated that SK treatment of preadipocytes may impact exosomal signaling and could alter breast tumor cell phenotype. To test our hypothesis, we first purified exosomes from preadipocytes using ultracentrifugation [23]. The purity of these exosomes was confirmed using an exosomal antibody array. This array includes well-characterized exosomal protein markers and allows for the confirmation of exosomal recovery.
The array revealed positive signals for CD81, ICAM, CD63, ALIX, TSG101, and FLOT-1 all frequently observed exosomal proteins, indicating successful exosome isolation. The isolated exosomes were free of cell debris and other contaminants, as demonstrated by the negative staining for GM130, a cis-Golgi matrix protein. Pre-adipocyte-derived exosomes were strongly positive for flotillin-1 (FLOT-1), a caveolae-associated integral membrane protein of unknown function (Fig. 2a). After confirming exosome purity, we isolated exosomes from 3T3L1 treated with or without SK and compared exosomal protein expression. Figure 2b shows that exosomes derived from 3T3L1 cells are highly enriched in SOX9 proteins. We observed a significant decrease in exosomal SOX9 expression in addition to several exosomal marker proteins (FLOT1 and CD81) in exosomes derived from SK-treated 3T3L1 cells (gel staining was used to control for total protein content). Since exosomes often contain growth factors and cytokines that can be transferred into recipient cells and modulate their functions, we performed a protein array to examine the impact of SK on exosomal secretion of growth factors and cytokines (Fig. 2c, d). We observed that treatment with SK leads to a dramatic decrease in exosomal secretion of many growth factors and cytokines (Fig. 2d) directly related to tumor growth, angio-genesis, and invasion.
Fig. 2.
Shikonin treatment inhibits exosomal secretion of growth factors and cytokines. a Exosomes from 3T3L1(L1) cells were collected and exosome protein array was used for detection of exosome markers commonly associated with normal and tumor-derived exosomes including negative control antibody to control for cellular debris (GM130). b Western blot was performed for exosomes from L1 cells treated with or without 2 µM shikonin (SK) for 48 h. FLOT1, SOX9, and CD81 were expressed in L1-derived exosomes, whereas SK treatment significantly reduced FLOT1, SOX9, and CD81 in Ll-derived exosomes. Colloidal blue-stained gel was used as a loading control. c Exosomes from L1 cells treated with or without 2 µM SK for 48 h were collected and exosome proteins were incubated with array membrane to detect cytokines. Signals were quantified using imageJ and alterations of exosome proteins were summarized in d
Shikonin inhibits preadipocyte-derived exosomal signaling that supports DCIS stem cell formation
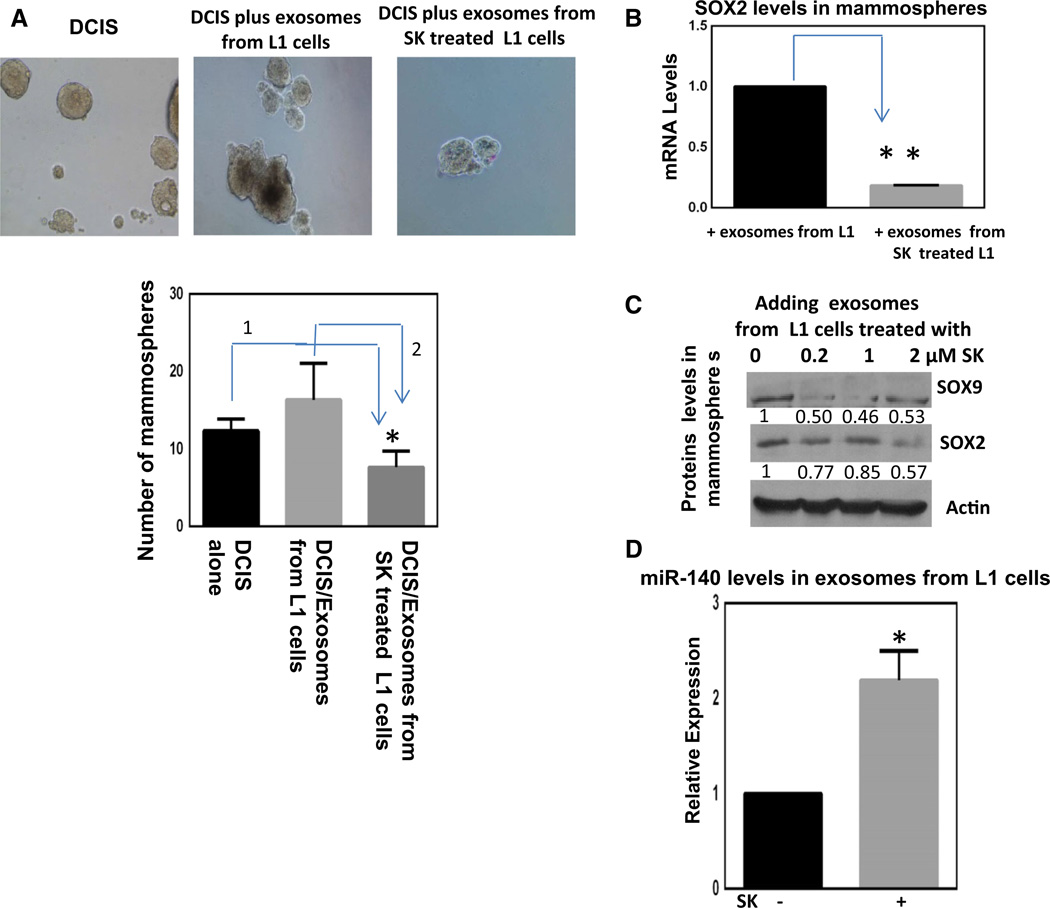
Exosomes express surface makers that recognize recipient cells and facilitate intercellular transfer of miRNAs and proteins [11]. To study the role of preadipocyte-derived exosomes in cancer stem cell signaling, MCF10DCIS (DCIS) cells, representing early-stage breast cancer cells [28], were treated with preadipocyte-derived exosomes in mammosphere cultures, where stem-like cells remain viable and grow as non-adherent spheres. We have previously shown that mammosphere culture of DCIS cells enriches for cancer stem cells with an enhanced migratory phenotype [28]. Exosomes were isolated from 3T3L1 cells treated with or without SK, and these exosomes were labeled with PKH26, a red fluorescent dye, to confirm that they indeed were taken up by the mammosphere cells (Fig. 3a). In the presence of exosomes isolated from 3T3L1 cells, DCIS cells showed significant increases in mammosphere formation, suggesting exosomes promote cancer stem cell renewal. In contrast, exosomes derived from SK-treated 3T3L1 cells failed to promote cancer stem cell renewal as evidenced by a decrease in mammosphere formation.
Fig. 3.
Preadipocyte-derived exosomes can modify DCIS stem cell renewal and shikonin treatment can alter exosomal signaling inhibiting DCIS stem cells, a MCF10DCIS (DCIS) mammosphere cultured with exosomes from preadipocytes (L1 cells) with or without 2 µM shikonin treatment for 48 h. Exosomes were stained with PKH26, a red fluorescent dye to visualize uptake into DCIS cells. b At the end of the experiment in a, DCIS mammospheres were collected and SOX2 mRNAs were measured via qRT-PCR, normalizing to β-actin levels. In parallel, proteins were collected and Western blotting was performed for SOX2 and SOX9 normalizing to β-actin (c). d qRT-PCR was conducted on exosomal RNA extracts from preadipocytes with or without shikonin treatment (2 µM) for 48 h. miR-140 levels were measured and normalized to U6 snRNA. Data represent the mean ± SE (n = 3). *p < 0.05, **p < 0.01
We have shown that exosomes derived from 3T3L1 cells contain detectable levels of SOX9 protein (Fig. 2b). In order to elucidate the mechanism through which exosomes derived from SK-treated 3T3L1 cells inhibit DCIS mammosphere formation, we checked the mRNA levels of SOX2 and SOX9 in DCIS mammospheres following addition of exosomes derived from SK-treated 3T3L1 cells. We have previously demonstrated that the pluripotency factor SOX2 is also directly targeted via miR-140 and can regulate stem cell signaling in breast cancer cells [26]. mRNA levels of SOX2 but not SOX9 (data not shown) were significantly reduced in DCIS mammospheres following incubation with exosomes isolated from SK-treated 3T3L1 cells (Fig. 3b). We then examined the effect of SK-treated exosomes on SOX2 and SOX9 protein levels in DCIS mammospheres. The protein levels of both SOX2 and SOX9 in DCIS mammospheres reduced in a dose-dependent manner upon incubation with SK-treated exosomes derived from 3T3L1 cells (Fig. 3c). Moreover, qRT-PCR of isolated exosomal RNA showed that exosomes from SK-treated 3T3 L1 cells have significantly higher levels of miR-140 (Fig. 3d), mirroring the effect that was seen in SK-treated 3T3L1 cells (Fig. 1c). These results suggest that treatment of tumor microenvironment with SK could inhibit cancer stem cell function via preadipocyte-derived exosomal miR-140 targeting SOX2/SOX9 in breast cancer stem cells.
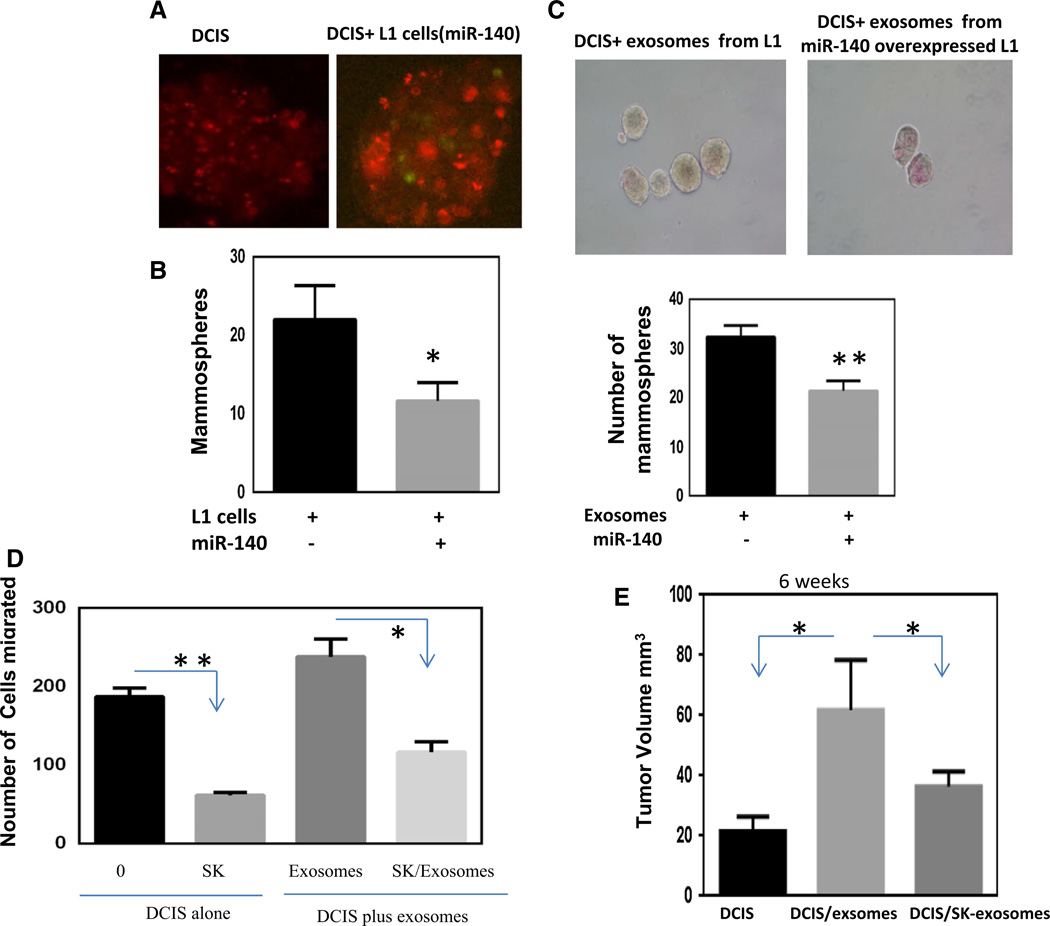
We further investigated the potential for exosomal miR-140 to impact stem cell signaling in breast cancer cells. DCIS cells labeled with PKH26 were mixed with 3T3L1 cells transfected with GFP-miR-140 expression vector at a 1:1 ratio and seeded for mammosphere formation. DCIS cells mixed with miR-140-transfected 3T3L1 cells formed a significantly lower number of mammospheres compared to DCIS + 3T3L1 control cells (Fig. 4a, b). Next, 3T3L1 cells were transfected with miR-140 and exosomes were collected. The exosomes were then labeled with PKH26 and mixed with DCIS cells and mammosphere formation was examined. DCIS cells treated with exosomes from miR-140-transfected 3T3L1 cells formed significantly fewer and smaller mammospheres compared to control exosomes (Fig. 4c). These data suggest that activation of miR-140 in preadipocyte-derived exosomes could inhibit breast cancer stem cell renewal.
Fig. 4.
Preadipocyte-derived exosomal miR-140 can target DCIS stem cells via inhibiting SOX9 expression. a MCF10DCIS (DCIS) cells labeled with PKH26 red fluorescent dye were cultured in mammosphere conditions with control 3T3L1 (L1, no GFP) cells or L1 cells transfected with miR-140 vector containing GFP. b Mam-mospheres were quantified using light microscopy (spheres >100 µrn were counted) from 5 random fields. c Exosomes derived from L1 cells treated with or without shikonin treatment (2 µM) for 48 h were added to mammosphere cultures of DCIS cells. c Exosomes were isolated from L1 control or miR-140 overexpressing L1 cells and labeled with PKH26 red fluorescent dye. Exosomes were then added to DCIS mammosphere cultures and mammospheres were quantified (spheres >100 µm) after 8 days. Data represent the mean ± SE (n = 3). Preadipocyte-derived exosomes can regulate cell migration and tumor formation. d Cell migration assay was performed on DCIS cells with or without shikonin treatment (2 µM) for 48 h. DCIS cells were also grown for 48 h in co-culture conditions with exosomes from preadipocytes (±2 µM shikonin for 48 h) and then cell migration assays were performed. Cells were plated in a transwell insert with 8 lm pore size in serum-free media. 10 % serum was added to the bottom chamber as a chemoattractant. Cells were stained with crystal violet and visualized by light microscopy, and 5 random fields were quantified. e Xenografts of 1 × 106 DCIS cells alone, DCIS + exosomes (100 µl) isolated from L1 cells, or DCIS + exosomes derived from L1 cells treated with shikonin (2 µM) for 48 h were injected into mammary glands of nude mice and tumor size was measured using digital calipers (length × width2)/2. Data represent the mean ± SE (n = 7). *p < 0.05. **p < 0.01
Shikonin-dependent inhibition of cell migration can be mediated by exosomes
Preadipocytes play a significant role in breast tumor progression mediated through cytokine and growth factor secretion, and exosomal signaling [6, 7]. We next investigated whether SK could inhibit preadipocyte-mediated increases in tumor cell invasion. We first cultured DCIS cells alone and checked their migration ability upon SK treatment. SK treatment significantly reduced the number of migrating DCIS cells (Fig. 4d). We then cocultured DCIS cells with exosomes from 3T3L1 cells for 48 h prior to seeding them for migration assay. Cell migration was significantly enhanced following co-culture with exosomes derived from preadipocytes. We then cocultured DCIS with exosomes from SK-pretreated 3T3L1 cells and observed a significant decrease in tumor cell migration. These data suggest that SK treatment inhibits breast cancer cell migration through targeting breast cancer cells as well as exosomes derived from preadipocytes.
Exosomes promotes tumor formation in vivo
In vitro studies showed that exosomes from 3T3L1 preadipocytes contribute significantly to the aggressive behavior of DCIS cells (Fig. 4d). To validate these results in vivo, DCIS cells with or without exosomes isolated from 3T3L1 cells were injected into the mammary gland of 5–6-week-old immunodeficient Nu/Nu mice. Six weeks after the injection, the mice were sacrificed and tumor growth was compared to the tumors of the mice injected with DCIS cells only. The tumor volume of the mice injected with DCIS with exosomes from 3T3L1 cells was significantly larger than the tumor volume of mice injected with DCIS cells alone (Fig. 4e). When the mice were injected with DCIS cells along with exosomes isolated from SK-treated 3T3L1 cells, the tumor volume was observed to be significantly smaller than tumors derived from DCIS and control exosomes. These data demonstrate that preadipocyte-derived exosomes within the tumor microenvironment are essential for tumor formation. Our data also confirm that SK inhibition of tumor formation can be mediated through preadipocyte-secreted exosomes in vivo.
Discussion
Many studies have reported on the strong antitumor activity of SK in breast tumor cells [18–20]. For the first time, here, we report on the potential for SK to inhibit tumor growth by targeting preadipocyte signaling in the tumor microenvironment. We found that SK treatment could inhibit preadipocyte differentiation via a miR-140/SOX9 mechanism. As preadipocyte cells can promote breast tumor progression, this suggests that SK may be a potent chemopreventive compound able to target both tumor cells and activated tumor microenvironment.
Signaling within the tumor microenvironment has been reported to occur via secreted cytokines and growth factors [24]. Recent reports indicate that exosomal secretion by both tumor and stromal cells may also play an important role in cell–cell signaling in the tumor microenvironment [14, 17]. We first observed that SK could dramatically alter exosomal protein content including SOX9 and numerous pro-tumorigenic growth factors and cytokines. Next, we observed altered miR-140 expression in exosomes derived from SK-treated preadipocytes. We found that incubation with exosomes derived from SK-treated preadipocytes could dramatically alter DCIS mammosphere formation. This suggests that preadipocyte-derived exosomes may impact breast tumorigenesis via signaling with cancer stem cells.
Previously, we have reported that miR-140 is a powerful regulator of DCIS stem cells [28]. In addition, we found that treatment with the chemopreventive compound sulforaphane altered exosomal secretion of miR-140 from DCIS cells. Here we have found that co-culture with miR-140 overexpressing preadipocytes can impact mammosphere formation of DCIS cells. Furthermore, we found that this response was dependent on expression of the miR-140 target gene SOX9 in DCIS cells. These results suggest that exosomal secretion of miR-140 from preadipocytes can impact DCIS stem cells via targeting SOX9 transcript in target cells.
It has been previously reported that co-culture of adipocytes and breast cancer cells results in a more aggressive phenotype, enhanced invasiveness, and increased tumor growth in vivo [7]. We further observed a significant increase in migration of DCIS cells following co-culture with exosomes secreted from preadipocytes. We also observed a significant decrease in tumor cell migration of DCIS cells co-cultured with exosomes derived from SK-pretreated preadipocytes. Finally, preadipocyte-derived exosomes dramatically increase tumor growth of DCIS cells in vivo. This is powerful evidence that the intercellular signaling mediated by exosomes plays an important part in regulating breast tumorigenesis.
Taken together, these results provide further demonstration of the importance of tumor microenvironmental signaling to tumor progression. We find that exosomal signaling between preadipocytes and breast cancer cells can regulate tumor stem cell formation and tumor cell migration. Finally, our results suggest a new potential strategy for inhibiting breast tumorigenesis, by targeting preadipocyte signaling within the tumor microenvironment.
Acknowledgments
This work was supported by Grants from the NCI R01 (Q.Z), the American Cancer Society (Q.Z.) and NCI 5F31CA183522 (G.E.).
Footnotes
Conflict of interest All authors declare that they have no conflict of interest.
Contributor Information
Ramkishore Gernapudi, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Yuan Yao, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Yongshu Zhang, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Benjamin Wolfson, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Sanchita Roy, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Nadire Duru, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Gabriel Eades, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Peixin Yang, Department of Obstetrics, Gynecology & Reproductive Sciences at University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Qun Zhou, Email: qzhou@som.umaryland.edu, Department of Biochemistry and Molecular Biology, Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
References
- 1.Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004;96:906–920. doi: 10.1093/jnci/djh164. [DOI] [PubMed] [Google Scholar]
- 2.Fowble B, Hanlon A, Fein D, Hoffman J, Sigurdson E, Patchefsky A, Kessler H. Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS) Int J Radiat Oncol. 1997;38:949–957. doi: 10.1016/s0360-3016(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 3.Cristancho A, Lazar M. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tebbe C, Chhina J, Dar S, Sarigiannis K, Giri S, Munkarah A, Rattan R. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget. 2014;5:4746. doi: 10.18632/oncotarget.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delort L, Lequeux C, Dubois V, Dubouloz A, Billard A, Mojallal A, Damour O, Vasson M, Caldefie-Chézet F. Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3D adipose equivalent model. PLoS One. 2013;8:431–137. doi: 10.1371/journal.pone.0066284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang Y, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Xia J, Yao Y, Gong D, Shi H, Zhou Q. Sulforaphane inhibits mammary adipogenesis by targeting adipose mesenchymal stem cells. Breast Cancer Res Treat. 2013;141:317–324. doi: 10.1007/s10549-013-2672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochet L, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5667. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 9.Barcellos-Hoff M, Lyden D, Wang T. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer. 2013;13:511–518. doi: 10.1038/nrc3536. [DOI] [PubMed] [Google Scholar]
- 10.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 11.Denzer K, Kleijmeer M, Heijnen H, Stoorvogel W, Geuze H. Exosomes from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;2113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 12.Van den Boom J, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2013;65:331–335. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Azmi A. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christoph K, Raghu K. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91:431–137. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang J, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Park S, Jung B, Jeon Y, Lee Y, Kim M, Kim Y, Jang J, Kim C. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Eades G, Yao Y, Zhang Y, Zhou Q. Characterization of a stem-like subpopulation in basal-like ductal carcinoma in situ (DCIS) lesions. J Biol Chem. 2014;289:1303–1312. doi: 10.1074/jbc.M113.502278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao Y, Zhou Q. A novel antiestrogen agent Shikonin inhibits estrogen-dependent gene transcription in human breast cancer cells. Breast Cancer Res Treat. 2010;121:233–240. doi: 10.1007/s10549-009-0547-2. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Brodie A, Davidson N, Kensler T, Zhou Q. Inhibition of estrogen signaling activates the NRF2 pathway in breast cancer. Breast Cancer Res Treat. 2010;124:585–591. doi: 10.1007/s10549-010-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Qian R, Li P. Shikonin, an ingredient of Lithos-permum erythrorhizon, down-regulates the expression of steroid sulfatase genes in breast cancer cells. Cancer Lett. 2009;284:47–54. doi: 10.1016/j.canlet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–26002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spangenburg E, Stephen JPP, Lindsay MW, Richard L. Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle. J Biomed Biotechnol. 2011;2011:598358. doi: 10.1155/2011/598358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 24.Iyengar P, Combs T, Shah S, Gouon-Evans V, Pollard J, Al-banese C, Flanagan L, Tenniswood M, Guha C, Lisanti M, Pestell R, Scherer P. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Sul H. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Eades G, Yao Y, Li Q, Zhou Q. Estrogen receptor α signaling regulates breast tumor-initiating cells by downregulating miR- 140 which targets the transcription factor SOX2. J Biol Chem. 2012;287:41514–41522. doi: 10.1074/jbc.M112.404871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyaki S, Nakasa T, Otsuki S, et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Yao Y, Eades G, Liu Z, Zhang Y, Zhou Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589–2600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessitore L, Vizio B, Pesola D, Cecchini F, Mussa A, Argiles J, Benedetto C. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int J Oncol. 2014;24:1529–1535. [PubMed] [Google Scholar]